Elan: erst der Anfang! - 500 Beiträge pro Seite (Seite 3)
eröffnet am 03.05.05 10:07:23 von
neuester Beitrag 14.04.09 20:29:58 von
neuester Beitrag 14.04.09 20:29:58 von
Beiträge: 1.421
ID: 978.699
ID: 978.699
Aufrufe heute: 0
Gesamt: 111.443
Gesamt: 111.443
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| 01.05.24, 18:36 | 1249 | |
| gestern 22:25 | 424 | |
| heute 00:17 | 312 | |
| gestern 23:38 | 244 | |
| heute 00:00 | 160 | |
| vor 1 Stunde | 142 | |
| gestern 23:53 | 138 | |
| gestern 23:59 | 120 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 18.001,60 | +0,59 | 240 | |||
| 2. | 2. | 181,19 | +0,66 | 87 | |||
| 3. | 3. | 9,7000 | +12,27 | 75 | |||
| 4. | 14. | 6,1400 | -1,35 | 69 | |||
| 5. | 11. | 0,1865 | 0,00 | 52 | |||
| 6. | 7. | 0,8750 | -12,50 | 47 | |||
| 7. | 12. | 0,1561 | +2,97 | 38 | |||
| 8. | 6. | 2.302,50 | 0,00 | 36 |
Antwort auf Beitrag Nr.: 24.644.268 von Birgit.Tersteegen am 15.10.06 21:22:55Hi Birgit!
das hier ist ein wirklich ein klasse Fund (dank sei investorvillage)
http://www.nature.com/nm/journal/v12/n7/full/nm0706-780.html
Für mich ein absoluter Basisartikel über Alzheimer:
+ Entwicklung der Erkrankungen
+ Entwicklung der Kosten
+ Aktuelle Medikamente
+ Medikamente/Wirkstoffe in Forschung
interessant ist dieser Satz:
"In our opinion, the most exciting drug in development is Elan and Wyeth's bapineuzumab (AAB-001), a humanized monoclonal antibody against amyloid-, which is in phase 2 trials ...."
Na dann lassen wir uns mal überraschen
das hier ist ein wirklich ein klasse Fund (dank sei investorvillage)

http://www.nature.com/nm/journal/v12/n7/full/nm0706-780.html
Für mich ein absoluter Basisartikel über Alzheimer:
+ Entwicklung der Erkrankungen
+ Entwicklung der Kosten
+ Aktuelle Medikamente
+ Medikamente/Wirkstoffe in Forschung
interessant ist dieser Satz:
"In our opinion, the most exciting drug in development is Elan and Wyeth's bapineuzumab (AAB-001), a humanized monoclonal antibody against amyloid-, which is in phase 2 trials ...."
Na dann lassen wir uns mal überraschen

Antwort auf Beitrag Nr.: 24.644.580 von tippse am 15.10.06 21:49:41Beide Funde freuen uns---der Erste weil es zeigt,dass auch die Presse langsam das Potential von TY erkennt...der Zweite belegt die wahnsinns Pipeline von Elan....Also:Auf viele weitere gute Funde und steigende Kurse....

NY Times Editorial on Alzheimer's
October 16, 2006
An Alzheimer’s Treatment Debunked
More and more often, it seems, drugs that were widely thought to be effective against serious illnesses turn out to show little or no value when tested in large, impartial clinical trials insulated from drug company influence. The latest example is a class of drugs known as atypical antipsychotics that are commonly used to soothe agitation, delusions and aggression in people with Alzheimer’s disease.
A government-sponsored study published in The New England Journal of Medicine last week found that the drugs are no more effective than placebos for most patients and carry troubling side effects, like sedation and confusion.
This was the third major study in the last year to cast doubt on the atypical antipsychotics, which were supposedly a significant advance over the first generation of antipsychotics. The earlier drugs had been enormously successful in alleviating the symptoms of schizophrenia, allowing patients to leave hospitals. But they often caused severe side effects that the newer drugs were designed to avoid.
Unfortunately, enthusiasm for the newer drugs proved misplaced. A government-sponsored study published last year found that three of the atypical antipsychotics were no better than an older, far cheaper drug for treating schizophrenia. A study sponsored by the National Health Service in Britain reported last week that schizophrenics did as well or better on the first-generation drugs.
The latest study focuses on the “off label” use of atypical antipsychotics to treat Alzheimer’s patients for the agitation and behavioral problems that at some point afflict most of them. Their popularity for this unapproved use — despite a label warning of dangerous side effects — reflects the dearth of better options, and word-of-mouth promotion by company-sponsored doctors.
The new study, which tested three of the atypical antipsychotics in a group of 421 patients around the country, found them no better than a placebo. That does not mean that the drugs are useless. They may be helpful to some patients who are carefully chosen and monitored by their physicians.
These discouraging results speak mostly to the desperate need for effective new treatments for Alzheimer’s, as well as the need to be wary of off-label uses that are not supported by well-conducted trials. But they also underscore the great value of impartial, government-sponsored studies that have — three times in the past year — punctured the myth that new and expensive brand-name drugs are necessarily superior to older and cheaper medications.
http://www.investorvillage.com/smbd.asp?mb=160&mn=33169&pt=m…
October 16, 2006
An Alzheimer’s Treatment Debunked
More and more often, it seems, drugs that were widely thought to be effective against serious illnesses turn out to show little or no value when tested in large, impartial clinical trials insulated from drug company influence. The latest example is a class of drugs known as atypical antipsychotics that are commonly used to soothe agitation, delusions and aggression in people with Alzheimer’s disease.
A government-sponsored study published in The New England Journal of Medicine last week found that the drugs are no more effective than placebos for most patients and carry troubling side effects, like sedation and confusion.
This was the third major study in the last year to cast doubt on the atypical antipsychotics, which were supposedly a significant advance over the first generation of antipsychotics. The earlier drugs had been enormously successful in alleviating the symptoms of schizophrenia, allowing patients to leave hospitals. But they often caused severe side effects that the newer drugs were designed to avoid.
Unfortunately, enthusiasm for the newer drugs proved misplaced. A government-sponsored study published last year found that three of the atypical antipsychotics were no better than an older, far cheaper drug for treating schizophrenia. A study sponsored by the National Health Service in Britain reported last week that schizophrenics did as well or better on the first-generation drugs.
The latest study focuses on the “off label” use of atypical antipsychotics to treat Alzheimer’s patients for the agitation and behavioral problems that at some point afflict most of them. Their popularity for this unapproved use — despite a label warning of dangerous side effects — reflects the dearth of better options, and word-of-mouth promotion by company-sponsored doctors.
The new study, which tested three of the atypical antipsychotics in a group of 421 patients around the country, found them no better than a placebo. That does not mean that the drugs are useless. They may be helpful to some patients who are carefully chosen and monitored by their physicians.
These discouraging results speak mostly to the desperate need for effective new treatments for Alzheimer’s, as well as the need to be wary of off-label uses that are not supported by well-conducted trials. But they also underscore the great value of impartial, government-sponsored studies that have — three times in the past year — punctured the myth that new and expensive brand-name drugs are necessarily superior to older and cheaper medications.
http://www.investorvillage.com/smbd.asp?mb=160&mn=33169&pt=m…
Antwort auf Beitrag Nr.: 24.651.760 von bernie55 am 16.10.06 10:01:32Falls AAB-001 (generic name "Bapineuzumab") diesen Winter frühzeitig in Phase 3 konvertiert, dann gibt es ein erstes "Aber Hallo"! Ich drück uns und den vielen Alzheimer-Erkrankten die Daumen.
In our opinion, the most exciting drug in development is Elan and Wyeth's bapineuzumab (AAB-001), a humanized monoclonal antibody against amyloid-, which is in phase 2 trials. As part of an alliance investigating a number of opportunities including the amyloid- vaccine ACC-001, Elan and Wyeth are concentrating on immunotherapeutic strategies for Alzheimer disease in the hope that they will lead to a truly disease-modifying drug. Evidence of efficacy with AAB-001 has already been obtained (Black, R.S. et al., presented at 9th Annual Geneva Springfield Symposium on Advances in Alzheimer's Therapy in Geneva, Switzerland). These drug candidates may also carry the highest risk, however; the highest dose of AAB-001 has now been discontinued owing to transient abnormalities observed through brain imaging (Black, R.S. et al., presented at 9th Annual Geneva Springfield Symposium on Advances in Alzheimer's Therapy in Geneva, Switzerland). An earlier candidate drug developed to trigger an immune response against amyloid-, AN-1792, was also discontinued owing to cases of encephalitis.17
http://www.nature.com/nm/journal/v12/n7/full/nm0706-780.html
In our opinion, the most exciting drug in development is Elan and Wyeth's bapineuzumab (AAB-001), a humanized monoclonal antibody against amyloid-, which is in phase 2 trials. As part of an alliance investigating a number of opportunities including the amyloid- vaccine ACC-001, Elan and Wyeth are concentrating on immunotherapeutic strategies for Alzheimer disease in the hope that they will lead to a truly disease-modifying drug. Evidence of efficacy with AAB-001 has already been obtained (Black, R.S. et al., presented at 9th Annual Geneva Springfield Symposium on Advances in Alzheimer's Therapy in Geneva, Switzerland). These drug candidates may also carry the highest risk, however; the highest dose of AAB-001 has now been discontinued owing to transient abnormalities observed through brain imaging (Black, R.S. et al., presented at 9th Annual Geneva Springfield Symposium on Advances in Alzheimer's Therapy in Geneva, Switzerland). An earlier candidate drug developed to trigger an immune response against amyloid-, AN-1792, was also discontinued owing to cases of encephalitis.17
http://www.nature.com/nm/journal/v12/n7/full/nm0706-780.html


G. Kelly Martin
President and Chief Executive Officer
Elan Corporation, plc
October 17, 2006
12:00 p.m. - 1:30 p.m.
Kelly Martin was named Elan President and Chief Executive Officer in January 2003. He is an officer of the Company, a member of the Elan Leadership Team, and a member of the Elan Board of Directors.
From 2001 to 2003, Mr. Martin was president of Merrill Lynch’s International Private Client group and a member of the company’s Executive Management and Operating Committees. During his career at Merrill Lynch, Mr. Martin oversaw the Global Debt Markets group (1998-2001), served as chief technology officer for the Corporate and Institutional Client Group (1995-1998), and was chief operating officer for the Investment Banking group (1993-1995). Mr. Martin began his career at Merrill Lynch with sales and management assignments in New York, Tokyo and London.
Mr. Martin has been on the Board of the Kennedy Center Corporate Fund since March 2003. He received his B.A. degree in politics from Princeton University.
http://www.biotech2006.org/content/view/16/33/
Antwort auf Beitrag Nr.: 24.672.180 von bernie55 am 17.10.06 09:50:14Throughout the two-day event, (www.biotech2006.org), more than 80 speakers, including 43 C-level executives, will discuss topics related to the financing and growth of the bioscience industry; cutting-edge science; the convergence of the biotechnology and medical device sectors; and opportunities in the global marketplace, among many other topics.
There will be a wealth of information and numerous interview opportunities for reporters who cover a variety of issues, including business, health, and science and human resources. Biotech 2006 is the largest such conference held in the region
...G. Kelly Martin, president & CEO of Elan Corporation, plc will
speak at the lunch on October 17th. He will highlight his
experience at the helm of Elan over the past three years,
overcoming the challenges through his focus on innovation,and
what is in store for the future.
......dann werden die " Business Reporters " mal das Potential von ELAN erkennen und etwas mehr über ELAN berichten....
http://www.omniomix.com/inthenews.php?id=66610
There will be a wealth of information and numerous interview opportunities for reporters who cover a variety of issues, including business, health, and science and human resources. Biotech 2006 is the largest such conference held in the region
...G. Kelly Martin, president & CEO of Elan Corporation, plc will
speak at the lunch on October 17th. He will highlight his
experience at the helm of Elan over the past three years,
overcoming the challenges through his focus on innovation,and
what is in store for the future.
......dann werden die " Business Reporters " mal das Potential von ELAN erkennen und etwas mehr über ELAN berichten....

http://www.omniomix.com/inthenews.php?id=66610
..ein sehr interessanter Videofilm über " Alzheimer Disease "....
http://cbs5.com/video/?id=8905@kpix.dayport.com
http://cbs5.com/video/?id=8905@kpix.dayport.com
Elan Corporation: Real Potential for Early Filing of AAB-001 for Alzheimer's Disease
The focus of Elan' s pipeline is on the lead Alzheimer' s Disease product, AAB-001, which is currently in Phase II studies. Over the next 3 months, interim analysis of the Phase II data with AAB-001 has the potential to transform Elan's investment case.
We believe that the market is not aware of the potential for AAB-001 to progress directly from Phase II to filing for marketing approval but rather has focused on progress to Phase III trials as the best case for AAB-001. Investors should be alert to the possibility of a more rapid progress to market of AAB-001 based on a review of both the trial design and FDA regulatory procedures. We believe that Elan/Wyeth will consider this option if the Phase II efficacy data is strong. In our view, coupling evidence of plaque reduction by AAB-001 with statistical efficacy from a cognitive/quality of life endpoint would provide a compelling case for filing AAB-001 for approval post completion of the Phase II study.
Assuming that AAB-001 has a tolerable level of safety and is effective in halting/slowing the clinical decline of patients with the disease, AAB-001 could become a significant new therapeutic option with disease-modifying effects. The requirements for a disease-modifying drug for this disease are huge considering that there are c.24m people globally with the disease with 4.5m affected in the US alone.
A valuation assessment based on NPV/sum-of-the-parts supports a price target of $16.55-$19.18.
In our analysis, we have not included the possibility of either AAB-001 progressing to Phase III studies or a filing for approval based on Phase II data alone.
Progress of AAB-001 to Phase III would increase our probability of success from + c.25% to c.50% and add c.30% to our valuation range.
A filing post Phase II would increase our probability of success to at least + c.60% and add c.50% to our valuation range.
We maintain our Buy recommendation !!!!.
http://www.investorvillage.com/smbd.asp?mb=160&mn=35395&pt=m…
The focus of Elan' s pipeline is on the lead Alzheimer' s Disease product, AAB-001, which is currently in Phase II studies. Over the next 3 months, interim analysis of the Phase II data with AAB-001 has the potential to transform Elan's investment case.
We believe that the market is not aware of the potential for AAB-001 to progress directly from Phase II to filing for marketing approval but rather has focused on progress to Phase III trials as the best case for AAB-001. Investors should be alert to the possibility of a more rapid progress to market of AAB-001 based on a review of both the trial design and FDA regulatory procedures. We believe that Elan/Wyeth will consider this option if the Phase II efficacy data is strong. In our view, coupling evidence of plaque reduction by AAB-001 with statistical efficacy from a cognitive/quality of life endpoint would provide a compelling case for filing AAB-001 for approval post completion of the Phase II study.
Assuming that AAB-001 has a tolerable level of safety and is effective in halting/slowing the clinical decline of patients with the disease, AAB-001 could become a significant new therapeutic option with disease-modifying effects. The requirements for a disease-modifying drug for this disease are huge considering that there are c.24m people globally with the disease with 4.5m affected in the US alone.
A valuation assessment based on NPV/sum-of-the-parts supports a price target of $16.55-$19.18.
In our analysis, we have not included the possibility of either AAB-001 progressing to Phase III studies or a filing for approval based on Phase II data alone.
Progress of AAB-001 to Phase III would increase our probability of success from + c.25% to c.50% and add c.30% to our valuation range.
A filing post Phase II would increase our probability of success to at least + c.60% and add c.50% to our valuation range.
We maintain our Buy recommendation !!!!.
http://www.investorvillage.com/smbd.asp?mb=160&mn=35395&pt=m…
bisher wird Alzheimer so gut wie gar nicht therapiert!
Anbei eine Zusammenfassung über Kosten und (bescheidener) Nutzen der am Markt etablierten Wirkstoffe:
http://www.crbestbuydrugs.org/drugreport_DR_alzheimers.html
Our Recommendations — A Summary
The medicines used to slow mental decline in people with Alzheimer's disease are not particularly effective. When compared with a placebo, only 10 to 20 percent more people taking an Alzheimer's drug seem to benefit at all. And it is the rare person who has a significant delay in the worsening of their symptoms over time.
Anbei eine Zusammenfassung über Kosten und (bescheidener) Nutzen der am Markt etablierten Wirkstoffe:
http://www.crbestbuydrugs.org/drugreport_DR_alzheimers.html
Our Recommendations — A Summary
The medicines used to slow mental decline in people with Alzheimer's disease are not particularly effective. When compared with a placebo, only 10 to 20 percent more people taking an Alzheimer's drug seem to benefit at all. And it is the rare person who has a significant delay in the worsening of their symptoms over time.
...ach ja, diese Botschaft sollten wir mit ins Wochenende nehmen:
Red wine slows Alzheimer's-like disease in mice
http://www.nlm.nih.gov/medlineplus/news/fullstory_39208.html
Red wine slows Alzheimer's-like disease in mice
http://www.nlm.nih.gov/medlineplus/news/fullstory_39208.html
Antwort auf Beitrag Nr.: 24.737.559 von Cyberhexe am 20.10.06 11:29:45neben Rotwein, scheint dies momentan wohl der aussichtsreichste Wirkstoff zur Therapie von AD zu sein:
http://www.clinicaltrials.gov/ct/show/NCT00112073
...sollte ein frühzeitiges Konvertieren in phaseIII stattfinden, dann werden wir unsere Freude haben!
http://www.clinicaltrials.gov/ct/show/NCT00112073
...sollte ein frühzeitiges Konvertieren in phaseIII stattfinden, dann werden wir unsere Freude haben!
Antwort auf Beitrag Nr.: 24.739.706 von Cyberhexe am 20.10.06 13:08:16...und auch noch eine interessante Zusammenfassung über den Stand der Forschung bei AD, herausgegeben von der FDA:
http://www.fda.gov/fdac/features/2003/403_alz.html
im folgenden Absatz ist AN-1792 gemeint, das erste Vaccin und Vorläufersubstanz von ACC-001, mit welchem die Immunreaktion stimuliert werden soll. AAB-001 ist der Antikörper dazu, der über eine passive Immunantwort das Fortschreiten der Plaquebildung verhindern soll:
Another approach to plaque attack is to stimulate the body's immune system to destroy the beta-amyloid. Scientists developed a vaccine that put amyloid into the blood in the hopes of making antibodies to destroy the plaques. The vaccine was successful in transgenic mice--special mice that were injected with human genes that caused them to develop AD-like plaques. But when tested in a human trial, some people showed inflammation of the brain (encephalitis). Further vaccination was stopped, but study participants continue to be followed. Although this particular vaccine may be disappointing, many scientists believe that the strategy of fighting AD by stimulating the immune system still remains an important potential avenue to slow or prevent the disease.
...und natürlich auch der neue Ansatz mit den Secretase-Hemmern:
New Drug Development
New drugs are emerging from the basic science laboratories and moving toward testing in human trials. "The ones furthest along are based on the amyloid hypothesis," says Thies. The hypothesis is that AD starts with the accumulation of amyloid plaques, and that limiting this accumulation will change the progress of AD.
Scientists have isolated enzymes called secretases, which are thought to lead to the formation of beta-amyloid. Secretases are categorized as proteases, the same type of enzymes that are targeted by protease inhibitors to treat AIDS. Drugs called secretase inhibitors are being developed to block beta-amyloid formation, and some of these drugs are now being tested.
http://www.elan.com/research_development/Pipeline_Products/d…
...summasummarum ist Elan bei den neurodegenerativen Erkrankungen bestens positioniert ("the reason for ou being!"). Demnächst werden ebenfalls auch Neuigkeiten zur Parkinson-Erkrankung erwartet. Es ist und bleibt eine spannende Geschichte!
http://www.fda.gov/fdac/features/2003/403_alz.html
im folgenden Absatz ist AN-1792 gemeint, das erste Vaccin und Vorläufersubstanz von ACC-001, mit welchem die Immunreaktion stimuliert werden soll. AAB-001 ist der Antikörper dazu, der über eine passive Immunantwort das Fortschreiten der Plaquebildung verhindern soll:
Another approach to plaque attack is to stimulate the body's immune system to destroy the beta-amyloid. Scientists developed a vaccine that put amyloid into the blood in the hopes of making antibodies to destroy the plaques. The vaccine was successful in transgenic mice--special mice that were injected with human genes that caused them to develop AD-like plaques. But when tested in a human trial, some people showed inflammation of the brain (encephalitis). Further vaccination was stopped, but study participants continue to be followed. Although this particular vaccine may be disappointing, many scientists believe that the strategy of fighting AD by stimulating the immune system still remains an important potential avenue to slow or prevent the disease.
...und natürlich auch der neue Ansatz mit den Secretase-Hemmern:
New Drug Development
New drugs are emerging from the basic science laboratories and moving toward testing in human trials. "The ones furthest along are based on the amyloid hypothesis," says Thies. The hypothesis is that AD starts with the accumulation of amyloid plaques, and that limiting this accumulation will change the progress of AD.
Scientists have isolated enzymes called secretases, which are thought to lead to the formation of beta-amyloid. Secretases are categorized as proteases, the same type of enzymes that are targeted by protease inhibitors to treat AIDS. Drugs called secretase inhibitors are being developed to block beta-amyloid formation, and some of these drugs are now being tested.
http://www.elan.com/research_development/Pipeline_Products/d…
...summasummarum ist Elan bei den neurodegenerativen Erkrankungen bestens positioniert ("the reason for ou being!"). Demnächst werden ebenfalls auch Neuigkeiten zur Parkinson-Erkrankung erwartet. Es ist und bleibt eine spannende Geschichte!
http://www.rte.ie/business/2006/Morningrep/download/1020ncb.…
an irish joke to brighten your day
A Scotsman, an Italian, and an Irishman are in a bar. They are having a
good time and all agree that the bar is a nice place. Then the Scotsman says,
“Aye, this is a nice bar, but where I come from, back in Glasgee, there’s a
better one. At MacDougal’s, ye buy a drink, ye buy another drink, and
MacDougal himself will buy yir third drink!“
The others agree that sounds like a good place.
Then the Italian says, „Yeah, dat’s a nice bar, but where I come from,
dere’s a better one. In Roma, dere’s this place, Vincenzo’s. At
Vincenzo’s, you buy a drink, Vincenzo buys you a drink. You buy anudda
drink, Vincenzo buys you anudda drink.“
Everyone agrees that sounds like a great bar.
Then the Irishman says, „You tink dat’s great? Where Oi come from in
Dublin, dere’s dis place called Morphy’s. At Morphy’s, they boy you your
forst drink, dey boy you your second drink, dey boy you your tird drink,
and
den, dey take you in de back and get you laid!“
“Wow!“ say the other two. „That’s fantastic! Did that actually happen to
you?“
“No,“ replies the Irish guy, „but it happened to my sister!“
an irish joke to brighten your day
A Scotsman, an Italian, and an Irishman are in a bar. They are having a
good time and all agree that the bar is a nice place. Then the Scotsman says,
“Aye, this is a nice bar, but where I come from, back in Glasgee, there’s a
better one. At MacDougal’s, ye buy a drink, ye buy another drink, and
MacDougal himself will buy yir third drink!“
The others agree that sounds like a good place.
Then the Italian says, „Yeah, dat’s a nice bar, but where I come from,
dere’s a better one. In Roma, dere’s this place, Vincenzo’s. At
Vincenzo’s, you buy a drink, Vincenzo buys you a drink. You buy anudda
drink, Vincenzo buys you anudda drink.“
Everyone agrees that sounds like a great bar.
Then the Irishman says, „You tink dat’s great? Where Oi come from in
Dublin, dere’s dis place called Morphy’s. At Morphy’s, they boy you your
forst drink, dey boy you your second drink, dey boy you your tird drink,
and
den, dey take you in de back and get you laid!“
“Wow!“ say the other two. „That’s fantastic! Did that actually happen to
you?“
“No,“ replies the Irish guy, „but it happened to my sister!“

Friday, 3.11.06, in der Zeit von 16.15 -16.30
Dale Schenk
- Aß immunotherapy prevents Alzheimer's disease neuropathology.
Saturday, 4.11.06, in der Zeit von 15.30 - 15.30
Dennis Selkoe
- The seventh age of man: solving senility.
...nochmal zur Erinnerung...
Elan to Present at the Cowen and Company 7th Annual Global Health Care Conference
DUBLIN, Ireland, Nov 01, 2006 (BUSINESS WIRE) -- Elan Corporation, plc announces today that its presentation at the Cowen and Company 7th Annual Global Health Care Conference in London will be webcast live via the internet on Tuesday, November 7, 2006 at 10.40 a.m. Greenwich Mean Time, 5.40 a.m. Eastern Standard Time. Interested parties may access a live audio webcast of the presentation by visiting Elan's website at www.elan.com and clicking on the Investor Relations section, then on the event icon.
Elan to Present at the Cowen and Company 7th Annual Global Health Care Conference
DUBLIN, Ireland, Nov 01, 2006 (BUSINESS WIRE) -- Elan Corporation, plc announces today that its presentation at the Cowen and Company 7th Annual Global Health Care Conference in London will be webcast live via the internet on Tuesday, November 7, 2006 at 10.40 a.m. Greenwich Mean Time, 5.40 a.m. Eastern Standard Time. Interested parties may access a live audio webcast of the presentation by visiting Elan's website at www.elan.com and clicking on the Investor Relations section, then on the event icon.
interessanter Link:
http://www.ifpma.org/clinicaltrials.html
http://www.ifpma.org/clinicaltrials.html
ACT-AD Coalition Calls FDA's New Focus on Neurological Diseases a Positive First Step
Coalition Urges Agency to Make Alzheimer's Disease a Priority
WASHINGTON, November 14, 2006 /PRNewswire/ -- The ACT-AD Coalition (Accelerate Cure/Treatments for Alzheimer's Disease) called today's announcement by the Food and Drug Administration (FDA) that it has taken steps to increase its focus on Alzheimer's disease (AD), a positive first step toward making the disease a priority.
"ACT-AD is committed to making Alzheimer's disease a national health priority," said Daniel Perry, chairman of ACT-AD. "Improved communications within FDA around Alzheimer's disease is a positive first step toward achieving our goal of rapid review for the growing number of products in the pipeline that address this devastating disease."
The FDA announced that it has created an Intra-agency Neurology Working Group that will include experts involved in the regulation of drugs, biologics and medical devices. According to FDA, the group will meet regularly to expand awareness of leading-edge developments, enable sharing of expertise and provide for greater consistency of review standards and processes. The group is chaired by Dr. Celia Witten of the Center for Biologics Evaluation and Research and Dr. Robert Temple of the Center for Drug Evaluation and Research.
The FDA also announced that it will provide opportunities for patient advocates and family caregivers to participate in the review of new treatments for neurological diseases. Specifically, FDA will expand its existing Patient Consultant program to include AD advocates and caregivers to advise FDA during the development of new products. FDA will also invite AD advocates to participate in FDA advisory committee meetings that address marketing approval decisions and in response to issues that arise with products in the marketplace.
Representatives of ACT-AD met with Acting FDA Commissioner Andrew von Eschenbach and other FDA leaders in July to explore ways of updating drug procedures and policy regarding AD. ACT-AD also asked FDA to find ways to engage patients and families in the development process. In calling for an increased focus, ACT-AD underscored both the growing impact of AD as the nation's population ages, as well as the need for FDA to keep pace with the discovery of emerging therapies that could potentially halt or reverse Alzheimer's disease. Currently available drugs only provide temporary relief of the symptoms rather than alter the course of the disease.
"As the country races toward an Alzheimer's explosion that will affect 5.6 million people by 2010 and could bankrupt the U.S. health care system, the FDA's new focus is significant, and could make meaningful treatments that change the course of the disease available before another generation of patients and their caregivers are lost," said Perry. "We look forward to working with the FDA to continue to explore ways to extend the rapid approval mechanisms it has so effectively used for other life-threatening diseases, such as cancer and HIV-AIDS, to promising drugs for Alzheimer's."
What is Alzheimer's Disease?
Discovered by Alois Alzheimer in 1906, AD affects 4.5 million Americans and causes millions more to leave the workforce to care for loved ones who eventually need around-the-clock attention. It is a universally fatal progressive neurodegenerative disorder that results in cognitive deterioration that affects many areas of daily function. As the disease progresses, people suffer severe cognitive deterioration, confusion, disorientation, and personality and behavior changes. The greatest risk factor for Alzheimer's is age. One in 10 people over age 65 and nearly half of those over 85 have this fatal disease.
AD costs the U.S. economy more than $100 billion/year and U.S. business costs are $61 billion annually of which almost half ($36.5 billion) results from lost productivity of employee caregivers. By 2050, it is estimated that Medicare will be spending more than $1 trillion to care for those with Alzheimer's and other dementias.
What is ACT-AD?
Formed in February 2006, the ACT-AD coalition is currently comprised of 47 national not-for-profit groups representing patients, caregivers, consumers, older Americans and health advocates. The Coalition is supported in part through an educational grant by Elan Corp. and Wyeth.
For more information about the ACT-AD Coalition, its collaboration with the FDA, and other Coalition efforts to fight AD, visit www.ACT-AD.org.
CONTACT: Joan Hurwitz of ACT-AD Coalition, +1-202-293-2856, or cell,+1-703-244-6783, jhurwitz@agingresearch.org
Web site: http://www.ACT-AD.org/
Terms and conditions of use apply
Copyright © 2006 PR Newswire Association LLC. All rights reserved.
A United Business Media Company
http://www.pharmalive.com/News/index.cfm?articleid=391618&ca…
Coalition Urges Agency to Make Alzheimer's Disease a Priority
WASHINGTON, November 14, 2006 /PRNewswire/ -- The ACT-AD Coalition (Accelerate Cure/Treatments for Alzheimer's Disease) called today's announcement by the Food and Drug Administration (FDA) that it has taken steps to increase its focus on Alzheimer's disease (AD), a positive first step toward making the disease a priority.
"ACT-AD is committed to making Alzheimer's disease a national health priority," said Daniel Perry, chairman of ACT-AD. "Improved communications within FDA around Alzheimer's disease is a positive first step toward achieving our goal of rapid review for the growing number of products in the pipeline that address this devastating disease."
The FDA announced that it has created an Intra-agency Neurology Working Group that will include experts involved in the regulation of drugs, biologics and medical devices. According to FDA, the group will meet regularly to expand awareness of leading-edge developments, enable sharing of expertise and provide for greater consistency of review standards and processes. The group is chaired by Dr. Celia Witten of the Center for Biologics Evaluation and Research and Dr. Robert Temple of the Center for Drug Evaluation and Research.
The FDA also announced that it will provide opportunities for patient advocates and family caregivers to participate in the review of new treatments for neurological diseases. Specifically, FDA will expand its existing Patient Consultant program to include AD advocates and caregivers to advise FDA during the development of new products. FDA will also invite AD advocates to participate in FDA advisory committee meetings that address marketing approval decisions and in response to issues that arise with products in the marketplace.
Representatives of ACT-AD met with Acting FDA Commissioner Andrew von Eschenbach and other FDA leaders in July to explore ways of updating drug procedures and policy regarding AD. ACT-AD also asked FDA to find ways to engage patients and families in the development process. In calling for an increased focus, ACT-AD underscored both the growing impact of AD as the nation's population ages, as well as the need for FDA to keep pace with the discovery of emerging therapies that could potentially halt or reverse Alzheimer's disease. Currently available drugs only provide temporary relief of the symptoms rather than alter the course of the disease.
"As the country races toward an Alzheimer's explosion that will affect 5.6 million people by 2010 and could bankrupt the U.S. health care system, the FDA's new focus is significant, and could make meaningful treatments that change the course of the disease available before another generation of patients and their caregivers are lost," said Perry. "We look forward to working with the FDA to continue to explore ways to extend the rapid approval mechanisms it has so effectively used for other life-threatening diseases, such as cancer and HIV-AIDS, to promising drugs for Alzheimer's."
What is Alzheimer's Disease?
Discovered by Alois Alzheimer in 1906, AD affects 4.5 million Americans and causes millions more to leave the workforce to care for loved ones who eventually need around-the-clock attention. It is a universally fatal progressive neurodegenerative disorder that results in cognitive deterioration that affects many areas of daily function. As the disease progresses, people suffer severe cognitive deterioration, confusion, disorientation, and personality and behavior changes. The greatest risk factor for Alzheimer's is age. One in 10 people over age 65 and nearly half of those over 85 have this fatal disease.
AD costs the U.S. economy more than $100 billion/year and U.S. business costs are $61 billion annually of which almost half ($36.5 billion) results from lost productivity of employee caregivers. By 2050, it is estimated that Medicare will be spending more than $1 trillion to care for those with Alzheimer's and other dementias.
What is ACT-AD?
Formed in February 2006, the ACT-AD coalition is currently comprised of 47 national not-for-profit groups representing patients, caregivers, consumers, older Americans and health advocates. The Coalition is supported in part through an educational grant by Elan Corp. and Wyeth.
For more information about the ACT-AD Coalition, its collaboration with the FDA, and other Coalition efforts to fight AD, visit www.ACT-AD.org.
CONTACT: Joan Hurwitz of ACT-AD Coalition, +1-202-293-2856, or cell,+1-703-244-6783, jhurwitz@agingresearch.org
Web site: http://www.ACT-AD.org/
Terms and conditions of use apply
Copyright © 2006 PR Newswire Association LLC. All rights reserved.
A United Business Media Company
http://www.pharmalive.com/News/index.cfm?articleid=391618&ca…
Eine der Bibeln der Naturwissenschaftler, das "Nature", äussert sich sehr viel versprechend zu Bapineuzumab.
Sollte ein vorzeitiges Konvertieren in Phase3 stattfinden, dann wird sich das sicherlich beim Kurs bemmerkbar machen. Der absolute Hammer wäre natürlich, wenn aufgrund der Ergebnisse aus Phase 2 ein Zulassungsantrag bei der FDA eingereicht würde.
http://www.nature.com/nm/journal/v12/n7/full/nm0706-780.html
...In our opinion, the most exciting drug in development is Elan and Wyeth's bapineuzumab (AAB-001),...
Sollte ein vorzeitiges Konvertieren in Phase3 stattfinden, dann wird sich das sicherlich beim Kurs bemmerkbar machen. Der absolute Hammer wäre natürlich, wenn aufgrund der Ergebnisse aus Phase 2 ein Zulassungsantrag bei der FDA eingereicht würde.
http://www.nature.com/nm/journal/v12/n7/full/nm0706-780.html
...In our opinion, the most exciting drug in development is Elan and Wyeth's bapineuzumab (AAB-001),...
Antwort auf Beitrag Nr.: 25.454.373 von Cyberhexe am 16.11.06 10:14:21ELND001 in der Klinik:
http://www.elan.com/research_development/Pipeline_Products/d…
...zur Behandlung von Rheumatoider Arthritis? Weiss jemand mehr?
http://www.elan.com/research_development/Pipeline_Products/d…
...zur Behandlung von Rheumatoider Arthritis? Weiss jemand mehr?
Passagen aus Lehman upgrades Biogen (positive on Ty)
20-Nov-06 07:08 am
"Like many, we have heard a divergent set of opinions from
neurologists about the current use of Tysabri, and the patients they consider candidates for the product. 2007 Tysabri sales should begin to pinpoint the market segment in which Tysabri flourishes, and our view continues to be that the efficacy of the product will position it as the product of choice in a 25% segment of the MS market."
"Actual PML Incidence Unknown
Another potential risk to our Tysabri forecast is the possible identification of new cases of PML. Based on information from the Tysabri clinical studies and limited post-market surveillance following the initial launch of Tysabri in 2004, the risk of developing PML on Tysabri is
roughly 1/1000. If the actual incidence from the post-market monitoring were found to be higher than 1/1000 we believe it would have an immediate negative impact on utilization and raise the possibility of negative regulatory action. However, it remains unclear if Biogen Idec
would be willing to make such data from the post-marketing surveillance studies publicly available. We understand that the company is obligated to report suspected cases of PML to the FDA, but do not know if a incidence threshold has been established with regulators regarding the formal re-evaluation of the risk/benefit profile of the product. Indeed, BIIB expects that suspected cases of Tysabri related
PML will likely appear in the press and that the company does not plan to comment on such reports."
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
20-Nov-06 07:08 am
"Like many, we have heard a divergent set of opinions from
neurologists about the current use of Tysabri, and the patients they consider candidates for the product. 2007 Tysabri sales should begin to pinpoint the market segment in which Tysabri flourishes, and our view continues to be that the efficacy of the product will position it as the product of choice in a 25% segment of the MS market."
"Actual PML Incidence Unknown
Another potential risk to our Tysabri forecast is the possible identification of new cases of PML. Based on information from the Tysabri clinical studies and limited post-market surveillance following the initial launch of Tysabri in 2004, the risk of developing PML on Tysabri is
roughly 1/1000. If the actual incidence from the post-market monitoring were found to be higher than 1/1000 we believe it would have an immediate negative impact on utilization and raise the possibility of negative regulatory action. However, it remains unclear if Biogen Idec
would be willing to make such data from the post-marketing surveillance studies publicly available. We understand that the company is obligated to report suspected cases of PML to the FDA, but do not know if a incidence threshold has been established with regulators regarding the formal re-evaluation of the risk/benefit profile of the product. Indeed, BIIB expects that suspected cases of Tysabri related
PML will likely appear in the press and that the company does not plan to comment on such reports."
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
NCB Morning News & Views
November 23, 2006
• Elan has completed the offering of $615m of Senior Notes, an increase on the
anticipated $500m offering previously planned. The Senior notes consist of $465m of
8.875% senior fixed rate notes due 2013 and $150m of senior floating rate notes due
2013. The floating rate notes will bear quarterly interest equal to 3 month LIBOR plus
4.125%.
• The net proceeds of the offering will be used to repay (within 90 days) all or a a portion
of the $613m senior notes due in 2008.
• Elan intends to issue a redemption notice for the $254m convertible notes (converting
to 34.2m shares) due also in 2008. Elan can redeem all of the notes outstanding at par
at any time after 2nd December 2006.
• With the completion of the $615m offering and including the conversion of the
convertible notes, Elan's debt profile reduces from $2bn to $1.77bn. Currently, Elan has
cash of $990m, which reduces the net debt position to $780m. Overall the refinancing
of Elan's debt due in 2008 extends the debt maturity profile while retaining cash
balances.
November 23, 2006
• Elan has completed the offering of $615m of Senior Notes, an increase on the
anticipated $500m offering previously planned. The Senior notes consist of $465m of
8.875% senior fixed rate notes due 2013 and $150m of senior floating rate notes due
2013. The floating rate notes will bear quarterly interest equal to 3 month LIBOR plus
4.125%.
• The net proceeds of the offering will be used to repay (within 90 days) all or a a portion
of the $613m senior notes due in 2008.
• Elan intends to issue a redemption notice for the $254m convertible notes (converting
to 34.2m shares) due also in 2008. Elan can redeem all of the notes outstanding at par
at any time after 2nd December 2006.
• With the completion of the $615m offering and including the conversion of the
convertible notes, Elan's debt profile reduces from $2bn to $1.77bn. Currently, Elan has
cash of $990m, which reduces the net debt position to $780m. Overall the refinancing
of Elan's debt due in 2008 extends the debt maturity profile while retaining cash
balances.
DOCTORS USE CAUTION WITH TYSABRI®
Doctors are proving more cautious than expected about prescribing Tysabri, which was re-launched in July after being suspended due to safety concerns.
A Reuters Primary Research survey of 63 neurologists indicates that Tysabri will be used in less than 1 percent of MS patients during 2006.
Since July, only 47 of more than 8,500 patients treated by physicians surveyed had used Tysabri, despite the fact that more than 700 patients had discussed using it.
The survey also showed that more than 75 percent of the patients who had used Tysabri prior to its suspension have decided not to use it since its reintroduction.
"At our center, we recommend Tysabri cautiously," confirms Dr. Roger Williams, Medical Director of the Northern Rockies MS Center in Billings, Montana. "Initially, we may have considered Tysabri as first-line therapy for relapsing-remitting MS. Now, we use it for patients with breakthrough disease after one or two of the C-R-A-B drugs. Hence, it now competes with Novantrone and Cytoxan as a potentially more aggressive and effective therapy, but one that is risky, riskier than the C-R-A-B drugs but comparable to chemotherapy. We have enrolled eight patients for Tysabri. All patients have been pre-approved by third party payers. However it may take three to four months to work out the glitches of reimbursement."
"I am also being cautious with Tysabri," adds Dr. Ben Thrower, Medical Director of the MS Center at Shepherd Center in Atlanta, Georgia. "I use it in individuals who have failed the A-B-C-R drugs and who have aggressive enough disease to warrant the risk. The problem is that we really don't know what the true, long-term risk of PML will be. We work with a little over 2,000 people with MS, but only consider about 17 of them to be Tysabri candidates."
Another issue may be the 'Touch' Program, a special training required for physicians prescribing and personnel administering Tysabri. "I have yet to be 'touched' by Biogen-Idec so I cannot prescribe Tysabri yet," says Dr. Greg Zarelli, Staff Physician in the Department of Neurology at Kaiser-Permanente NW in Clackamas, Oregon.
http://multiplesclerosisnewsblog.blogspot.com/2006/11/multip…
Die allgemeine Unsicherheit bezüglich des Umgangs mit Tysabri scheint bei einzelnen Fachärzten wohl doch noch sehr stark vorhanden zu sein...
..es wird sicherlich interessant werden,was die Verkaufszahlen von Tysabri bei den nächsten Quartalszahlen sprechen werden.....
Werden sich o.g. Unsicherheitstendenzen bestätigen ????
TIME WILL TELL
Doctors are proving more cautious than expected about prescribing Tysabri, which was re-launched in July after being suspended due to safety concerns.
A Reuters Primary Research survey of 63 neurologists indicates that Tysabri will be used in less than 1 percent of MS patients during 2006.
Since July, only 47 of more than 8,500 patients treated by physicians surveyed had used Tysabri, despite the fact that more than 700 patients had discussed using it.
The survey also showed that more than 75 percent of the patients who had used Tysabri prior to its suspension have decided not to use it since its reintroduction.
"At our center, we recommend Tysabri cautiously," confirms Dr. Roger Williams, Medical Director of the Northern Rockies MS Center in Billings, Montana. "Initially, we may have considered Tysabri as first-line therapy for relapsing-remitting MS. Now, we use it for patients with breakthrough disease after one or two of the C-R-A-B drugs. Hence, it now competes with Novantrone and Cytoxan as a potentially more aggressive and effective therapy, but one that is risky, riskier than the C-R-A-B drugs but comparable to chemotherapy. We have enrolled eight patients for Tysabri. All patients have been pre-approved by third party payers. However it may take three to four months to work out the glitches of reimbursement."
"I am also being cautious with Tysabri," adds Dr. Ben Thrower, Medical Director of the MS Center at Shepherd Center in Atlanta, Georgia. "I use it in individuals who have failed the A-B-C-R drugs and who have aggressive enough disease to warrant the risk. The problem is that we really don't know what the true, long-term risk of PML will be. We work with a little over 2,000 people with MS, but only consider about 17 of them to be Tysabri candidates."
Another issue may be the 'Touch' Program, a special training required for physicians prescribing and personnel administering Tysabri. "I have yet to be 'touched' by Biogen-Idec so I cannot prescribe Tysabri yet," says Dr. Greg Zarelli, Staff Physician in the Department of Neurology at Kaiser-Permanente NW in Clackamas, Oregon.
http://multiplesclerosisnewsblog.blogspot.com/2006/11/multip…
Die allgemeine Unsicherheit bezüglich des Umgangs mit Tysabri scheint bei einzelnen Fachärzten wohl doch noch sehr stark vorhanden zu sein...
..es wird sicherlich interessant werden,was die Verkaufszahlen von Tysabri bei den nächsten Quartalszahlen sprechen werden.....
Werden sich o.g. Unsicherheitstendenzen bestätigen ????
TIME WILL TELL
erste Tysabri-Applikationen in Dänemark:
http://www.scleroseforeningen.dk/Nyheder/2006/Rigshospitalet…
http://www.scleroseforeningen.dk/Nyheder/2006/Rigshospitalet…
"DAVY Research" Kommentar von Heute zu Alzheimer -
Die Möglichkeit mit den Phase2-Daten eine Zulassung zu beantragen wird nicht kategorisch ausgeschlossen!
Price $14.73 Target: $18.00 Issued: 31/01/06 Previous: $13.00 Issued: 09/08/05
Eli Lilly highlighted two important disease-modifying programmes in Alzheimers
Disease at its investor day on December 7th.
Its passive immunotherapy is in phase II studies and is designed to increase amyloid
clearance. LY 450139, its gamma secretase inhibitor, is scheduled to begin pivotal
trials in late 2007 or early 2008. A 12–18 month study, allowance for recruitment
time, and a standard FDA review would imply a potential market launch by 2011.
By way of comparison, this could be up to two years behind Elan/Wyeth’s AAB-001
candidate, should the companies decide (and the data allow them) to use its
current phase II as a pivotal trial for FDA review purposes.
Die Möglichkeit mit den Phase2-Daten eine Zulassung zu beantragen wird nicht kategorisch ausgeschlossen!
Price $14.73 Target: $18.00 Issued: 31/01/06 Previous: $13.00 Issued: 09/08/05
Eli Lilly highlighted two important disease-modifying programmes in Alzheimers
Disease at its investor day on December 7th.
Its passive immunotherapy is in phase II studies and is designed to increase amyloid
clearance. LY 450139, its gamma secretase inhibitor, is scheduled to begin pivotal
trials in late 2007 or early 2008. A 12–18 month study, allowance for recruitment
time, and a standard FDA review would imply a potential market launch by 2011.
By way of comparison, this could be up to two years behind Elan/Wyeth’s AAB-001
candidate, should the companies decide (and the data allow them) to use its
current phase II as a pivotal trial for FDA review purposes.
Antwort auf Beitrag Nr.: 26.017.204 von Cyberhexe am 08.12.06 10:20:20...im IVMB wird bereits vermutet, dass die Entscheidung der Scottish Medicines Consortium, Tysabri u.a. aus Kostengründen nicht zu empfehlen, die beste Werbung sein könnte. Dies bringt Tysabri über verschiedenste Pressemitteilungen in den Focus der Öffentlichkeit . Und eine entsprechende Reaktion der schottischen MS-Gesellschaft lässt erwarten, dass man diese Entscheidung nicht widerstandslos hinnehmen wird:
http://news.scotsman.com/health.cfm?id=1842272006
http://news.scotsman.com/health.cfm?id=1842272006
Elan to Present at the 25th Annual JP Morgan Healthcare Conference
Wednesday December 20, 8:30 am ET
DUBLIN, Ireland--(BUSINESS WIRE)--Elan Corporation, plc announces that it will present at the 25th Annual JP Morgan Healthcare Conference in San Francisco on Tuesday, January 9th, 2007 at 8.30 a.m. Pacific Standard Time, 11.30 a.m. Eastern Standard Time and 4.30 p.m. Greenwich Mean Time. Interested parties may access a live audio webcast of the presentation by visiting Elan's website at www.elan.com and clicking on the Investor Relations section, then on the event icon.
http://biz.yahoo.com/bw/061220/20061220005392.html?.v=1
Wednesday December 20, 8:30 am ET
DUBLIN, Ireland--(BUSINESS WIRE)--Elan Corporation, plc announces that it will present at the 25th Annual JP Morgan Healthcare Conference in San Francisco on Tuesday, January 9th, 2007 at 8.30 a.m. Pacific Standard Time, 11.30 a.m. Eastern Standard Time and 4.30 p.m. Greenwich Mean Time. Interested parties may access a live audio webcast of the presentation by visiting Elan's website at www.elan.com and clicking on the Investor Relations section, then on the event icon.
http://biz.yahoo.com/bw/061220/20061220005392.html?.v=1
ELAN . to Present at the JP Morgan Healthcare Conference on Tuesday, January 9, 2007
WYETH to Present at the JP Morgan Healthcare Conference on Tuesday, January 9, 2007
Von Goodbody
In the various fora for companies to update investors on their prospects at the beginning of the year, we have already heard Wyeth play down the first interim look at data from an on-going Phase II trial of AAB-001 for the treatment of Alzheimer’s being conducted with partner Elan. In a brief reference towards the end of a 50 minute session, researchers noted that there has been no announcement on progress to Phase III to date (evident), that Phase II is on-going (again evident) and that the next interim look will be in late spring 2007 (always flagged by both companies).
This week, both companies again have opportunities to update the market, where their wording of AAB-001’s progress will be closely monitored. In particular, we will be looking for any inference on drug efficacy rather than Phase II progress, a factor which could take longer to analyse than safety data. [uIn addition for Elan, the market will be awaiting any indication on
the number of patients receiving Tysabri infusions][/u] for the treatment of Multiple Sclerosis and the progress of reimbursement talks across Europe. We very conservatively have 5,000 patients pencilled in for the start of 2007, ramping up to 45,900 by December, as the main European markets approve the drug’s reimbursement. Any number above 5,000 would give us increasing comfort in our total projected Tysabri revenue of $664.3m for 2007.
http://www.rte.ie/business/2007/morningrep/download/0108good…
WYETH to Present at the JP Morgan Healthcare Conference on Tuesday, January 9, 2007
Von Goodbody
In the various fora for companies to update investors on their prospects at the beginning of the year, we have already heard Wyeth play down the first interim look at data from an on-going Phase II trial of AAB-001 for the treatment of Alzheimer’s being conducted with partner Elan. In a brief reference towards the end of a 50 minute session, researchers noted that there has been no announcement on progress to Phase III to date (evident), that Phase II is on-going (again evident) and that the next interim look will be in late spring 2007 (always flagged by both companies).
This week, both companies again have opportunities to update the market, where their wording of AAB-001’s progress will be closely monitored. In particular, we will be looking for any inference on drug efficacy rather than Phase II progress, a factor which could take longer to analyse than safety data. [uIn addition for Elan, the market will be awaiting any indication on
the number of patients receiving Tysabri infusions][/u] for the treatment of Multiple Sclerosis and the progress of reimbursement talks across Europe. We very conservatively have 5,000 patients pencilled in for the start of 2007, ramping up to 45,900 by December, as the main European markets approve the drug’s reimbursement. Any number above 5,000 would give us increasing comfort in our total projected Tysabri revenue of $664.3m for 2007.
http://www.rte.ie/business/2007/morningrep/download/0108good…
Study: Gene Could Be Signal Of Alzheimer's
NEW YORK, Jan. 14, 2007
A huge international study has identified a gene that apparently can raise the risk of developing the most common form of Alzheimer's disease, a discovery that may help scientists develop new treatments.
Scientists analyzed DNA from more than 6,000 people from a variety of ethnic groups and found evidence implicating certain versions of the gene, called SORL1.
It's too soon to tell how much those gene versions raise the risk of getting Alzheimer's, or what percentage of cases they account for, the researchers said. They said the effect on risk appears to be modest.
Still, if the finding is confirmed by other scientists, it would be "a very substantial step forward in our understanding of the genetics of Alzheimer's disease," said one expert not involved in the work, Jonathan Haines of Vanderbilt University.
By shedding light on the biology of the illness, the discovery could help lead scientists to find new treatments, he and other experts said.
Up to 4.5 million Americans are estimated to have Alzheimer's, which gradually destroys memory and other mental abilities. No cure has been found.
The study, released Sunday on the Web site of the journal Nature Genetics, focused on Alzheimer's that appears after age 65, the most common type.
Only one gene, called APOE, has been firmly linked to raising susceptibility to the common form. A Harvard-based group lists about 20 other genes it considers promising candidates, based on research. Some authors of the new paper said they believe the evidence for SORL1 is unusually strong.
Experts familiar with the new work said SORL1 probably is involved in Alzheimer's, but cautioned that it will be important for other scientists to confirm that. "People will jump on it pretty fast," said Rudy Tanzi of Harvard Medical School.
Gerard Schellenberg of the University of Washington said the paper's evidence is "pretty solid, so I'm optimistic that it will be replicated."
Dr. Sam Gandy, a researcher who chairs the medical and scientific advisory council of the Alzheimer's Association, which helped finance the new study, said that he doubted SORL1 or any other gene plays as big a role in causing the disease as APOE does.
The new work was reported by Dr. Peter St. George-Hyslop of the University of Toronto, Lindsay Farrer of Boston University, Dr. Richard Mayeux of the Columbia University in New York, and others in the U.S., Germany, Israel and Japan.
The new paper implicates SORL1 in Alzheimer's in two ways. First, it shows that inheriting certain variants was associated with developing the disease in seven out of nine samples of people examined. The association appeared in African-American, Caribbean Hispanic, northern European and Israeli Arab groups.
In laboratory studies, researchers also found that when they suppressed the activity of SORL1, cells made greater amounts of amyloid beta, a substance thought to play a key role in causing Alzheimer's. Researchers believe the disease-promoting variants of SORL1 act by suppressing the gene's activity.
http://www.cbsnews.com/stories/2007/01/14/health/main2359062…
NEW YORK, Jan. 14, 2007
A huge international study has identified a gene that apparently can raise the risk of developing the most common form of Alzheimer's disease, a discovery that may help scientists develop new treatments.
Scientists analyzed DNA from more than 6,000 people from a variety of ethnic groups and found evidence implicating certain versions of the gene, called SORL1.
It's too soon to tell how much those gene versions raise the risk of getting Alzheimer's, or what percentage of cases they account for, the researchers said. They said the effect on risk appears to be modest.
Still, if the finding is confirmed by other scientists, it would be "a very substantial step forward in our understanding of the genetics of Alzheimer's disease," said one expert not involved in the work, Jonathan Haines of Vanderbilt University.
By shedding light on the biology of the illness, the discovery could help lead scientists to find new treatments, he and other experts said.
Up to 4.5 million Americans are estimated to have Alzheimer's, which gradually destroys memory and other mental abilities. No cure has been found.
The study, released Sunday on the Web site of the journal Nature Genetics, focused on Alzheimer's that appears after age 65, the most common type.
Only one gene, called APOE, has been firmly linked to raising susceptibility to the common form. A Harvard-based group lists about 20 other genes it considers promising candidates, based on research. Some authors of the new paper said they believe the evidence for SORL1 is unusually strong.
Experts familiar with the new work said SORL1 probably is involved in Alzheimer's, but cautioned that it will be important for other scientists to confirm that. "People will jump on it pretty fast," said Rudy Tanzi of Harvard Medical School.
Gerard Schellenberg of the University of Washington said the paper's evidence is "pretty solid, so I'm optimistic that it will be replicated."
Dr. Sam Gandy, a researcher who chairs the medical and scientific advisory council of the Alzheimer's Association, which helped finance the new study, said that he doubted SORL1 or any other gene plays as big a role in causing the disease as APOE does.
The new work was reported by Dr. Peter St. George-Hyslop of the University of Toronto, Lindsay Farrer of Boston University, Dr. Richard Mayeux of the Columbia University in New York, and others in the U.S., Germany, Israel and Japan.
The new paper implicates SORL1 in Alzheimer's in two ways. First, it shows that inheriting certain variants was associated with developing the disease in seven out of nine samples of people examined. The association appeared in African-American, Caribbean Hispanic, northern European and Israeli Arab groups.
In laboratory studies, researchers also found that when they suppressed the activity of SORL1, cells made greater amounts of amyloid beta, a substance thought to play a key role in causing Alzheimer's. Researchers believe the disease-promoting variants of SORL1 act by suppressing the gene's activity.
http://www.cbsnews.com/stories/2007/01/14/health/main2359062…
More support for A-b hypothesis
For proponents of the so-called amyloid hypothesis of Alzheimer’s, the new finding provides one more piece of evidence that A-beta is a critical player in the development of the disease. It’s a piece of evidence that’s hard to dismiss. Unlike most studies, which involve just one population group and need to be replicated by other labs before scientists will accept them, this study was conducted in nine different groups comprising 6,800 individuals from several ethnic populations—namely, European and American whites, African-Americans, Dominican Hispanics and Israeli Arabs....
http://www.msnbc.msn.com/id/16622458/site/newsweek/
For proponents of the so-called amyloid hypothesis of Alzheimer’s, the new finding provides one more piece of evidence that A-beta is a critical player in the development of the disease. It’s a piece of evidence that’s hard to dismiss. Unlike most studies, which involve just one population group and need to be replicated by other labs before scientists will accept them, this study was conducted in nine different groups comprising 6,800 individuals from several ethnic populations—namely, European and American whites, African-Americans, Dominican Hispanics and Israeli Arabs....
http://www.msnbc.msn.com/id/16622458/site/newsweek/
Prof. Gass, einer der beiden Experten am Kantonsspital bzw. Uniklinik in Basel hat am Samstag Vormittag einen Vortrag über MS gehalten. Dieser Vortrag war ausgezeichnet besucht, überwiegensd antürlich von MS-Betroffenen. Der überwiegende Teil seiner ppt-Vorführung mit insegesamt 28 Folien hat sich mit Natalizumab beschäftigt (SENTINEL, AFFIRM und über den Wirkmechanismus von Natalizumab)...darüber hinaus hat Prof. Gass in der Eskalationstherapie die in der CH bevorstehende Zulassung von Tysabri als Fortschritt erwähnt und bei den Zuhörern, wie mir schien, grosse Erwartungen geweckt. Ich denke, dass Tysabri mittlerweile von vielen MS-Patienten in der CH mit Sehnsucht erwartet wird!
Ein langjähriger Elanweggefährte noch aus Comdirectzeiten freut sich über dein Lebenszeichen. posimist
Demnächst könnte es Umsatzzuwächse beim Schmerzmittel 'Prialt' geben. Ein Expertengremium schlägt Prialt als First-Line Alternative zu Morphinen vor.
Als Vorbild für Prialt diente das Nervengift der Kegelschnecke, welches Nervenreize unterbindet. Dadurch können chronische Schmerzpatienten behandelt werden. Prialt hat den Vorteil, daß es nicht das Suchtpotential der Morphine besitzt.
Prialt ist momentan das Produkt von Elan mit den geringsten Umsätzen (3,1 Mil im letzten Quartal).
http://www.marketwatch.com/News/Story/Story.aspx?guid=%7B6D5…
http://www.investorvillage.com/smbd.asp?mb=160&mn=77265&pt=m…
Als Vorbild für Prialt diente das Nervengift der Kegelschnecke, welches Nervenreize unterbindet. Dadurch können chronische Schmerzpatienten behandelt werden. Prialt hat den Vorteil, daß es nicht das Suchtpotential der Morphine besitzt.
Prialt ist momentan das Produkt von Elan mit den geringsten Umsätzen (3,1 Mil im letzten Quartal).
http://www.marketwatch.com/News/Story/Story.aspx?guid=%7B6D5…
http://www.investorvillage.com/smbd.asp?mb=160&mn=77265&pt=m…
Antwort auf Beitrag Nr.: 27.504.702 von posimist am 08.02.07 10:08:20hi posi
...vielleicht werden wir ja auch bei Dendreon Weggefährten - auf dem NCI/FDA workshop (8./9.2.07) wird die Zukunft in der Onkologie diskutiert und hierbei scheinen die Vakzine eine entscheidende Rolle zu spielen.
Nun bin ich mir fast sicher, dass Dendreon´s Provenge zugelassen wird, zumal die der lebensverlängernde Effekt in Kombination mit Chemo (Docetaxel) beeindruckend ist - wurde von J.Schlom vom "National Cancer Institute" in einer Präsentation konkret thematisiert.
Hier könnt etwas ganz Grosses entstehen - bin mit 50% investiert...die anderen 50% natürlich immer noch in Elan.
Grüsse
ch
Im Dendreon-Forum könnten wir noch "fixe" Teilnehmer gebrauchen ;-)
...vielleicht werden wir ja auch bei Dendreon Weggefährten - auf dem NCI/FDA workshop (8./9.2.07) wird die Zukunft in der Onkologie diskutiert und hierbei scheinen die Vakzine eine entscheidende Rolle zu spielen.
Nun bin ich mir fast sicher, dass Dendreon´s Provenge zugelassen wird, zumal die der lebensverlängernde Effekt in Kombination mit Chemo (Docetaxel) beeindruckend ist - wurde von J.Schlom vom "National Cancer Institute" in einer Präsentation konkret thematisiert.
Hier könnt etwas ganz Grosses entstehen - bin mit 50% investiert...die anderen 50% natürlich immer noch in Elan.
Grüsse
ch
Im Dendreon-Forum könnten wir noch "fixe" Teilnehmer gebrauchen ;-)
aus dem Jahresbericht von biogenIdec:
Global in-market net sales of TYSABRI in the fourth quarter of 2006 were $30 million, comprised of $23 million in the U.S. and $7 million in Europe. Based on our collaboration structure with Elan, Biogen Idec recognized revenue of $18 million related to TYSABRI in the fourth quarter of 2006
To date, nearly 10,000 patients have been prescribed TYSABRI worldwide. Almost 8,000 patients in the U.S. have enrolled in the TOUCH Program and of these, approximately 5,000 are on therapy. Approximately 1,600 patients internationally have received TYSABRI infusions, primarily in Germany and Sweden.
...und ich rechne mit progressivem Wachstum!
Global in-market net sales of TYSABRI in the fourth quarter of 2006 were $30 million, comprised of $23 million in the U.S. and $7 million in Europe. Based on our collaboration structure with Elan, Biogen Idec recognized revenue of $18 million related to TYSABRI in the fourth quarter of 2006
To date, nearly 10,000 patients have been prescribed TYSABRI worldwide. Almost 8,000 patients in the U.S. have enrolled in the TOUCH Program and of these, approximately 5,000 are on therapy. Approximately 1,600 patients internationally have received TYSABRI infusions, primarily in Germany and Sweden.
...und ich rechne mit progressivem Wachstum!
Antwort auf Beitrag Nr.: 27.723.870 von Cyberhexe am 15.02.07 15:06:14To date, nearly 10,000 patients have been prescribed TYSABRI worldwide...
...im 1.Quartal werden dann im Durchschnitt über die 3 Monate 10.000 Patienten mit Tysabri behandelt werden.
Ty-Umsatz im 1.Quartal:
10.000 Patienten x 3 (Monate) x $2.290 (Kosten einer Infusion)= ca. 68 Mio$
...und wie gesagt, die Akzeptanz sollte zunehmen und der Markt sollte grösser werden (z.B. Schweiz, Italien etc. sowie zusätzliche Indikation - MorbusCrohn).
...im 1.Quartal werden dann im Durchschnitt über die 3 Monate 10.000 Patienten mit Tysabri behandelt werden.
Ty-Umsatz im 1.Quartal:
10.000 Patienten x 3 (Monate) x $2.290 (Kosten einer Infusion)= ca. 68 Mio$
...und wie gesagt, die Akzeptanz sollte zunehmen und der Markt sollte grösser werden (z.B. Schweiz, Italien etc. sowie zusätzliche Indikation - MorbusCrohn).
Kommentar von Ian Hunter, Goodbody stockbrokers, zum Heute veröffentlichten Q-Ergebnis bzw. Jahresergebnis 2006:
Elan (ADD, Closing Price $14.81); Core business performing strongly
Analyst: Ian Hunter T +353-1-6410498 E ian.g.hunter@goodbody.ie
Elan this morning reported a solid set of Q4’06 results. Losses per share of $0.12, were 24.9% worse than the losses reported in Q4’05, but well ahead of our forecast of $0.21. This is a
combination of greater than forecast revenue, strong cost control and large tax rebate. The company also recorded an exceptional gain of $43.2m on legal settlements. The company reported an 18.5% increase in revenue over the same period last year to $166.4m, 14.0% ahead our forecast of $146.0m. Within the revenue line, Maxipime contributed $46.2m, 32.0% ahead of expectations. Azactam recorded revenue of $21.3m over the quarter, 31.5% ahead of our forecast. From this, it is evident that it has not yet come under generic competition. The contract manufacturing and royalties revenue of $67.3m was up 15.4% on the same quarter last year and
20.6% ahead of our forecast of $55.8m. This was primarily driven by strong revenue from Tricor ($16.1m) and Skelaxin ($11.8m). For a loss-making company, cost control remains an imperative. In Q4’06, Elan (despite strong top-line growth) reported a 2.3% decrease in SG&A costs over Q3’06 to $89.6m. R&D costs (at $15.4m) are $2.2m under our estimates.
Management continues to demonstrate strong cost management. In the short to medium term, the company is dependent on the progress of Tysabri in the market for the treatment of MS and AAB-001 in the pipeline for Alzheimer’s. Both have already been covered by Q4’06 results from Elan’s partners. To re-iterate, Biogen Idec reported that there are 6,600 patients to date on Tysabri with a further 3,400 in the queue. The current global run rate is an additional 300 patients on the drug per week. These metrics would suggest a FY07 revenue number in the $370m to $500m mark, with potential for considerable upside as more EU countries approve reimbursement and efficacy overtakes safety concerns as the growth driver. On AAB-001, we are awaiting a second look at the data from patients currently on a phase II trial. This will take place in mid-2007 and will determine whether or not a Phase III trial can be initiated before the current Phase II trial concludes. This is due in late 2007, early 2008. A positive outcome from the mid-2007 “look” would augur well for the drug. In forward guidance Elan is looking for FY’07 (ex-Tysabri) revenue to exceed $500m, with R&D and SG&A expenses to be in the range of $600m to $650m. Currently we are forecasting revenue of $499.5m, however, our R&D and SG&A costs total $450m. We will, therefore, be looking to revise our numbers downwards, accordingly.
Despite this marked increase in cost, Elan management believes that Tysabri revenues will drive its return to profitability in FY’07. This would imply confidence in strong revenue for Tysabri
through FY’07.
Elan (ADD, Closing Price $14.81); Core business performing strongly
Analyst: Ian Hunter T +353-1-6410498 E ian.g.hunter@goodbody.ie
Elan this morning reported a solid set of Q4’06 results. Losses per share of $0.12, were 24.9% worse than the losses reported in Q4’05, but well ahead of our forecast of $0.21. This is a
combination of greater than forecast revenue, strong cost control and large tax rebate. The company also recorded an exceptional gain of $43.2m on legal settlements. The company reported an 18.5% increase in revenue over the same period last year to $166.4m, 14.0% ahead our forecast of $146.0m. Within the revenue line, Maxipime contributed $46.2m, 32.0% ahead of expectations. Azactam recorded revenue of $21.3m over the quarter, 31.5% ahead of our forecast. From this, it is evident that it has not yet come under generic competition. The contract manufacturing and royalties revenue of $67.3m was up 15.4% on the same quarter last year and
20.6% ahead of our forecast of $55.8m. This was primarily driven by strong revenue from Tricor ($16.1m) and Skelaxin ($11.8m). For a loss-making company, cost control remains an imperative. In Q4’06, Elan (despite strong top-line growth) reported a 2.3% decrease in SG&A costs over Q3’06 to $89.6m. R&D costs (at $15.4m) are $2.2m under our estimates.
Management continues to demonstrate strong cost management. In the short to medium term, the company is dependent on the progress of Tysabri in the market for the treatment of MS and AAB-001 in the pipeline for Alzheimer’s. Both have already been covered by Q4’06 results from Elan’s partners. To re-iterate, Biogen Idec reported that there are 6,600 patients to date on Tysabri with a further 3,400 in the queue. The current global run rate is an additional 300 patients on the drug per week. These metrics would suggest a FY07 revenue number in the $370m to $500m mark, with potential for considerable upside as more EU countries approve reimbursement and efficacy overtakes safety concerns as the growth driver. On AAB-001, we are awaiting a second look at the data from patients currently on a phase II trial. This will take place in mid-2007 and will determine whether or not a Phase III trial can be initiated before the current Phase II trial concludes. This is due in late 2007, early 2008. A positive outcome from the mid-2007 “look” would augur well for the drug. In forward guidance Elan is looking for FY’07 (ex-Tysabri) revenue to exceed $500m, with R&D and SG&A expenses to be in the range of $600m to $650m. Currently we are forecasting revenue of $499.5m, however, our R&D and SG&A costs total $450m. We will, therefore, be looking to revise our numbers downwards, accordingly.
Despite this marked increase in cost, Elan management believes that Tysabri revenues will drive its return to profitability in FY’07. This would imply confidence in strong revenue for Tysabri
through FY’07.
...und dann auch noch Orla Hartford von NCB Stockbrokers:
Q4 2006 results this morning were ahead of our expectations. At the revenue level product sales of $161.4m were 15% ahead of our $140.3m forecast and with lower than anticipated operating costs along with a tax benefit, the EPS loss (adjusted) of $0.13
was significantly lower than our $0.23 loss expectation. For the year Elan exceeded the $500m revenue target (revenues of $560.4m reported of which $532.9m related to product sales) and reported operating costs of $536m significantly lower than the operating cost target for FY2006. Guidance for 2007 is for revenues (ex-Tysabri) to exceed $500m and for group to be breakeven on an EBITDA basis by the end of the year. We will be revising our 2007 forecasts to reflect the stronger than anticipated revenue performance in 2006.
• The outperformance of product revenues of $161.4m, a 15% increase y-o-y were underpinned by stronger than expected revenues from contract manufacturing and the
hospital antibiotics. Product gross margins of 59% were ahead of our 55% expectation. Operating costs reduced 11% year-on-year (and were 13% lower than our forecast) primarily on lower SG&A leaving the operating loss (pre-charges) of $38.8m compared to our $72.4m loss expectation. A net charge of $43.2m on an arbitration award along with a tax benefit of $9.3m, resulted in a net loss of $16.5m. Negative adjusted EBITDA for the group was $9.2m, which resulted in Elan reporting negative adjusted EBITDA for
the full year of $91.1m. Excluding Tysabri positive adjusted EBITDA of $10.6m for 2006 was reported.
• This quarter revenues of Maxipime were broadly flat but Azactam increased 22% y-o-y and both products were significantly ahead of our expectations. The performance of Prialt was modest again this quarter with revenues of $3.4m. Notably, contract manufacturing was strong this quarter with revenues increasing 15% y-o-y to $67.3m, 8% ahead of expectations. Contract manufacturing revenues continue to be primarily underpinned by Tricor ($16.1m), Skelaxin ($11.8m) and Verelan ($8.5m).
• Elan expects total revenues (excluding Tysabri) in 2007 to exceed $500m with operating expenses anticipated to be in the range of $600m-$650m. Adjusted EBITDA is targeted to be less than negative $50m for the full year and for the group to reach
break-even on an EBITDA (adjusted) by the end of 2007. The group ex-Tysabri has
reached breakeven and use of Tysabri in c.15k patients is expected for the Tysabri brand to breakeven. Our 2007 forecasts assume use of Tysabri in c.25K patients by the
end of the year. On the pipeline Elan anticipate regulatory decisions in EU and the US this year on Tysabri for Crohn’s disease and anticipate progressing three of the Alzheimer’s Disease products to the next stage of clinical trials including the initiation, interim analyses dependent, of Phase III trials with AAB-001.
• The post results conference call is at 1.30 GMT/8.30 EST where further details are expected on the guidance and R&D objectives for the coming year.
• See attachment for Further Details
Orla Hartford +353 1 611 5844 orla.hartford@ncb.ie
Q4 2006 results this morning were ahead of our expectations. At the revenue level product sales of $161.4m were 15% ahead of our $140.3m forecast and with lower than anticipated operating costs along with a tax benefit, the EPS loss (adjusted) of $0.13
was significantly lower than our $0.23 loss expectation. For the year Elan exceeded the $500m revenue target (revenues of $560.4m reported of which $532.9m related to product sales) and reported operating costs of $536m significantly lower than the operating cost target for FY2006. Guidance for 2007 is for revenues (ex-Tysabri) to exceed $500m and for group to be breakeven on an EBITDA basis by the end of the year. We will be revising our 2007 forecasts to reflect the stronger than anticipated revenue performance in 2006.
• The outperformance of product revenues of $161.4m, a 15% increase y-o-y were underpinned by stronger than expected revenues from contract manufacturing and the
hospital antibiotics. Product gross margins of 59% were ahead of our 55% expectation. Operating costs reduced 11% year-on-year (and were 13% lower than our forecast) primarily on lower SG&A leaving the operating loss (pre-charges) of $38.8m compared to our $72.4m loss expectation. A net charge of $43.2m on an arbitration award along with a tax benefit of $9.3m, resulted in a net loss of $16.5m. Negative adjusted EBITDA for the group was $9.2m, which resulted in Elan reporting negative adjusted EBITDA for
the full year of $91.1m. Excluding Tysabri positive adjusted EBITDA of $10.6m for 2006 was reported.
• This quarter revenues of Maxipime were broadly flat but Azactam increased 22% y-o-y and both products were significantly ahead of our expectations. The performance of Prialt was modest again this quarter with revenues of $3.4m. Notably, contract manufacturing was strong this quarter with revenues increasing 15% y-o-y to $67.3m, 8% ahead of expectations. Contract manufacturing revenues continue to be primarily underpinned by Tricor ($16.1m), Skelaxin ($11.8m) and Verelan ($8.5m).
• Elan expects total revenues (excluding Tysabri) in 2007 to exceed $500m with operating expenses anticipated to be in the range of $600m-$650m. Adjusted EBITDA is targeted to be less than negative $50m for the full year and for the group to reach
break-even on an EBITDA (adjusted) by the end of 2007. The group ex-Tysabri has
reached breakeven and use of Tysabri in c.15k patients is expected for the Tysabri brand to breakeven. Our 2007 forecasts assume use of Tysabri in c.25K patients by the
end of the year. On the pipeline Elan anticipate regulatory decisions in EU and the US this year on Tysabri for Crohn’s disease and anticipate progressing three of the Alzheimer’s Disease products to the next stage of clinical trials including the initiation, interim analyses dependent, of Phase III trials with AAB-001.
• The post results conference call is at 1.30 GMT/8.30 EST where further details are expected on the guidance and R&D objectives for the coming year.
• See attachment for Further Details
Orla Hartford +353 1 611 5844 orla.hartford@ncb.ie
...und "last but not least" Jack Gorman von Davy Research:
Elan Corp (USc)
ELN US
Better-than-expected Q4 outturn;
2007 guidance targets
positive adj EBITDA by end 2007
Elan’s Q4 results were better than expected – revenues of $161m were 10% ahead of forecast. Combined with lower SG&A costs, this meant that the adjusted EBITDA loss was $9.2m versus our $37.4m forecast. Adjusted loss per share was an
estimated 16c versus 25c forecast.
To recap on Tysabri, Q4 global revenues were $30.2m ($23m US, $7.2m EU]. A total of 6,600 patients are on the drug to date, with 9,600 enrolled. As we flagged last week, we will be reducing our 2007 Tysabri forecasts to circa $430m to reflect
enrolment trends. Our forecast had been top of the range.
Of the other products, Maxipime and Azactam had a stronger Q4 after supply issues in Q3. Elan ex Tysabri posted $14.4m EBITDA in Q4, driven by the higher
revenues but also by lower SG&A costs. Overall SG&A costs were also 15% below forecast in Q4 – the majority are related to lower costs on Tysabri. Elan’s consistent
SG&A costs discipline is to be highlighted and applauded.
As expected, there was no material newsflow on R&D contained in the statement.
Elan also provided its first guidance for 2007. Revenues ex Tysabri should exceed $500m, in line with our existing $522.5m forecast. Group operating costs of $600-650m are substantially below our $746m forecast. Adjusted EBITDA loss should be lower than $50m, reaching positive territory by end 2007 – in line with our original forecasts.
Although we will reduce our 2007 Tysabri forecasts, much of this is offset by Elan’s guidance on lower operating costs and means that our adjusted EBITDA losses for
2007 (-$14m) should be broadly intact.
Conference call at 13.30 (Ireland/UK time) on February 20th; US part
Elan Corp (USc)
ELN US
Better-than-expected Q4 outturn;
2007 guidance targets
positive adj EBITDA by end 2007
Elan’s Q4 results were better than expected – revenues of $161m were 10% ahead of forecast. Combined with lower SG&A costs, this meant that the adjusted EBITDA loss was $9.2m versus our $37.4m forecast. Adjusted loss per share was an
estimated 16c versus 25c forecast.
To recap on Tysabri, Q4 global revenues were $30.2m ($23m US, $7.2m EU]. A total of 6,600 patients are on the drug to date, with 9,600 enrolled. As we flagged last week, we will be reducing our 2007 Tysabri forecasts to circa $430m to reflect
enrolment trends. Our forecast had been top of the range.
Of the other products, Maxipime and Azactam had a stronger Q4 after supply issues in Q3. Elan ex Tysabri posted $14.4m EBITDA in Q4, driven by the higher
revenues but also by lower SG&A costs. Overall SG&A costs were also 15% below forecast in Q4 – the majority are related to lower costs on Tysabri. Elan’s consistent
SG&A costs discipline is to be highlighted and applauded.
As expected, there was no material newsflow on R&D contained in the statement.
Elan also provided its first guidance for 2007. Revenues ex Tysabri should exceed $500m, in line with our existing $522.5m forecast. Group operating costs of $600-650m are substantially below our $746m forecast. Adjusted EBITDA loss should be lower than $50m, reaching positive territory by end 2007 – in line with our original forecasts.
Although we will reduce our 2007 Tysabri forecasts, much of this is offset by Elan’s guidance on lower operating costs and means that our adjusted EBITDA losses for
2007 (-$14m) should be broadly intact.
Conference call at 13.30 (Ireland/UK time) on February 20th; US part
...da fehlt ja noch Einer : Stuart Draper von Dolmen Daily
Today’s Recommendation
Elan ($14.81) Q4 results announced Stuart Draper
• Q4 results : This morning, Elan announced its results for the 3
months ended 31/12/06. Net loss and loss per share of $26.5m and $0.06 respectively were improvements on the Q4 2005 loss
and loss per share of $58.3m and $0.14. The loss per share of
$0.06 was better than the consensus forecast of a loss per share
of $0.24, but this was largely driven by a $49.8m gain on an
arbitration award.
• Tysabri update : The full year 2006 net sales result of $38.1m
achieved by Tysabri will also not be sufficient to drive any
upgrades, and represents a slower level of sales uptake than
some would have originally expected prior to the drug’s 2004
approval. The figure of 10,000 patients currently signed up for
Tysabri, with c.300 more per week being added, is also a lower
level of growth than some previous forecasts.
• Sales required : Our view was always that Tysabri would receive
approval to return to the market given the unmet MS clinical
need for symptom alleviation rather than symptom relief, and
impaired function improvement. However, our continued
cautiousness in relation to Elan’s share price is based on the
very high level of Tysabri sales which are necessary to sustain it
at current levels. There is still no indication that Tysabri will be
approved as a first-choice treatment for categories of MS
patients such as “new to drug therapy” patients and “individual
benefit-risk” patients.
• Addressable market : The entire MS market is currently worth
c.$4 bn and given that there are some categories of MS patients
for which the drug is unlikely to be considered suitable, the
addressable market for Elan is unlikely to exceed $3 bn. Even
assuming that Elan achieves 50% of this addressable market,
with peak Tysabri sales of $1.5 bn, then the shares are still not
a BUY at current levels. There are currently 4 competing drugs
on the MS market, Avonex, Betaseron, Rebif and Capoxone, and
consensus forecasts for peak Tysabri sales have recently been
downgraded from $2 bn to $1.6 bn.
• Debt repayment : With Elan’s operating cash burn still running
at c.$250m per annum, there is still a risk that it will have
difficulty in repaying the debt of $1.1 bn due in 2008.
Therefore, our current NEUTRAL recommendation remains in
place until there is better visibility available that Tysabri’s
sales will significantly exceed $1.5 bn. Our view continues to be
that there are other share prices elsewhere offering similar
upside potential to Elan, for taking much less risk : NEUTRAL.
Today’s Recommendation
Elan ($14.81) Q4 results announced Stuart Draper
• Q4 results : This morning, Elan announced its results for the 3
months ended 31/12/06. Net loss and loss per share of $26.5m and $0.06 respectively were improvements on the Q4 2005 loss
and loss per share of $58.3m and $0.14. The loss per share of
$0.06 was better than the consensus forecast of a loss per share
of $0.24, but this was largely driven by a $49.8m gain on an
arbitration award.
• Tysabri update : The full year 2006 net sales result of $38.1m
achieved by Tysabri will also not be sufficient to drive any
upgrades, and represents a slower level of sales uptake than
some would have originally expected prior to the drug’s 2004
approval. The figure of 10,000 patients currently signed up for
Tysabri, with c.300 more per week being added, is also a lower
level of growth than some previous forecasts.
• Sales required : Our view was always that Tysabri would receive
approval to return to the market given the unmet MS clinical
need for symptom alleviation rather than symptom relief, and
impaired function improvement. However, our continued
cautiousness in relation to Elan’s share price is based on the
very high level of Tysabri sales which are necessary to sustain it
at current levels. There is still no indication that Tysabri will be
approved as a first-choice treatment for categories of MS
patients such as “new to drug therapy” patients and “individual
benefit-risk” patients.
• Addressable market : The entire MS market is currently worth
c.$4 bn and given that there are some categories of MS patients
for which the drug is unlikely to be considered suitable, the
addressable market for Elan is unlikely to exceed $3 bn. Even
assuming that Elan achieves 50% of this addressable market,
with peak Tysabri sales of $1.5 bn, then the shares are still not
a BUY at current levels. There are currently 4 competing drugs
on the MS market, Avonex, Betaseron, Rebif and Capoxone, and
consensus forecasts for peak Tysabri sales have recently been
downgraded from $2 bn to $1.6 bn.
• Debt repayment : With Elan’s operating cash burn still running
at c.$250m per annum, there is still a risk that it will have
difficulty in repaying the debt of $1.1 bn due in 2008.
Therefore, our current NEUTRAL recommendation remains in
place until there is better visibility available that Tysabri’s
sales will significantly exceed $1.5 bn. Our view continues to be
that there are other share prices elsewhere offering similar
upside potential to Elan, for taking much less risk : NEUTRAL.
Was hälst Du von diesem Statement, Cyberhexe ???
Ist denn AAB-001 mit AN-1792 vergleichbar ????
AAB-001 DEADLY SIDE EFFECTS CAUSE HAULT TO DOSAGE LEVEL!!!!
23-Feb-07 09:47 pm Anybody with half a brain can find this (below) from an article published by nature.com just 4 or 5 months ago. Read it and weep you pathetic drunk. Sober up and get your money out of the March horror coming!
Do you really want to lose all your money AGAIN in elan? Be smart this time, even if you don't have much money after last black monday you idiot.
""These drug candidates may also carry the highest risk, however; the highest dose of AAB-001 has now been discontinued owing to transient abnormalities observed through brain imaging (Black, R.S. et al., presented at 9th Annual Geneva Springfield Symposium on Advances in Alzheimer's Therapy in Geneva, Switzerland). An earlier candidate drug developed to trigger an immune response against amyloid-, AN-1792, was also discontinued owing to cases of encephalitis.17""
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Ist denn AAB-001 mit AN-1792 vergleichbar ????
AAB-001 DEADLY SIDE EFFECTS CAUSE HAULT TO DOSAGE LEVEL!!!!
23-Feb-07 09:47 pm Anybody with half a brain can find this (below) from an article published by nature.com just 4 or 5 months ago. Read it and weep you pathetic drunk. Sober up and get your money out of the March horror coming!
Do you really want to lose all your money AGAIN in elan? Be smart this time, even if you don't have much money after last black monday you idiot.
""These drug candidates may also carry the highest risk, however; the highest dose of AAB-001 has now been discontinued owing to transient abnormalities observed through brain imaging (Black, R.S. et al., presented at 9th Annual Geneva Springfield Symposium on Advances in Alzheimer's Therapy in Geneva, Switzerland). An earlier candidate drug developed to trigger an immune response against amyloid-, AN-1792, was also discontinued owing to cases of encephalitis.17""
http://messages.finance.yahoo.com/Stocks_%28A_to_Z%29/Stocks…
Antwort auf Beitrag Nr.: 28.044.637 von bernie55 am 01.03.07 12:33:17Ist denn AAB-001 mit AN-1792 vergleichbar ????
Bernie, der Vergleich ist nicht korrekt. AN-1792 ist ein Vakzin, also ein aktives Eingreifen in das Immunsystem, während AAB-001 ein Antikörper ist. Bei der Vakzinierung mit AN-1792 kam es zu Überreaktionen (Hirnhautentzündungen), die zum Studienabbruch geführt haben. Bei der Antikörpergabe findet jedoch keine Stimulanz der Immunabwehr statt, so dass derartige Reaktionen wie bei AN-1792 fast vollständig auszuschliessen sind.
ACC-001 ist hingegen eine Weiterentwicklung von AN-1792 und soll wie bei dessen Vorgänger die Plaquebildung über eine Stimulanz der Immunabwehr verhindern.
http://www.elan.com/research_development/Pipeline_Products/d…
Bernie, der Vergleich ist nicht korrekt. AN-1792 ist ein Vakzin, also ein aktives Eingreifen in das Immunsystem, während AAB-001 ein Antikörper ist. Bei der Vakzinierung mit AN-1792 kam es zu Überreaktionen (Hirnhautentzündungen), die zum Studienabbruch geführt haben. Bei der Antikörpergabe findet jedoch keine Stimulanz der Immunabwehr statt, so dass derartige Reaktionen wie bei AN-1792 fast vollständig auszuschliessen sind.
ACC-001 ist hingegen eine Weiterentwicklung von AN-1792 und soll wie bei dessen Vorgänger die Plaquebildung über eine Stimulanz der Immunabwehr verhindern.
http://www.elan.com/research_development/Pipeline_Products/d…
Antwort auf Beitrag Nr.: 28.072.323 von Cyberhexe am 02.03.07 17:09:12Gut dass wir Dich haben! Danke!
Danke!
 Danke!
Danke!
Antwort auf Beitrag Nr.: 28.072.323 von Cyberhexe am 02.03.07 17:09:12 THX + THX + THX + THX + THX
THX + THX + THX + THX + THX
 THX + THX + THX + THX + THX
THX + THX + THX + THX + THX
Auszug aus NCB
Wyeth Comments on Alzheimer’s Disease
Wyeth’s CFO made comments (see below) yesterday at an investor presentation related to the Alzheimer’s disease programs where he stated that the company’s Alzheimer’s research program will probably fail given the difficulty of developing
products for this disease.
• Wyeth’s comments are as follows: “We have a program which we call the “War on Alzheimer’s”, and our Alzheimer’s program involves 10 different programs in clinical development, 17 projects overall involving Alzheimer’s. We have a tremendous amount of resources committed to this and the bottom line is that we will probably fail and I say that not because I have deep insight into this but I say it because it is a highly risky proposition and statistics say that if you go after a disease like this you will probably fail but we might not. If we fail, Wyeth will still be a strong company, if we succeed we will change the world because Alzheimer’s is an incredibly costly, burdensome problem for society….”
• When considered in context Wyeth’s CFO, in our view, was highlighting the high risk high reward nature of the company’s Alzheimer’s disease programs. Elan collaborates with Wyeth on approximately half of the programs mentioned. AAB-001, which is in Phase II studies, is the most advanced program.
In our NPV analysis, we are assuming a 25% probability of success of AAB-001.
http://www.rte.ie/business/2007/morningrep/download/0321ncb.…
AHA !!!!???
FRAGE an DICH, Cyberhexe
Was ist denn überhaupt eine - NPV analysis ?????
Wie kommen die auf eine 25% ige Wahrscheinlichkeit bezüglich des Erfolges von AAB-01 ???
Wyeth Comments on Alzheimer’s Disease
Wyeth’s CFO made comments (see below) yesterday at an investor presentation related to the Alzheimer’s disease programs where he stated that the company’s Alzheimer’s research program will probably fail given the difficulty of developing
products for this disease.
• Wyeth’s comments are as follows: “We have a program which we call the “War on Alzheimer’s”, and our Alzheimer’s program involves 10 different programs in clinical development, 17 projects overall involving Alzheimer’s. We have a tremendous amount of resources committed to this and the bottom line is that we will probably fail and I say that not because I have deep insight into this but I say it because it is a highly risky proposition and statistics say that if you go after a disease like this you will probably fail but we might not. If we fail, Wyeth will still be a strong company, if we succeed we will change the world because Alzheimer’s is an incredibly costly, burdensome problem for society….”
• When considered in context Wyeth’s CFO, in our view, was highlighting the high risk high reward nature of the company’s Alzheimer’s disease programs. Elan collaborates with Wyeth on approximately half of the programs mentioned. AAB-001, which is in Phase II studies, is the most advanced program.
In our NPV analysis, we are assuming a 25% probability of success of AAB-001.
http://www.rte.ie/business/2007/morningrep/download/0321ncb.…
AHA !!!!???
FRAGE an DICH, Cyberhexe
Was ist denn überhaupt eine - NPV analysis ?????
Wie kommen die auf eine 25% ige Wahrscheinlichkeit bezüglich des Erfolges von AAB-01 ???
very interesting,
bin ja mal gespannt ob bei GNTA noch ein As aus dem Ärmel gezogen wird oder ob ende allende ist für das Nasdaq-Gastspiel.
grüße hb
bin ja mal gespannt ob bei GNTA noch ein As aus dem Ärmel gezogen wird oder ob ende allende ist für das Nasdaq-Gastspiel.
grüße hb
http://www.transitiontherapeutics.com/news/article.php
Apr 03, 2007
FDA Grants Fast Track Designation to Alzheimer’s Disease Drug Candidate AZD-103/ELND005
TORONTO, ON, April 3rd, 2007 - Transition Therapeutics Inc. ("Transition") (TSX: TTH) announced today that the United States Food and Drug Administration ("FDA") has granted Fast Track designation to investigational drug candidate AZD-103/ELND005, being developed in collaboration with Elan Corporation, plc. ("Elan") (NYSE: ELN) for the treatment of Alzheimer's disease. AZD-103/ELND005 is currently being evaluated in multiple Phase I clinical studies, and the companies anticipate starting Phase II clinical studies around the end of 2007.
"The decision by the FDA is very encouraging news for Alzheimer's disease patients and their families, and we believe it reflects the considerable potential for AZD-103 in this area," said Dr. Tony Cruz, Chairman and Chief Executive Officer of Transition. "Transition and Elan welcome this decision and look forward to working with the FDA and the clinical community to make continued progress on AZD-103/ELND005."
Under the FDA Modernization Act of 1997, Fast Track designation is intended to facilitate the development and expedite the review of a drug or biologic if it is intended for the treatment of a serious or life-threatening condition, and it demonstrates the potential to address unmet medical needs for such a condition.
http://www.elan.com/research_development/Pipeline_Products/d…" target="_blank" rel="nofollow ugc noopener">
http://www.elan.com/research_development/Pipeline_Products/d…
Apr 03, 2007
FDA Grants Fast Track Designation to Alzheimer’s Disease Drug Candidate AZD-103/ELND005
TORONTO, ON, April 3rd, 2007 - Transition Therapeutics Inc. ("Transition") (TSX: TTH) announced today that the United States Food and Drug Administration ("FDA") has granted Fast Track designation to investigational drug candidate AZD-103/ELND005, being developed in collaboration with Elan Corporation, plc. ("Elan") (NYSE: ELN) for the treatment of Alzheimer's disease. AZD-103/ELND005 is currently being evaluated in multiple Phase I clinical studies, and the companies anticipate starting Phase II clinical studies around the end of 2007.
"The decision by the FDA is very encouraging news for Alzheimer's disease patients and their families, and we believe it reflects the considerable potential for AZD-103 in this area," said Dr. Tony Cruz, Chairman and Chief Executive Officer of Transition. "Transition and Elan welcome this decision and look forward to working with the FDA and the clinical community to make continued progress on AZD-103/ELND005."
Under the FDA Modernization Act of 1997, Fast Track designation is intended to facilitate the development and expedite the review of a drug or biologic if it is intended for the treatment of a serious or life-threatening condition, and it demonstrates the potential to address unmet medical needs for such a condition.
http://www.elan.com/research_development/Pipeline_Products/d…" target="_blank" rel="nofollow ugc noopener">
http://www.elan.com/research_development/Pipeline_Products/d…
...ich frage mich, weshalb Elan eine so restriktive Kommunikationspolitik betreibt und beispielsweise die Erfolge der Nanotechnologie in den Quartalsberichten fast vollständig unterschlägt?
Als ehemaliger Investment-Banker bei Merrill Lynch & Co weiss CEO Kelly Martin sicherlich genau, dass Elans Wert nur dann nachhaltig ist, wenn institutionelle Anleger investieren.
Und der Anteil von institutionellen Anlegern ist mittlerweile auf über 50% angestiegen - Tendenz steigend:
http://holdings.nasdaq.com/asp/Institutional.asp?FormType=In…
Eine restriktive Kommunikationspolitik ist vor allem im Interesse von aktuellen und zukünftigen Käufern - was sagt uns dies?
Als ehemaliger Investment-Banker bei Merrill Lynch & Co weiss CEO Kelly Martin sicherlich genau, dass Elans Wert nur dann nachhaltig ist, wenn institutionelle Anleger investieren.
Und der Anteil von institutionellen Anlegern ist mittlerweile auf über 50% angestiegen - Tendenz steigend:
http://holdings.nasdaq.com/asp/Institutional.asp?FormType=In…
Eine restriktive Kommunikationspolitik ist vor allem im Interesse von aktuellen und zukünftigen Käufern - was sagt uns dies?
Antwort auf Beitrag Nr.: 28.653.993 von Cyberhexe am 04.04.07 10:52:00die "unzureichende" PR und IR Abteilungen bei ELAN verwundern mich schon seit mehreren Monaten. Gleichfalls bin ich über die große Anzahl an Aktien verwundert, die in den letzten Monaten zu diesen niedrigen Kursen veräußert wurden.
Allerdings fällt auch auf, dass der Kurs immer wieder mit kleinen Positionen gedrückt werden kann, um dann wieder günstig einkaufen zu können.
Ist halt ein großes Spiel, dass hier gespielt wird und das hoffentlich bald ein Ende gefunden hat, so dass der Kurs endlich in die Höhen steigen kann, auf die er schon seit langem gehört. Das an der Börse mit ELAN-Aktien auch die Zukunft es Unternehmens gehandelt wird, kann ich nicht feststellen. Momentan habe ich nicht einmal mehr das Gefühl, dass es sich um die Gegenwart handelt. ELAN hat in der Vergangenheit wohl zu viele Anleger enttäuscht, so dass der Einstieg erst mit dem Erreichen der Gewinnschwelle für viele Investoren und Institutionellen in Frage kommt.

Allerdings fällt auch auf, dass der Kurs immer wieder mit kleinen Positionen gedrückt werden kann, um dann wieder günstig einkaufen zu können.
Ist halt ein großes Spiel, dass hier gespielt wird und das hoffentlich bald ein Ende gefunden hat, so dass der Kurs endlich in die Höhen steigen kann, auf die er schon seit langem gehört. Das an der Börse mit ELAN-Aktien auch die Zukunft es Unternehmens gehandelt wird, kann ich nicht feststellen. Momentan habe ich nicht einmal mehr das Gefühl, dass es sich um die Gegenwart handelt. ELAN hat in der Vergangenheit wohl zu viele Anleger enttäuscht, so dass der Einstieg erst mit dem Erreichen der Gewinnschwelle für viele Investoren und Institutionellen in Frage kommt.

Goodbody...Q1’07 preview - Tysabri and AAB-001 centre stage.
Elan will release its Q1’07 results on Tuesday, 24th April. We forecast that the company will report a fully diluted loss per share of $0.12 from revenue of $170.5m. We have pencilled in total worldwide Q1’07 sales Tysabri of $46.7m, of which $28.7m will be recorded as revenue by Elan under its accounting agreement with Biogen Idec.
Under our current model, to allow for a ramp up of sales to an FY’07 total of $468.8m, we are looking for c.8,100 patients to have been on the drug by the end of March, rising to 9,800 by the end of April.
The other driver for the stock, at present, is the progress of AAB-001 for the treatment of Alzheimer’s Disease. A second interim look at the Phase II data is due in “mid” 2007. By the time of Elan’s results, the timing of potential newsflow will already have been discussed in the market, with the issue of its development partner Wyeth’s results tomorrow, 19th of April. On the rest of the business, attention will focus on impending generic pressure on Maxipime. We forecast the drug will contribute $41.7m to Q1’07 revenue. The initial patent expired in March and BMS (the patent holder) has been notified by Apotex (the marketing partner of Indian anti-infectives specialists Orchid Chemicals) that it intends to enter the US market on receiving approval from the FDA. Azactam lost patent protection in October 2005, but to date, there has been no indication that a generic is in the offing. In anticipation of generic challenges to both drugs, we
have already factored in sequential quarterly reductions in revenue through 2007. The recent strength in share price has seen it break through our price target. While anticipating further price momentum through April and early May on AAB-001 speculation, given the inherent risk still associated with Tysabri’s progress and the binary decision on AAB-001, we are retaining our Add recommendation, increasing our price target to $17.15. This reflects the weighting we place on the price should the AAB-001 decision come through (0.7 probability of $19.35) versus the price should there be no progress announced (0.3 probability of $12.00 - valuation of Elan with AAB-001 as a Phase II drug on a normal development time frame).
http://www.rte.ie/business/2007/morningrep/download/0418good…
Elan will release its Q1’07 results on Tuesday, 24th April. We forecast that the company will report a fully diluted loss per share of $0.12 from revenue of $170.5m. We have pencilled in total worldwide Q1’07 sales Tysabri of $46.7m, of which $28.7m will be recorded as revenue by Elan under its accounting agreement with Biogen Idec.
Under our current model, to allow for a ramp up of sales to an FY’07 total of $468.8m, we are looking for c.8,100 patients to have been on the drug by the end of March, rising to 9,800 by the end of April.
The other driver for the stock, at present, is the progress of AAB-001 for the treatment of Alzheimer’s Disease. A second interim look at the Phase II data is due in “mid” 2007. By the time of Elan’s results, the timing of potential newsflow will already have been discussed in the market, with the issue of its development partner Wyeth’s results tomorrow, 19th of April. On the rest of the business, attention will focus on impending generic pressure on Maxipime. We forecast the drug will contribute $41.7m to Q1’07 revenue. The initial patent expired in March and BMS (the patent holder) has been notified by Apotex (the marketing partner of Indian anti-infectives specialists Orchid Chemicals) that it intends to enter the US market on receiving approval from the FDA. Azactam lost patent protection in October 2005, but to date, there has been no indication that a generic is in the offing. In anticipation of generic challenges to both drugs, we
have already factored in sequential quarterly reductions in revenue through 2007. The recent strength in share price has seen it break through our price target. While anticipating further price momentum through April and early May on AAB-001 speculation, given the inherent risk still associated with Tysabri’s progress and the binary decision on AAB-001, we are retaining our Add recommendation, increasing our price target to $17.15. This reflects the weighting we place on the price should the AAB-001 decision come through (0.7 probability of $19.35) versus the price should there be no progress announced (0.3 probability of $12.00 - valuation of Elan with AAB-001 as a Phase II drug on a normal development time frame).
http://www.rte.ie/business/2007/morningrep/download/0418good…
Eine gute Elan-Analyse von Goldman
Habe leider den Orginallink nicht gefunden, aber der zum IV sollte erstmal genügen.
Die erwarten übrigens auch, daß CD nicht so der Hammer wird ...
http://www.investorvillage.com/smbd.asp?mb=160&mn=99883&pt=m…
Goldman Initiates Bond Coverage....text pasted - apparently occurred March 7 - only rec'd last week
Elan has reached an important milestone, the re-launch of Tysabri, and we expect the
trajectory of the company’s bonds to match the trajectory of the drug. If, as we expect,
the adoption of Tysabri grows to the EBITDA breakeven level of 15,000 patients by
mid-2007, we look for the value of Elan’s bonds to appreciate meaningfully. With
Elan’s negative EBITDA and its previous market withdrawal of the franchise product, we
acknowledge the risk, and bad memories, inherent in the ELN bonds. Nonetheless,
multiple sclerosis patients remain underserved by existing therapies and the superior
efficacy of Tysabri as a second line treatment for MS is not in dispute. For Elan to reach
positive EBITDA it must garner a relatively small 3% of the MS population. In addition,
the risk in the re-launch is mitigated by the company’s large cash balance: The year-end
2006 cash balance of $898 million pro forma for the redemption of the Athena notes is
large enough to cover almost five years of interest and capex, assuming the rest of the
business is EBITDA neutral. We view the incremental risk from extending maturity to
2013 from 2011 as small relative to the near-term risks, so we recommend buying the
longer-dated bonds to pick up yield.
Upcoming catalysts. We expect trading in ELN bonds to be heavily influenced by news
flow in 2007:
• Late April: Biogen (BIIB) announces 1Q2007 results and should provide an update on
Tysabri dosing in the US and EU.
• Late April/early May: Elan reports 1Q2007 results. In addition to Tysabri results, we
expect to see the ex-Tysabri business remain EBITDA positive.
• April 28-May 5: American Academy of Neurology Annual Meeting. ELN will review
performance and safety data of Tysabri (i.e., reporting any incidences of PML).
• May 19-24: Digestive Disease Week 2007. Expect incremental data on the use of
Tysabri for treating Crohn’s disease.
• 1H2007: Potential approval of Tysabri for treatment of Crohn’s in EU.
• Midsummer: Could advance Alzheimer’s drug AAB-001 into Phase III trials.
• Widely recognized superior efficacy of Tysabri in an underserved patient
population. The existing MS market is fragmented among four drugs, with each
posting over $1.1 billion in sales and the aggregate sales of the four drugs
growing at 7.8% in 2006. Based on trial data, Tysabri yielded a 67% reduction in
relapses while the other four drugs are believed to yield approximately a onethird
reduction in relapses. In addition, Tysabri creates fewer common side
effects such as flu-like symptoms and tiredness. The three past Tysabri-related
incidences of PML, a very rare brain infection, occurred in patients in dual
therapy with Avonex and a patient recently treated with immuno-suppressants.
While it remains to be seen if Tysabri in mono-therapy causes PML, our
unscientific survey of MS blogs indicates that the MS community is supportive of
the risk/benefit trade-off of the drug. This leads us to believe that the
reintroduction of Tysabri may result in an increase in the total number of
patients being treated as well as steal share from the competing therapies.
Exhibit 1: Existing $5.5 billion MS market is fragmented
(2006 sales data in billions)
Betaseron
(Schering/Berlex)
$1.1, 20%
Copaxone
(Teva/Aventis)
$1.4, 25% Avonex (Biogen)
$1.7, 31%
Rebif
(Serono/Pfizer)
$1.3, 24%
Source: BIIB company filings
• Tysabri needs only 3% market penetration to be EBITDA breakeven. Because of
the high gross margin of biologics, Elan’s EBITDA and cash flow are highly
leveraged to Tysabri sales. The Tysabri business achieves positive EBITDA with
approximately 15,000 patients, which is 3% of the approximately 490,000 MS
patients worldwide (including 350,000 currently on other treatments and 100,000
who have stopped treatments and 40,000 who have not yet been on any
treatment)1. We estimate that 15,000 patients equate to annual sales (meaning both
Elan and Biogen) of $405 million and gross margin of $312 million. Elan’s 50%
share of that gross margin approximates its 50% share of sales and R&D expense.
Each additional 15,000 patients adds approximately $156 million of cash flow to
ELN. See Exhibit 14 for details of these estimates.
• No near-term maturities. Elan’s tender for the Athena notes in January 2007
extended the nearest maturity to 2011. This provides the company with almost a
four years to build a market for Tysabri and possibly launch an Alzheimer’s
treatment. Also, with the recent forced conversion of the $254 million converts,
the company has shown its willingness to issue equity for the benefit of the
balance sheet.
Exhibit 2: Elan has no debt maturities until 2011
Debt maturity profile in $ millions
$0
$200
$400
$600
$800
$1,000
$1,200
$1,400
2007 2008 2009 2010 2011 2012 2013
Source: Company filings
• Large cash balance. As of December 31, 2006, Elan had $898 million of cash pro
forma for the redemption of the $613 million Athena notes, which occurred in
January 2007. Between now and early 2009 when we expect free cash flow to
become positive, the cash balance does not drop below $660 million, according to
our model. See the credit risk section for a sensitivity analysis of Tysabri
adoption.
Elan Corporation PLC Healthcare
4 Goldman Sachs Credit Research
Exhibit 3: Cash balance provides a large cushion
($ in millions, spike due to timing difference in refinancing of Athena debt)
$0
$200
$400
$600
$800
$1,000
$1,200
$1,400
$1,600
1
Q05
3Q05
1Q
06
3Q06
1Q
07
3Q07
1Q08
3Q
08
1Q09
3Q09
Tysabri re-launch
Tysabri reaches
15,000 patients
Tysabri reaches
30,000 patients
Source: Company filings and Goldman Sachs Credit Research estimates
Credit risks
• Dependence on one drug with an unknown safety profile. While Elan does
have other businesses, its bonds are likely to underperform if Tysabri
underperforms. Despite the expected growth in the NanoCrystal business and
lack of generic competition so far in the company’s other marketed products, we
believe Elan would remain cash flow negative if less than 30,000 patients were to
use Tysabri. Risks to broad adoption of Tysabri include incidences of PML in
mono-therapy and commercialization of a new oral MS therapy. Previous cases
of PML occurred in two patients on dual therapy with Avonex and one patient
whose immune system was compromised. The TOUCH prescribing program is
designed to screen patients in order to reduce the risk of PML. On the
competitive front, Serono’s Cladribine is an oral therapy that is just beginning
Phase III trials, meaning it is unlikely to come to market before early 2010,
assuming it is successful. Elan management points to Sanofi-Aventis’s
Teriflunomide and Novartis’s FTY720 as the therapies under development that
are the most likely to succeed.
Exhibit 4: Several Competing MS therapies are under development
Compound Manufacturer Trial Phase Dosing Notes
Cladribine Serono Enrolling patients for a two-year
Phase III trial
Oral dosing
Teriflunomide Sanofi-Aventis Recruiting patients for Phase III trial Oral dosing
FTY 720 Novartis Recruiting patients for a 24-month
Phase III trial
Daily oral dosing
Laquinimod Active Biotech Additional Phase II studies ongoing Oral dosing
MBP 8298 BIOMS Being developed in three late-stage
trials (Phase II/Phase III)
Intravenous
Source: Company websites
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 5
• We estimate Elan will be free cash flow negative until early 2009. We are
projecting a 2007 cash burn of $179 million and a 2008 cash burn of $43 million
before the company turns free cash flow positive in early 2009. Estimates of cash
flow and leverage are highly sensitive to estimates of Tysabri use. Our base case
assumes maximum weekly adds of Tysabri patients of 350, with a steady-state
rate in 2009 and beyond of 225 per week. This results in 2009 leverage of 6.3x. We
have also tested downside cases with maximum weekly adds of 275 and 200
patients; in these cases, leverage in 2009 is 10.1x and 25.6x, respectively. See
Exhibit 5 for a summary of the sensitivity analysis. Note that the downside cases
do not assume any reduction in sales force expense.
Exhibit 5: Elan cash flow is sensitive to Tysabri adoption
$ in millions
Base Case A Little Worse Much Worse
Maximum weekly adoption rate 350 275 200
Steady-state weekly adoption rate 225 150 75
Tysabri WW 2009 revenue $1,239 $967 $695
ELN 2009 EBITDA $281 $175 $69
Debt/2009 EBITDA 6.3x 10.1x 25.6x
Min cash balance through YE2011* $660 $563 $384
* Assumes refinancing of 2011 maturity bonds
Source: Goldman Sachs Credit Research estimates
• Failure of AAB-001 to move to Phase III trial in 2007. While near-term cash
flows depend on Tysabri, the company’s Alzheimer’s pipeline is a key source of
Elan’s value. AAB-001 is scheduled to complete its Phase II trial by the end of
2007, and Elan has said the move to Phase III could be accelerated if the data at a
mid-2007 interim look are compelling. The AAB-001 trial is being run by an
independent clinical research firm, so Elan and co-developer Wyeth do not have
access to interim look data. Note that after the year-end 2006 interim look there
was also a possibility that the trial would be advanced to Phase III; however, that
did not happen, indicating the data were not “excellent.” We believe
Alzheimer’s, in conjunction with the NanoCrystal business, represents an
important path for Elan to diversify its revenues to reduce risk.
Potential catalyst
• Quarterly updates on Tysabri dosing. We are expecting patient adds to
accelerate somewhat from the 300 per week reported in February as
reimbursement is solidified in the individual European countries.
• American Academy of Neurology Annual Meeting. The company intends to
present clinical and safety data at research conferences. We also view the
conferences as an opportunity for the company to continue to educate doctors
about the superior features of Tysabri relative to other MS treatments. We expect
competing drug companies to announce trial data during the conferences.
Elan Corporation PLC Healthcare
6 Goldman Sachs Credit Research
Digestive Disease Week on May 19-24 will present a similar opportunity
regarding the treatment of Crohn’s.
• Potential approval of Tysabri for treatment of Crohn’s in the EU. A response,
whether positive or negative, is expected in the first half of 2007. While Tysabri
has shown a lasting positive therapeutic effect in Crohn’s patients, approval is far
from certain. One of the three Tysabri-related cases of PML was in a Crohn’s
patient who had previously been treated with immuno-supressants. Biogen CEO
James Mullen told the Boston Globe that he believes European approval is not
likely without additional data; he noted that Tysabri may not be well-suited to
Crohn’s patients because that patient population has been through several
rounds of immunosuppressing drugs and is therefore already at risk for PML.
An application has also been filed with the FDA.
• Alzheimer’s drug AAB-001 moves into Phase III trials. There will be an interim
look in mid-2007. If the data are compelling, the study will move to Phase III
immediately; otherwise, Phase II work will be completed by the end of 2007,
with a decision on continuing to Phase III to follow.
Relative value
Comparables. Assessing Elan’s relative value is difficult because of the unique
business risks at the company and the huge growth potential in EBITDA. We are
comparing Elan with a group of similarly rated hospitals companies. Business risk at
those hospitals is manageable, with the key concerns being persistently weak
volumes and declining operating margins. The hospitals also have relatively high
capex needs, which significantly cut into cash flow, particularly for the highly
levered companies such as Tenet. By contrast, the risk at Elan is more focused, as it
hinges on the success of one product. Although Elan has minimal capex needs, we
are projecting free cash flow to be negative until early 2009.
Leverage. We are projecting only $20 million of EBITDA in 2007 for Elan because
Tysabri is just beginning to ramp up; therefore, we are using debt/enterprise value
as our key leverage metric. We believe this is a useful ratio because Elan’s enterprise
value is a better measure of the company’s value than 2007 EBITDA since it
incorporates the market outlook for Tysabri and the Alzheimer’s pipeline. Given the
company’s large cash balance, we think it is acceptable to use this future value
measure instead of next year’s EBITDA because liquidity is not an issue. Elan’s debt
to enterprise value is 26%, which compares favorably with a debt-to-EV of 39% and
66% for Community Health and Tenet, respectively. In addition, we consider debt to
2009E EBITDA. We are projecting a debt to 2009E EBITDA figure of 6.3x for Elan,
which compares with 5.6x for HCA and 5.0x for THC.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 7
Exhibit 6: Relative value comparison with similarly rated hospitals
Estimated 2007
ELN CYH HCA IAS THC
Financials
EBITDA $20 $688 $4,402 $253 $700
Cash $704 $80 $729 $108 $509
Total Debt 1,765 2,192 28,216 1,060 4,879
Net Debt $1,061 $2,112 $27,486 $952 $4,370
CFO ($149) $361 $2,052 $153 $375
Capex 31 325 1,800 200 750
FCF ($179) $36 $252 ($47) ($375)
Enterprise Value $6,717 $5,563 NA NA $7,368
Ratios
Debt/EBITDA 89.1x 3.2x 6.4x 4.2x 7.0x
Debt/EV 26% 39% NA NA 66%
Debt/2009 EBITDA 6.3x 2.6x 5.6x 2.9x 5.0x
Benchmark Bond Pricing
Bond 8.875% of 2013 6.5% of 2012 6.5% of 2016 8.75% of 2014 9.25% of 2015
Priority Sr. Notes Sr. Sub. Sr. Notes Sr. Sub. Sr. Notes
Rating (Moody's/S&P) B3/B B3/B Caa1/B- B3/B- Caa1/CCC+
Price $100.88 $99.25 $84.30 $102.75 $99.00
Yield to Worst 8.67% 6.66% 9.10% 8.09% 9.43%
Spread to Worst 420 218 460 426 496
Source: Goldman Sachs Credit Research and company reports
Total return. Because the risks are so different at Elan, we believe it needs to be
considered on an expected total return basis, not just on a relative value basis. In our
view, none of the bonds in the comparable group have a potential for capital
appreciation similar to that of the Elan bonds. Exhibit 7 shows the sensitivity of the
price of the 8.875% senior notes of 2013 to assumed spread levels. We use it to
illustrate the potential for capital appreciation at various yields. As neurologists
weigh the risks of Tysabri and the adoption of the drug progresses, some business
risk is reduced. There will always be the possibility of additional PML cases, so we
are not suggesting the bonds should trade all the way to pre-PML levels; however,
we do believe there is at least 100 bp of spread upside if Tysabri reaches 15,000
patients by mid-2007.
Exhibit 7: A return to near pre-PML spreads creates a large jump in bond price
8.875% senior notes of 2013
Spread Price Notes
700 $88 Average level in month after PML first reported in Feb 2005
442 $100 Par
419 $101 Current level
350 $104
300 $107
250 $109 Average level in month prior to initial FDA approval in Nov 2004
175 $112 Tightest spread prior to PML report
Source: Goldman Sachs Credit Research
Elan Corporation PLC Healthcare
8 Goldman Sachs Credit Research
Company description
Biotech, with history. Elan is a well-established biotech company with a history in
drug development going back to 1969. The company operates in two business units:
Biopharmaceuticals and Elan Drug Technology (EDT). Biopharmaceutical focuses on
three areas: (1) autoimmune diseases such as multiple sclerosis; (2)
neurodegenerative disease, with a specialty in Alzheimer’s; (3) specialty
pharmaceuticals for severe pain and infectious diseases. EDT is a contract
manufacturer and licenses drug delivery technologies. The company maintains R&D,
manufacturing, and marketing facilities in the United States and Europe, and
employs roughly 1,700 people worldwide. The Ireland-based public company has
been listed on the NYSE since 1984.
Elan grew through acquisitions in the 1990s and 2000; however, the company then
suffered a number of setbacks – both corporate and clinical – which led to large
divestitures. In January 2002, Elan became the subject of an investigation by the SEC
sparked by a Wall Street Journal report that questioned the company’s accounting
practices. Subsequently, Elan became the target of a shareholder lawsuit regarding
false and misleading statements. In 1Q2002, clinical trials of an Alzheimer’s drug
were abruptly halted after 5% of patients suffered complications. These events led to
multiple credit rating downgrades by Moody’s and S&P, and the forced resignation
of top-level management. In July 2002, Elan’s current CEO, Kelly Martin, announced
plans to sell products worth $1.5 billion and drastically decrease the workforce.
While Elan does have some history, all related investigations and lawsuits have been
resolved and management has proved itself willing to take drastic action quickly to
right the company.
Exhibit 8: Elan’s share price still trades significantly off of its 2001 highs
(January 1, 2000 – February 27, 2007)
0
10
20
30
40
50
60
70
Jan-00 Jan-01 Jan-02 Jan-03 Jan-04 Jan-05 Jan-06 Jan-07
Stock price (US$)
Source: Bloomberg
Capitalization. In November 2006, Elan issued $615 million of 2013 bonds in two
tranches: a $465 million 8.875% tranche and a $150 million floating rate L+412.5
tranche. The proceeds were used to call the old Athena 7.25% senior notes of 2008. As
part of the same broad refinancing, the company issued a redemption notice for its
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 9
$254 million of 6.5% convertible notes of 2008, thereby forcing conversion at a share
price of $7.42. The transaction reduced debt by $254 million and extended the nearest
maturity from 2008 to 2011. The new bonds, as well as the existing 2011 bonds, were
issued by Elan Corporation and co-issuer Elan Finance. All bonds are guaranteed by
Elan and subsidiaries.
Exhibit 9: Elan Corporation – capitalization and ratios
($, millions)
Description Amount % of EV
No bank debt $0
Total Sr Sec debt 0 0.0%
7.75% Sr. Notes due 11/15/2011 $850
L+400 Sr. Notes due 11/15/2011 300
8.875% Sr. Notes due 2013 465
L+412.5 Sr. Notes due 2013 150
Total Sr debt 1,765 26.2%
Total Sub debt 0 26.2%
Other $0
Total Debt 1,765 26.2%
Market Cap 5,813
Enterprise Value 6,736 100.0%
Source: Goldman Sachs Credit Research and company reports
Marketed products. In addition to Tysabri, Elan currently markets three products:
Maxipime, Azactam, and Prialt (US only). Maxipime and Azactam are used to fight
hospital-born infection and Prialt is used to treat acute pain. All three are linked
because their use is hospital-based, so a single sales force can effectively market the
drugs. Azactam’s patent expired in October 2005 but to date no competitors have
filed for approval to produce a generic version. With 2006 sales of $78 million,
Azactam may be too small to warrant the interest of many generic manufacturers.
Maxipime is protected by several patents, with the basic patent expiring in March
2007 and a formulation patent coming off in February 2008. Elan sold the European
rights to Prialt in March 2006 for $50 million, plus $10 million to be received within
two years and $40 million of contingent payments. Our model assumes generic
competition enters the market as shown in Exhibit 10.
Elan Corporation PLC Healthcare
10 Goldman Sachs Credit Research
Exhibit 10: Tysabri revenue should more than offset other lost revenue
(Sales in $, millions)
0
50
100
150
200
1Q06 2Q06 3Q06 4Q06 1Q07E 2Q07E 3Q07E 4Q07E 1Q08E 2Q08E 3Q08E 4Q08E
Tysabri Maxipime Azactam Prialt Maxipime
generic
Supply
shortage
Azactam
generic
Source: Company filings and Goldman Sachs Credit Research
EDT. Elan Drug Technology (EDT) consists of a drug delivery technology licensing
business, headlined by NanoCrystal, and a contract manufacturing business. EDT
contributed $235 million of revenue in 2006. NanoCrystal technology is licensed to
other drug manufacturers in exchange for royalty payments in the mid- to high
single digits. The technology allows the slow release of crystalline drugs by reducing
the particle size to under 400nm. NanoCrystal patents expire in 2011 in the US and
2012 outside the US. A summary of the current portfolio of NanoCrystal compounds
is shown in Exhibit 11. The largest royalty-generating drug, Tricor from Abbott,
contributed $52 million in revenue in 2006, or 9% of Elan’s total.
Exhibit 11: Several products currently license NanoCrystal
Compound Manufacturer Launch date
2005 YTD sales
($ in millions)
2006 royalties
($ in millions)
TriCor Abbott/Fournier December 2004 $1,048 $52.1
Emend Merck April 2003 $131 NA
Rapamune Wyeth August 2000 $337 NA
Megace ES Par July 2005 $32* NA
BiDil NitroMed Announced Feb 2007 NA NA
Source: Company web sites and filings
* 1Q2006 sales annualized. More recent data not yet available.
While limited information on the NanoCrystal pipeline has been disclosed, we
believe NanoCrystal revenue should grow in the coming quarters. Exhibit 12 shows
development projects that are under way. A reformulation of Johnson & Johnson’s
paliperidone represents a large opportunity owing to the size of the schizophrenia
market. In addition, Elan signed an agreement with Abbott to develop a combination
of TriCor and Crestor that would use NanoCrystal technology. Recently, Elan has
started taking on a larger role in the development of new applications in exchange
for higher royalty rates. This tact is not without risk, however, as it requires Elan to
make a larger up-front investment.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 11
Exhibit 12: Several products are under development to license NanoCrystal
Compound Manufacturer Indication Status Projected launch
Paliperidone palmitate J&J Schizophrenia Phase III 2008
Budesonide MAP Asthma Phase III 2009
Fencrestor Abbott Cholesterol Phase III 2010
Source: Company web sites and filings
Pipeline. Elan’s drug development pipeline is dominated by Alzheimer’s studies,
with six different compounds along three different pathways in development. The
Alzheimer’s market is large, as an estimated 4.5 million people suffer from
Alzheimer’s in the US and 5 million in the EU. However, the market is also very
competitive, with basically all the major pharmaceutical companies working in the
space. Two of Elan’s studies are in conjunction with partners, Wyeth on the
Immunotherapy pathway and Transition Therapeutics on the A-beta Aggregation
Inhibitor pathway. While we do see the value of the pipeline as a buffer for the
minimum value of Elan’s bonds, we note that the development agreement with
Wyeth includes a change of control provision whereby Wyeth has the right of first
refusal to buy out Elan at a price determined by a formula.
The most advanced of Elan’s Alzheimer’s studies is AAB-001, which is in Phase II
trials. AAB-001, which is being developed in collaboration with Wyeth, has been
granted fast track status by the FDA because there is currently no breakthrough
treatment for Alzheimer’s disease. The drug is scheduled to complete Phase II by the
end of 2007, and Elan has said the move to Phase III could be accelerated if the data
at a mid-2007 interim look are compelling. The AAB-001 trial is being run by an
independent clinical research firm, so Elan does not have access to interim look data.
Note that after the year-end 2006 interim look, it was also possible that the trial
would be advanced to Phase III; however, that did not happen, indicating the data
were not “excellent.” Only Phase I data have been released; and while results do
show improvement in memory over the placebo, the p values are very high,
indicating there is a relatively high probability that the difference is due to chance.
Elan’s key objectives for 2007 include moving two other Alzheimer’s compounds into
Phase II.
Tysabri
The MS population. There are approximately 350,000 MS patients in the US and
360,000 in Europe. Of these, approximately 220,000 are patients diagnosed in the past
who have not started treatment (most likely because the diagnosis is not definitive or
the symptoms are mild). Therefore, we believe the market for Tysabri is
approximately 490,000 patients who are currently in treatment, have stopped
treatment, or have not yet tried treatment. According to the National MS Society,
roughly 200 people are diagnosed with the disease every week. The majority of those
diagnosed with MS are women between 20 and 50 years old. The disease is more
common among people of northern European descent.
MS is a progressive neurological disease in which the body loses the ability to
transmit messages along nerve cells, leading to a loss of muscle control, and in some
cases paralysis. Nerve fibers of the central nervous system are protected by a fatty
substance known as myelin. It is believed that MS is an autoimmune disease wherein
Elan Corporation PLC Healthcare
12 Goldman Sachs Credit Research
the body attacks its own myelin sheath surrounding the nerve fibers, creating scars
(or sclerosis) and oftentimes damaging the nerve fibers as well. Messages that travel
through the damaged nerve fibers to the brain then become distorted and
misinterpreted.
The difficulty of diagnosing MS is that individuals with the disease show different
symptoms. More common symptoms include tingling, numbness, slurred speech,
and vision problems. Other symptoms are muscle weakness, loss of balance and
coordination, tremors, and even paralysis, which may be temporary or permanent.
The National MS Society reports that one-third of MS suffers are wheelchair-bound.
Symptoms also vary over time, and usually come and go. For this reason, it can
sometimes take several months and even years to diagnose definitively.
Available treatments. There is no known cure for MS. There are, however, four
treatments in common use that can reduce the frequency of relapses: Avonex, Rebif,
Betaseron, and Copaxone. Among the four, Avonex, which was introduced by
Biogen in 1996, has the greatest market share. It is administered once weekly by
injection into muscle. In contrast, three to seven doses of Rebif, Betaseron, or
Copaxone are required per week, but they are administered through less-painful
injections just under the skin. Owing to the frequent and uncomfortable dosing and
the difficult side effects such as flu-like symptoms and severe fatigue, patient
compliance with the drug regiments is a challenge. Further, some patients develop
antibodies to the beta interferons (Avonex, Rebif, and Betaseron), making the drugs
less effective over time2. Maintaining a “normal” life between relapses is a big issue
for MS patients, and Elan believes that dosing by monthly infusion, as Tysabri is
administered, may be preferred over weekly or more frequent self-administered
injections.
History of Tysabri: Tysabri was introduced to the MS market in November 2004
after much anticipation. Three months later, in February 2005, Elan and Biogen
pulled the drug and stopped clinical trials. Two cases of PML, one of which resulted
in death, were discovered in the clinical trial of dual therapy with Avonex. In March,
it was discovered that a third patient (who died in December 2003) had contracted
PML after eight doses of Tysabri. This patient was in a Crohn’s study and had been
treated with immuno-suppressants. Elan and Biogen completed a safety review,
which uncovered no additional cases of PML, and then submitted a supplemental
Biologics License Application for re-approval with the FDA in September 2005.
The FDA re-approved the drug in June 2006 following a review process that included
emotional patient testimonies on the drug’s powerful effects, and Tysabri was
reintroduced to the US market the following month. The drug was also approved for
commercial distribution in July 2006 in the European Union. In the US, patients on
Tysabri must enroll in the TOUCH (Tysabri Outreach: Unified Commitment to
Health) program. TOUCH includes screening patients for factors that could
contribute to a weakened immune system (such as AIDS or a recent course of
immuno-suppressant therapy) and taking a baseline MRI in order to facilitate any
diagnoses of PML.
Tysabri efficacy. During the drug’s two-year plus clinical study, Tysabri was shown
to reduce the frequency of relapses significantly. In addition, Tysabri has been shown
2 Mayo Clinic web site on MS.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 13
to reduce the risk of disability progression by 42%.3 This contrasts with the other
treatments, which can reduce the frequency of flare-ups but have not been
definitively shown to alter the long-term development of permanent disability.4
There has not been a head-to-head study of the efficacy of Tysabri compared with
other treatments (the SENTINEL trial evaluated Tysabri in dual therapy with Avonex
compared with Avonex alone, and the AFFIRM trial compared Tysabri with a
placebo). Nonetheless, Tysabri is believed to be more effective. For example,
Copaxone reduced the relapse rate by about one-third while Tysabri mono-therapy
reduced the relapse rate by 67%5.
The National MS Society has endorsed Tysabri, noting the following: “We feel that
it’s likely that Tysabri is a very strong efficacious drug for people with MS.” The
Society says that the PML cases were “not a disaster” for MS treatment, and that MS
patients and doctors must learn to evaluate risks and benefits of treatments much as
RA and lupus doctors have. While the Society’s Web site provides information on
Tysabri, the Society’s Disease Management Consensus Statement has not been
updated since the re-approval of Tysabri. The FDA recommended Tysabri for
patients who have had inadequate response to or difficulty tolerating the side effects
of other MS therapies, and the MS Society echoes that recommendation.
A cursory survey of blog comments posted by MS patients regarding Tysabri shows
that most patients who have been infused report a very positive result. Comments
about “getting my life back” are common. A few indicated that they had expected a
more dramatic impact but that they were pleased so far. On the negative side, a
handful reported that they were considering taking the drug but that they were
afraid of the unknown, presumably PML, with one patient saying his neurologist
said “no way” to Tysabri.
Patents. While there is a move afoot in the US to create a pathway for generic
biologics, we do not view this as a risk to Tysabri. First, the drug is protected by
several patents both in the US and outside, covering the protein, DNA encoding the
protein, manufacturing methods, and pharmaceutical compositions. In the US, the
principal patents expire between 2014 and 2020, and outside the US they expire
between 2014 and 2016. Second, biologics are difficult to manufacture; so even if
bioequivalence can be shown, the would-be generic manufacturer would still need to
develop commercial manufacturing capabilities.
Adoption rate. The Elan financial model is very sensitive to the assumptions made
about Tysabri adoption. We believe that the adoption rate will accelerate from the
300 per week reported for February, as US insurance companies and European
national health systems determine reimbursement. So far, US private insurers have
been providing good access to the drug, with 40% of patients having no prior
treatment requirements and 55% having a one prior treatment requirement. In
Europe, the launch has not been rolled out to approximately half of the countries and
reimbursement is not yet in place for several countries, including UK, Finland,
Norway, and Austria.
3 The New England Journal of Medicine 2006; 354:899-910.
4 Mayo Clinic website on MS.
5 National Multiple Sclerosis Society video, “Tysabri: What you need to know”
Elan Corporation PLC Healthcare
14 Goldman Sachs Credit Research
Exhibit 13: Adoption rate estimates for Tysabri
0
50
100
150
200
250
300
350
400
4Q06 1Q07 2Q07 3Q07 4Q07 1Q08 2Q08 3Q08 4Q08 1Q09 2Q09 3Q09 4Q09
Weekly Adds to Tysabri
0
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
Average Tysabri Patients
Average patients (rt axis) Weekly adds (lt axis)
200 Diagnoses per week
Source: Company filings and Goldman Sachs Credit Research estimates
PML. Progressive Multifocal Leukoencephalopathy, or PML, is a very rare but often
fatal brain disease caused by the JC virus. It occurs in people with extremely weak
immune systems, owing to either AIDS or drug therapies. The Tysabri patients that
developed PML were in trials where patients had taken an average of 17.9 doses of
Tysabri. A total of 3,417 patients participated in the MS, Crohn’s, and RA trials and
three were diagnosed with PML. None of the three Tysabri-related PML cases were
in patients from the AFFIRM trial, which tested mono-therapy Tysabri for over two
years against placebo.6 If PML is detected early, it can sometimes be stabilized.
A few drugs are thought to be linked to PML and in certain disease categories the
benefits of treatment weigh favorably against the risk. The drugs that have been
linked to PML are Rituxan (cancer and severe rheumatoid arthritis (RA) drug from
Genentech and Biogen), Enbrel (RA and Psoriatic Arthritis drug from Amgen and
Wyeth), Humira (Abbott drug approved for RA, Psoriatic Arthritis, and, recently,
Crohn’s), Imuran (immunosuppressant used to prevent rejection of kidney
transplants), and Tysabri.
Accounting. Tysabri is marketed through a 50/50 collaboration with Biogen; the
product operating profit is effectively split 50/50 between the two companies,
however the accounting is a bit convoluted (an illustrative example is shown in
Exhibit 14). In the US, Elan purchases the drug from Biogen and is responsible for
distribution. Therefore, Elan books revenue equal to 100% of US sales and then books
COG at a price equal to the cost of manufacturing plus Biogen’s gross margin and
any third party royalties. Elan’s income statement also shows 50% of the combined
US sales and marketing expense. In the EU, Biogen is responsible for distribution, so
Elan books revenue that is equal to its share of EU operating profits plus any directly
incurred costs. Therefore, Elan’s EU revenue can be negative if operating profit in the
region is negative. Biogen is responsible for the manufacture of Tysabri for both
markets. The wholesale price of Tysabri has been set at $2,185 per vial, or $28,400 per
6 New England Journal of Medicine 2006.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 15
year for 13 doses.7 We have assumed a 7% discount in this price.
Exhibit 14: Illustrative accounting for Tysabri
Assuming nominal D&A, EBITDA breakeven with 15,000 patients
Average patients dosing 15,000
Price per year, net of discounts $26,980
Percentage of patients in US 65%
Assumed gross margin % 77%
Reality ($ in millions) Accounting for ELN ($ in millions)
In the US
Total US sales $263.1 Revenue $263.1
COGS $60.5 COGS (Mfg cost plus BIIB's share of GM) $161.8
Gross margin $202.6 Gross margin $101.3
SG&A $175.0 50% share of SG&A $87.5
Operating profit bf R&D $27.6 Operating profit bf R&D $13.8
In the EU
Total EU sales $141.6 Revenue (ELN's share of Op Profit + direct costs) $23.0
COGS $32.6 COGS $6.0
Gross margin $109.1 Gross margin $17.0
SG&A $75.0 SG&A $0.0
Operating profit bf R&D $34.1 Operating profit bf R&D $17.0
Direct ELN costs $6.0
Total
Worldwide R&D $65.0 50% share of R&D $32.5
Worldwide operating profit -$3.4
ELN share of operating profit -$1.7 Total operating profit -$1.7
Source: Company filings and Goldman Sachs Credit Research estimates
Crohn’s and other indications. Elan has filed applications in the US and Europe to
market Tysabri as a treatment for Crohn’s disease. Crohn’s disease is a chronic
inflammation of the gastrointestinal tract. It is most commonly diagnosed in 15- to
35-year-olds and is more prevalent among people of European Jewish ancestry.
Symptoms include abdominal pain, nausea and vomiting, severe diarrhea, and
weight loss. If approved, the drug would be administered though a TOUCH-like
program in the US, would probably not be recommended as a first line treatment,
and would probably come with strong safety warnings.
We believe the potential market for Tysabri is smaller for Crohn’s disease than it is
for MS. Although approximately 500,000 people in the US have been diagnosed with
Crohn’s disease8, there is a wide variety of treatments (anti-inflammatories such as
steroids, immuno-suppressants, and antibiotics) that would probably be tried before
Tysabri. A further difficulty in the Crohn’s market is that many of the patients have
been on immuno-suppressants for extended periods, perhaps putting them at greater
risk for PML. However, the fact that approximately 40% of Crohn’s patients do not
respond to anti-TNF treatments and the difficult side effects of steroids make Tysabri
potentially viable as a second line treatment.9 Because the Crohn’s indication has not
yet been approved and approval is not assured, our model does not include revenue
from Crohn’s. Elan is also studying the use of Tysabri in the treatment of ulcerative
colitis and RA.
7 National Multiple Sclerosis Society website.
8 Mayo Clinic website on Crohn's disease.
9 Elan fourth quarter conference call.
Habe leider den Orginallink nicht gefunden, aber der zum IV sollte erstmal genügen.
Die erwarten übrigens auch, daß CD nicht so der Hammer wird ...
http://www.investorvillage.com/smbd.asp?mb=160&mn=99883&pt=m…
Goldman Initiates Bond Coverage....text pasted - apparently occurred March 7 - only rec'd last week
Elan has reached an important milestone, the re-launch of Tysabri, and we expect the
trajectory of the company’s bonds to match the trajectory of the drug. If, as we expect,
the adoption of Tysabri grows to the EBITDA breakeven level of 15,000 patients by
mid-2007, we look for the value of Elan’s bonds to appreciate meaningfully. With
Elan’s negative EBITDA and its previous market withdrawal of the franchise product, we
acknowledge the risk, and bad memories, inherent in the ELN bonds. Nonetheless,
multiple sclerosis patients remain underserved by existing therapies and the superior
efficacy of Tysabri as a second line treatment for MS is not in dispute. For Elan to reach
positive EBITDA it must garner a relatively small 3% of the MS population. In addition,
the risk in the re-launch is mitigated by the company’s large cash balance: The year-end
2006 cash balance of $898 million pro forma for the redemption of the Athena notes is
large enough to cover almost five years of interest and capex, assuming the rest of the
business is EBITDA neutral. We view the incremental risk from extending maturity to
2013 from 2011 as small relative to the near-term risks, so we recommend buying the
longer-dated bonds to pick up yield.
Upcoming catalysts. We expect trading in ELN bonds to be heavily influenced by news
flow in 2007:
• Late April: Biogen (BIIB) announces 1Q2007 results and should provide an update on
Tysabri dosing in the US and EU.
• Late April/early May: Elan reports 1Q2007 results. In addition to Tysabri results, we
expect to see the ex-Tysabri business remain EBITDA positive.
• April 28-May 5: American Academy of Neurology Annual Meeting. ELN will review
performance and safety data of Tysabri (i.e., reporting any incidences of PML).
• May 19-24: Digestive Disease Week 2007. Expect incremental data on the use of
Tysabri for treating Crohn’s disease.
• 1H2007: Potential approval of Tysabri for treatment of Crohn’s in EU.
• Midsummer: Could advance Alzheimer’s drug AAB-001 into Phase III trials.
• Widely recognized superior efficacy of Tysabri in an underserved patient
population. The existing MS market is fragmented among four drugs, with each
posting over $1.1 billion in sales and the aggregate sales of the four drugs
growing at 7.8% in 2006. Based on trial data, Tysabri yielded a 67% reduction in
relapses while the other four drugs are believed to yield approximately a onethird
reduction in relapses. In addition, Tysabri creates fewer common side
effects such as flu-like symptoms and tiredness. The three past Tysabri-related
incidences of PML, a very rare brain infection, occurred in patients in dual
therapy with Avonex and a patient recently treated with immuno-suppressants.
While it remains to be seen if Tysabri in mono-therapy causes PML, our
unscientific survey of MS blogs indicates that the MS community is supportive of
the risk/benefit trade-off of the drug. This leads us to believe that the
reintroduction of Tysabri may result in an increase in the total number of
patients being treated as well as steal share from the competing therapies.
Exhibit 1: Existing $5.5 billion MS market is fragmented
(2006 sales data in billions)
Betaseron
(Schering/Berlex)
$1.1, 20%
Copaxone
(Teva/Aventis)
$1.4, 25% Avonex (Biogen)
$1.7, 31%
Rebif
(Serono/Pfizer)
$1.3, 24%
Source: BIIB company filings
• Tysabri needs only 3% market penetration to be EBITDA breakeven. Because of
the high gross margin of biologics, Elan’s EBITDA and cash flow are highly
leveraged to Tysabri sales. The Tysabri business achieves positive EBITDA with
approximately 15,000 patients, which is 3% of the approximately 490,000 MS
patients worldwide (including 350,000 currently on other treatments and 100,000
who have stopped treatments and 40,000 who have not yet been on any
treatment)1. We estimate that 15,000 patients equate to annual sales (meaning both
Elan and Biogen) of $405 million and gross margin of $312 million. Elan’s 50%
share of that gross margin approximates its 50% share of sales and R&D expense.
Each additional 15,000 patients adds approximately $156 million of cash flow to
ELN. See Exhibit 14 for details of these estimates.
• No near-term maturities. Elan’s tender for the Athena notes in January 2007
extended the nearest maturity to 2011. This provides the company with almost a
four years to build a market for Tysabri and possibly launch an Alzheimer’s
treatment. Also, with the recent forced conversion of the $254 million converts,
the company has shown its willingness to issue equity for the benefit of the
balance sheet.
Exhibit 2: Elan has no debt maturities until 2011
Debt maturity profile in $ millions
$0
$200
$400
$600
$800
$1,000
$1,200
$1,400
2007 2008 2009 2010 2011 2012 2013
Source: Company filings
• Large cash balance. As of December 31, 2006, Elan had $898 million of cash pro
forma for the redemption of the $613 million Athena notes, which occurred in
January 2007. Between now and early 2009 when we expect free cash flow to
become positive, the cash balance does not drop below $660 million, according to
our model. See the credit risk section for a sensitivity analysis of Tysabri
adoption.
Elan Corporation PLC Healthcare
4 Goldman Sachs Credit Research
Exhibit 3: Cash balance provides a large cushion
($ in millions, spike due to timing difference in refinancing of Athena debt)
$0
$200
$400
$600
$800
$1,000
$1,200
$1,400
$1,600
1
Q05
3Q05
1Q
06
3Q06
1Q
07
3Q07
1Q08
3Q
08
1Q09
3Q09
Tysabri re-launch
Tysabri reaches
15,000 patients
Tysabri reaches
30,000 patients
Source: Company filings and Goldman Sachs Credit Research estimates
Credit risks
• Dependence on one drug with an unknown safety profile. While Elan does
have other businesses, its bonds are likely to underperform if Tysabri
underperforms. Despite the expected growth in the NanoCrystal business and
lack of generic competition so far in the company’s other marketed products, we
believe Elan would remain cash flow negative if less than 30,000 patients were to
use Tysabri. Risks to broad adoption of Tysabri include incidences of PML in
mono-therapy and commercialization of a new oral MS therapy. Previous cases
of PML occurred in two patients on dual therapy with Avonex and one patient
whose immune system was compromised. The TOUCH prescribing program is
designed to screen patients in order to reduce the risk of PML. On the
competitive front, Serono’s Cladribine is an oral therapy that is just beginning
Phase III trials, meaning it is unlikely to come to market before early 2010,
assuming it is successful. Elan management points to Sanofi-Aventis’s
Teriflunomide and Novartis’s FTY720 as the therapies under development that
are the most likely to succeed.
Exhibit 4: Several Competing MS therapies are under development
Compound Manufacturer Trial Phase Dosing Notes
Cladribine Serono Enrolling patients for a two-year
Phase III trial
Oral dosing
Teriflunomide Sanofi-Aventis Recruiting patients for Phase III trial Oral dosing
FTY 720 Novartis Recruiting patients for a 24-month
Phase III trial
Daily oral dosing
Laquinimod Active Biotech Additional Phase II studies ongoing Oral dosing
MBP 8298 BIOMS Being developed in three late-stage
trials (Phase II/Phase III)
Intravenous
Source: Company websites
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 5
• We estimate Elan will be free cash flow negative until early 2009. We are
projecting a 2007 cash burn of $179 million and a 2008 cash burn of $43 million
before the company turns free cash flow positive in early 2009. Estimates of cash
flow and leverage are highly sensitive to estimates of Tysabri use. Our base case
assumes maximum weekly adds of Tysabri patients of 350, with a steady-state
rate in 2009 and beyond of 225 per week. This results in 2009 leverage of 6.3x. We
have also tested downside cases with maximum weekly adds of 275 and 200
patients; in these cases, leverage in 2009 is 10.1x and 25.6x, respectively. See
Exhibit 5 for a summary of the sensitivity analysis. Note that the downside cases
do not assume any reduction in sales force expense.
Exhibit 5: Elan cash flow is sensitive to Tysabri adoption
$ in millions
Base Case A Little Worse Much Worse
Maximum weekly adoption rate 350 275 200
Steady-state weekly adoption rate 225 150 75
Tysabri WW 2009 revenue $1,239 $967 $695
ELN 2009 EBITDA $281 $175 $69
Debt/2009 EBITDA 6.3x 10.1x 25.6x
Min cash balance through YE2011* $660 $563 $384
* Assumes refinancing of 2011 maturity bonds
Source: Goldman Sachs Credit Research estimates
• Failure of AAB-001 to move to Phase III trial in 2007. While near-term cash
flows depend on Tysabri, the company’s Alzheimer’s pipeline is a key source of
Elan’s value. AAB-001 is scheduled to complete its Phase II trial by the end of
2007, and Elan has said the move to Phase III could be accelerated if the data at a
mid-2007 interim look are compelling. The AAB-001 trial is being run by an
independent clinical research firm, so Elan and co-developer Wyeth do not have
access to interim look data. Note that after the year-end 2006 interim look there
was also a possibility that the trial would be advanced to Phase III; however, that
did not happen, indicating the data were not “excellent.” We believe
Alzheimer’s, in conjunction with the NanoCrystal business, represents an
important path for Elan to diversify its revenues to reduce risk.
Potential catalyst
• Quarterly updates on Tysabri dosing. We are expecting patient adds to
accelerate somewhat from the 300 per week reported in February as
reimbursement is solidified in the individual European countries.
• American Academy of Neurology Annual Meeting. The company intends to
present clinical and safety data at research conferences. We also view the
conferences as an opportunity for the company to continue to educate doctors
about the superior features of Tysabri relative to other MS treatments. We expect
competing drug companies to announce trial data during the conferences.
Elan Corporation PLC Healthcare
6 Goldman Sachs Credit Research
Digestive Disease Week on May 19-24 will present a similar opportunity
regarding the treatment of Crohn’s.
• Potential approval of Tysabri for treatment of Crohn’s in the EU. A response,
whether positive or negative, is expected in the first half of 2007. While Tysabri
has shown a lasting positive therapeutic effect in Crohn’s patients, approval is far
from certain. One of the three Tysabri-related cases of PML was in a Crohn’s
patient who had previously been treated with immuno-supressants. Biogen CEO
James Mullen told the Boston Globe that he believes European approval is not
likely without additional data; he noted that Tysabri may not be well-suited to
Crohn’s patients because that patient population has been through several
rounds of immunosuppressing drugs and is therefore already at risk for PML.
An application has also been filed with the FDA.
• Alzheimer’s drug AAB-001 moves into Phase III trials. There will be an interim
look in mid-2007. If the data are compelling, the study will move to Phase III
immediately; otherwise, Phase II work will be completed by the end of 2007,
with a decision on continuing to Phase III to follow.
Relative value
Comparables. Assessing Elan’s relative value is difficult because of the unique
business risks at the company and the huge growth potential in EBITDA. We are
comparing Elan with a group of similarly rated hospitals companies. Business risk at
those hospitals is manageable, with the key concerns being persistently weak
volumes and declining operating margins. The hospitals also have relatively high
capex needs, which significantly cut into cash flow, particularly for the highly
levered companies such as Tenet. By contrast, the risk at Elan is more focused, as it
hinges on the success of one product. Although Elan has minimal capex needs, we
are projecting free cash flow to be negative until early 2009.
Leverage. We are projecting only $20 million of EBITDA in 2007 for Elan because
Tysabri is just beginning to ramp up; therefore, we are using debt/enterprise value
as our key leverage metric. We believe this is a useful ratio because Elan’s enterprise
value is a better measure of the company’s value than 2007 EBITDA since it
incorporates the market outlook for Tysabri and the Alzheimer’s pipeline. Given the
company’s large cash balance, we think it is acceptable to use this future value
measure instead of next year’s EBITDA because liquidity is not an issue. Elan’s debt
to enterprise value is 26%, which compares favorably with a debt-to-EV of 39% and
66% for Community Health and Tenet, respectively. In addition, we consider debt to
2009E EBITDA. We are projecting a debt to 2009E EBITDA figure of 6.3x for Elan,
which compares with 5.6x for HCA and 5.0x for THC.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 7
Exhibit 6: Relative value comparison with similarly rated hospitals
Estimated 2007
ELN CYH HCA IAS THC
Financials
EBITDA $20 $688 $4,402 $253 $700
Cash $704 $80 $729 $108 $509
Total Debt 1,765 2,192 28,216 1,060 4,879
Net Debt $1,061 $2,112 $27,486 $952 $4,370
CFO ($149) $361 $2,052 $153 $375
Capex 31 325 1,800 200 750
FCF ($179) $36 $252 ($47) ($375)
Enterprise Value $6,717 $5,563 NA NA $7,368
Ratios
Debt/EBITDA 89.1x 3.2x 6.4x 4.2x 7.0x
Debt/EV 26% 39% NA NA 66%
Debt/2009 EBITDA 6.3x 2.6x 5.6x 2.9x 5.0x
Benchmark Bond Pricing
Bond 8.875% of 2013 6.5% of 2012 6.5% of 2016 8.75% of 2014 9.25% of 2015
Priority Sr. Notes Sr. Sub. Sr. Notes Sr. Sub. Sr. Notes
Rating (Moody's/S&P) B3/B B3/B Caa1/B- B3/B- Caa1/CCC+
Price $100.88 $99.25 $84.30 $102.75 $99.00
Yield to Worst 8.67% 6.66% 9.10% 8.09% 9.43%
Spread to Worst 420 218 460 426 496
Source: Goldman Sachs Credit Research and company reports
Total return. Because the risks are so different at Elan, we believe it needs to be
considered on an expected total return basis, not just on a relative value basis. In our
view, none of the bonds in the comparable group have a potential for capital
appreciation similar to that of the Elan bonds. Exhibit 7 shows the sensitivity of the
price of the 8.875% senior notes of 2013 to assumed spread levels. We use it to
illustrate the potential for capital appreciation at various yields. As neurologists
weigh the risks of Tysabri and the adoption of the drug progresses, some business
risk is reduced. There will always be the possibility of additional PML cases, so we
are not suggesting the bonds should trade all the way to pre-PML levels; however,
we do believe there is at least 100 bp of spread upside if Tysabri reaches 15,000
patients by mid-2007.
Exhibit 7: A return to near pre-PML spreads creates a large jump in bond price
8.875% senior notes of 2013
Spread Price Notes
700 $88 Average level in month after PML first reported in Feb 2005
442 $100 Par
419 $101 Current level
350 $104
300 $107
250 $109 Average level in month prior to initial FDA approval in Nov 2004
175 $112 Tightest spread prior to PML report
Source: Goldman Sachs Credit Research
Elan Corporation PLC Healthcare
8 Goldman Sachs Credit Research
Company description
Biotech, with history. Elan is a well-established biotech company with a history in
drug development going back to 1969. The company operates in two business units:
Biopharmaceuticals and Elan Drug Technology (EDT). Biopharmaceutical focuses on
three areas: (1) autoimmune diseases such as multiple sclerosis; (2)
neurodegenerative disease, with a specialty in Alzheimer’s; (3) specialty
pharmaceuticals for severe pain and infectious diseases. EDT is a contract
manufacturer and licenses drug delivery technologies. The company maintains R&D,
manufacturing, and marketing facilities in the United States and Europe, and
employs roughly 1,700 people worldwide. The Ireland-based public company has
been listed on the NYSE since 1984.
Elan grew through acquisitions in the 1990s and 2000; however, the company then
suffered a number of setbacks – both corporate and clinical – which led to large
divestitures. In January 2002, Elan became the subject of an investigation by the SEC
sparked by a Wall Street Journal report that questioned the company’s accounting
practices. Subsequently, Elan became the target of a shareholder lawsuit regarding
false and misleading statements. In 1Q2002, clinical trials of an Alzheimer’s drug
were abruptly halted after 5% of patients suffered complications. These events led to
multiple credit rating downgrades by Moody’s and S&P, and the forced resignation
of top-level management. In July 2002, Elan’s current CEO, Kelly Martin, announced
plans to sell products worth $1.5 billion and drastically decrease the workforce.
While Elan does have some history, all related investigations and lawsuits have been
resolved and management has proved itself willing to take drastic action quickly to
right the company.
Exhibit 8: Elan’s share price still trades significantly off of its 2001 highs
(January 1, 2000 – February 27, 2007)
0
10
20
30
40
50
60
70
Jan-00 Jan-01 Jan-02 Jan-03 Jan-04 Jan-05 Jan-06 Jan-07
Stock price (US$)
Source: Bloomberg
Capitalization. In November 2006, Elan issued $615 million of 2013 bonds in two
tranches: a $465 million 8.875% tranche and a $150 million floating rate L+412.5
tranche. The proceeds were used to call the old Athena 7.25% senior notes of 2008. As
part of the same broad refinancing, the company issued a redemption notice for its
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 9
$254 million of 6.5% convertible notes of 2008, thereby forcing conversion at a share
price of $7.42. The transaction reduced debt by $254 million and extended the nearest
maturity from 2008 to 2011. The new bonds, as well as the existing 2011 bonds, were
issued by Elan Corporation and co-issuer Elan Finance. All bonds are guaranteed by
Elan and subsidiaries.
Exhibit 9: Elan Corporation – capitalization and ratios
($, millions)
Description Amount % of EV
No bank debt $0
Total Sr Sec debt 0 0.0%
7.75% Sr. Notes due 11/15/2011 $850
L+400 Sr. Notes due 11/15/2011 300
8.875% Sr. Notes due 2013 465
L+412.5 Sr. Notes due 2013 150
Total Sr debt 1,765 26.2%
Total Sub debt 0 26.2%
Other $0
Total Debt 1,765 26.2%
Market Cap 5,813
Enterprise Value 6,736 100.0%
Source: Goldman Sachs Credit Research and company reports
Marketed products. In addition to Tysabri, Elan currently markets three products:
Maxipime, Azactam, and Prialt (US only). Maxipime and Azactam are used to fight
hospital-born infection and Prialt is used to treat acute pain. All three are linked
because their use is hospital-based, so a single sales force can effectively market the
drugs. Azactam’s patent expired in October 2005 but to date no competitors have
filed for approval to produce a generic version. With 2006 sales of $78 million,
Azactam may be too small to warrant the interest of many generic manufacturers.
Maxipime is protected by several patents, with the basic patent expiring in March
2007 and a formulation patent coming off in February 2008. Elan sold the European
rights to Prialt in March 2006 for $50 million, plus $10 million to be received within
two years and $40 million of contingent payments. Our model assumes generic
competition enters the market as shown in Exhibit 10.
Elan Corporation PLC Healthcare
10 Goldman Sachs Credit Research
Exhibit 10: Tysabri revenue should more than offset other lost revenue
(Sales in $, millions)
0
50
100
150
200
1Q06 2Q06 3Q06 4Q06 1Q07E 2Q07E 3Q07E 4Q07E 1Q08E 2Q08E 3Q08E 4Q08E
Tysabri Maxipime Azactam Prialt Maxipime
generic
Supply
shortage
Azactam
generic
Source: Company filings and Goldman Sachs Credit Research
EDT. Elan Drug Technology (EDT) consists of a drug delivery technology licensing
business, headlined by NanoCrystal, and a contract manufacturing business. EDT
contributed $235 million of revenue in 2006. NanoCrystal technology is licensed to
other drug manufacturers in exchange for royalty payments in the mid- to high
single digits. The technology allows the slow release of crystalline drugs by reducing
the particle size to under 400nm. NanoCrystal patents expire in 2011 in the US and
2012 outside the US. A summary of the current portfolio of NanoCrystal compounds
is shown in Exhibit 11. The largest royalty-generating drug, Tricor from Abbott,
contributed $52 million in revenue in 2006, or 9% of Elan’s total.
Exhibit 11: Several products currently license NanoCrystal
Compound Manufacturer Launch date
2005 YTD sales
($ in millions)
2006 royalties
($ in millions)
TriCor Abbott/Fournier December 2004 $1,048 $52.1
Emend Merck April 2003 $131 NA
Rapamune Wyeth August 2000 $337 NA
Megace ES Par July 2005 $32* NA
BiDil NitroMed Announced Feb 2007 NA NA
Source: Company web sites and filings
* 1Q2006 sales annualized. More recent data not yet available.
While limited information on the NanoCrystal pipeline has been disclosed, we
believe NanoCrystal revenue should grow in the coming quarters. Exhibit 12 shows
development projects that are under way. A reformulation of Johnson & Johnson’s
paliperidone represents a large opportunity owing to the size of the schizophrenia
market. In addition, Elan signed an agreement with Abbott to develop a combination
of TriCor and Crestor that would use NanoCrystal technology. Recently, Elan has
started taking on a larger role in the development of new applications in exchange
for higher royalty rates. This tact is not without risk, however, as it requires Elan to
make a larger up-front investment.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 11
Exhibit 12: Several products are under development to license NanoCrystal
Compound Manufacturer Indication Status Projected launch
Paliperidone palmitate J&J Schizophrenia Phase III 2008
Budesonide MAP Asthma Phase III 2009
Fencrestor Abbott Cholesterol Phase III 2010
Source: Company web sites and filings
Pipeline. Elan’s drug development pipeline is dominated by Alzheimer’s studies,
with six different compounds along three different pathways in development. The
Alzheimer’s market is large, as an estimated 4.5 million people suffer from
Alzheimer’s in the US and 5 million in the EU. However, the market is also very
competitive, with basically all the major pharmaceutical companies working in the
space. Two of Elan’s studies are in conjunction with partners, Wyeth on the
Immunotherapy pathway and Transition Therapeutics on the A-beta Aggregation
Inhibitor pathway. While we do see the value of the pipeline as a buffer for the
minimum value of Elan’s bonds, we note that the development agreement with
Wyeth includes a change of control provision whereby Wyeth has the right of first
refusal to buy out Elan at a price determined by a formula.
The most advanced of Elan’s Alzheimer’s studies is AAB-001, which is in Phase II
trials. AAB-001, which is being developed in collaboration with Wyeth, has been
granted fast track status by the FDA because there is currently no breakthrough
treatment for Alzheimer’s disease. The drug is scheduled to complete Phase II by the
end of 2007, and Elan has said the move to Phase III could be accelerated if the data
at a mid-2007 interim look are compelling. The AAB-001 trial is being run by an
independent clinical research firm, so Elan does not have access to interim look data.
Note that after the year-end 2006 interim look, it was also possible that the trial
would be advanced to Phase III; however, that did not happen, indicating the data
were not “excellent.” Only Phase I data have been released; and while results do
show improvement in memory over the placebo, the p values are very high,
indicating there is a relatively high probability that the difference is due to chance.
Elan’s key objectives for 2007 include moving two other Alzheimer’s compounds into
Phase II.
Tysabri
The MS population. There are approximately 350,000 MS patients in the US and
360,000 in Europe. Of these, approximately 220,000 are patients diagnosed in the past
who have not started treatment (most likely because the diagnosis is not definitive or
the symptoms are mild). Therefore, we believe the market for Tysabri is
approximately 490,000 patients who are currently in treatment, have stopped
treatment, or have not yet tried treatment. According to the National MS Society,
roughly 200 people are diagnosed with the disease every week. The majority of those
diagnosed with MS are women between 20 and 50 years old. The disease is more
common among people of northern European descent.
MS is a progressive neurological disease in which the body loses the ability to
transmit messages along nerve cells, leading to a loss of muscle control, and in some
cases paralysis. Nerve fibers of the central nervous system are protected by a fatty
substance known as myelin. It is believed that MS is an autoimmune disease wherein
Elan Corporation PLC Healthcare
12 Goldman Sachs Credit Research
the body attacks its own myelin sheath surrounding the nerve fibers, creating scars
(or sclerosis) and oftentimes damaging the nerve fibers as well. Messages that travel
through the damaged nerve fibers to the brain then become distorted and
misinterpreted.
The difficulty of diagnosing MS is that individuals with the disease show different
symptoms. More common symptoms include tingling, numbness, slurred speech,
and vision problems. Other symptoms are muscle weakness, loss of balance and
coordination, tremors, and even paralysis, which may be temporary or permanent.
The National MS Society reports that one-third of MS suffers are wheelchair-bound.
Symptoms also vary over time, and usually come and go. For this reason, it can
sometimes take several months and even years to diagnose definitively.
Available treatments. There is no known cure for MS. There are, however, four
treatments in common use that can reduce the frequency of relapses: Avonex, Rebif,
Betaseron, and Copaxone. Among the four, Avonex, which was introduced by
Biogen in 1996, has the greatest market share. It is administered once weekly by
injection into muscle. In contrast, three to seven doses of Rebif, Betaseron, or
Copaxone are required per week, but they are administered through less-painful
injections just under the skin. Owing to the frequent and uncomfortable dosing and
the difficult side effects such as flu-like symptoms and severe fatigue, patient
compliance with the drug regiments is a challenge. Further, some patients develop
antibodies to the beta interferons (Avonex, Rebif, and Betaseron), making the drugs
less effective over time2. Maintaining a “normal” life between relapses is a big issue
for MS patients, and Elan believes that dosing by monthly infusion, as Tysabri is
administered, may be preferred over weekly or more frequent self-administered
injections.
History of Tysabri: Tysabri was introduced to the MS market in November 2004
after much anticipation. Three months later, in February 2005, Elan and Biogen
pulled the drug and stopped clinical trials. Two cases of PML, one of which resulted
in death, were discovered in the clinical trial of dual therapy with Avonex. In March,
it was discovered that a third patient (who died in December 2003) had contracted
PML after eight doses of Tysabri. This patient was in a Crohn’s study and had been
treated with immuno-suppressants. Elan and Biogen completed a safety review,
which uncovered no additional cases of PML, and then submitted a supplemental
Biologics License Application for re-approval with the FDA in September 2005.
The FDA re-approved the drug in June 2006 following a review process that included
emotional patient testimonies on the drug’s powerful effects, and Tysabri was
reintroduced to the US market the following month. The drug was also approved for
commercial distribution in July 2006 in the European Union. In the US, patients on
Tysabri must enroll in the TOUCH (Tysabri Outreach: Unified Commitment to
Health) program. TOUCH includes screening patients for factors that could
contribute to a weakened immune system (such as AIDS or a recent course of
immuno-suppressant therapy) and taking a baseline MRI in order to facilitate any
diagnoses of PML.
Tysabri efficacy. During the drug’s two-year plus clinical study, Tysabri was shown
to reduce the frequency of relapses significantly. In addition, Tysabri has been shown
2 Mayo Clinic web site on MS.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 13
to reduce the risk of disability progression by 42%.3 This contrasts with the other
treatments, which can reduce the frequency of flare-ups but have not been
definitively shown to alter the long-term development of permanent disability.4
There has not been a head-to-head study of the efficacy of Tysabri compared with
other treatments (the SENTINEL trial evaluated Tysabri in dual therapy with Avonex
compared with Avonex alone, and the AFFIRM trial compared Tysabri with a
placebo). Nonetheless, Tysabri is believed to be more effective. For example,
Copaxone reduced the relapse rate by about one-third while Tysabri mono-therapy
reduced the relapse rate by 67%5.
The National MS Society has endorsed Tysabri, noting the following: “We feel that
it’s likely that Tysabri is a very strong efficacious drug for people with MS.” The
Society says that the PML cases were “not a disaster” for MS treatment, and that MS
patients and doctors must learn to evaluate risks and benefits of treatments much as
RA and lupus doctors have. While the Society’s Web site provides information on
Tysabri, the Society’s Disease Management Consensus Statement has not been
updated since the re-approval of Tysabri. The FDA recommended Tysabri for
patients who have had inadequate response to or difficulty tolerating the side effects
of other MS therapies, and the MS Society echoes that recommendation.
A cursory survey of blog comments posted by MS patients regarding Tysabri shows
that most patients who have been infused report a very positive result. Comments
about “getting my life back” are common. A few indicated that they had expected a
more dramatic impact but that they were pleased so far. On the negative side, a
handful reported that they were considering taking the drug but that they were
afraid of the unknown, presumably PML, with one patient saying his neurologist
said “no way” to Tysabri.
Patents. While there is a move afoot in the US to create a pathway for generic
biologics, we do not view this as a risk to Tysabri. First, the drug is protected by
several patents both in the US and outside, covering the protein, DNA encoding the
protein, manufacturing methods, and pharmaceutical compositions. In the US, the
principal patents expire between 2014 and 2020, and outside the US they expire
between 2014 and 2016. Second, biologics are difficult to manufacture; so even if
bioequivalence can be shown, the would-be generic manufacturer would still need to
develop commercial manufacturing capabilities.
Adoption rate. The Elan financial model is very sensitive to the assumptions made
about Tysabri adoption. We believe that the adoption rate will accelerate from the
300 per week reported for February, as US insurance companies and European
national health systems determine reimbursement. So far, US private insurers have
been providing good access to the drug, with 40% of patients having no prior
treatment requirements and 55% having a one prior treatment requirement. In
Europe, the launch has not been rolled out to approximately half of the countries and
reimbursement is not yet in place for several countries, including UK, Finland,
Norway, and Austria.
3 The New England Journal of Medicine 2006; 354:899-910.
4 Mayo Clinic website on MS.
5 National Multiple Sclerosis Society video, “Tysabri: What you need to know”
Elan Corporation PLC Healthcare
14 Goldman Sachs Credit Research
Exhibit 13: Adoption rate estimates for Tysabri
0
50
100
150
200
250
300
350
400
4Q06 1Q07 2Q07 3Q07 4Q07 1Q08 2Q08 3Q08 4Q08 1Q09 2Q09 3Q09 4Q09
Weekly Adds to Tysabri
0
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
Average Tysabri Patients
Average patients (rt axis) Weekly adds (lt axis)
200 Diagnoses per week
Source: Company filings and Goldman Sachs Credit Research estimates
PML. Progressive Multifocal Leukoencephalopathy, or PML, is a very rare but often
fatal brain disease caused by the JC virus. It occurs in people with extremely weak
immune systems, owing to either AIDS or drug therapies. The Tysabri patients that
developed PML were in trials where patients had taken an average of 17.9 doses of
Tysabri. A total of 3,417 patients participated in the MS, Crohn’s, and RA trials and
three were diagnosed with PML. None of the three Tysabri-related PML cases were
in patients from the AFFIRM trial, which tested mono-therapy Tysabri for over two
years against placebo.6 If PML is detected early, it can sometimes be stabilized.
A few drugs are thought to be linked to PML and in certain disease categories the
benefits of treatment weigh favorably against the risk. The drugs that have been
linked to PML are Rituxan (cancer and severe rheumatoid arthritis (RA) drug from
Genentech and Biogen), Enbrel (RA and Psoriatic Arthritis drug from Amgen and
Wyeth), Humira (Abbott drug approved for RA, Psoriatic Arthritis, and, recently,
Crohn’s), Imuran (immunosuppressant used to prevent rejection of kidney
transplants), and Tysabri.
Accounting. Tysabri is marketed through a 50/50 collaboration with Biogen; the
product operating profit is effectively split 50/50 between the two companies,
however the accounting is a bit convoluted (an illustrative example is shown in
Exhibit 14). In the US, Elan purchases the drug from Biogen and is responsible for
distribution. Therefore, Elan books revenue equal to 100% of US sales and then books
COG at a price equal to the cost of manufacturing plus Biogen’s gross margin and
any third party royalties. Elan’s income statement also shows 50% of the combined
US sales and marketing expense. In the EU, Biogen is responsible for distribution, so
Elan books revenue that is equal to its share of EU operating profits plus any directly
incurred costs. Therefore, Elan’s EU revenue can be negative if operating profit in the
region is negative. Biogen is responsible for the manufacture of Tysabri for both
markets. The wholesale price of Tysabri has been set at $2,185 per vial, or $28,400 per
6 New England Journal of Medicine 2006.
Healthcare Elan Corporation PLC
Goldman Sachs Credit Research 15
year for 13 doses.7 We have assumed a 7% discount in this price.
Exhibit 14: Illustrative accounting for Tysabri
Assuming nominal D&A, EBITDA breakeven with 15,000 patients
Average patients dosing 15,000
Price per year, net of discounts $26,980
Percentage of patients in US 65%
Assumed gross margin % 77%
Reality ($ in millions) Accounting for ELN ($ in millions)
In the US
Total US sales $263.1 Revenue $263.1
COGS $60.5 COGS (Mfg cost plus BIIB's share of GM) $161.8
Gross margin $202.6 Gross margin $101.3
SG&A $175.0 50% share of SG&A $87.5
Operating profit bf R&D $27.6 Operating profit bf R&D $13.8
In the EU
Total EU sales $141.6 Revenue (ELN's share of Op Profit + direct costs) $23.0
COGS $32.6 COGS $6.0
Gross margin $109.1 Gross margin $17.0
SG&A $75.0 SG&A $0.0
Operating profit bf R&D $34.1 Operating profit bf R&D $17.0
Direct ELN costs $6.0
Total
Worldwide R&D $65.0 50% share of R&D $32.5
Worldwide operating profit -$3.4
ELN share of operating profit -$1.7 Total operating profit -$1.7
Source: Company filings and Goldman Sachs Credit Research estimates
Crohn’s and other indications. Elan has filed applications in the US and Europe to
market Tysabri as a treatment for Crohn’s disease. Crohn’s disease is a chronic
inflammation of the gastrointestinal tract. It is most commonly diagnosed in 15- to
35-year-olds and is more prevalent among people of European Jewish ancestry.
Symptoms include abdominal pain, nausea and vomiting, severe diarrhea, and
weight loss. If approved, the drug would be administered though a TOUCH-like
program in the US, would probably not be recommended as a first line treatment,
and would probably come with strong safety warnings.
We believe the potential market for Tysabri is smaller for Crohn’s disease than it is
for MS. Although approximately 500,000 people in the US have been diagnosed with
Crohn’s disease8, there is a wide variety of treatments (anti-inflammatories such as
steroids, immuno-suppressants, and antibiotics) that would probably be tried before
Tysabri. A further difficulty in the Crohn’s market is that many of the patients have
been on immuno-suppressants for extended periods, perhaps putting them at greater
risk for PML. However, the fact that approximately 40% of Crohn’s patients do not
respond to anti-TNF treatments and the difficult side effects of steroids make Tysabri
potentially viable as a second line treatment.9 Because the Crohn’s indication has not
yet been approved and approval is not assured, our model does not include revenue
from Crohn’s. Elan is also studying the use of Tysabri in the treatment of ulcerative
colitis and RA.
7 National Multiple Sclerosis Society website.
8 Mayo Clinic website on Crohn's disease.
9 Elan fourth quarter conference call.
Press Release Source: Biogen Idec and Elan Corporation, plc
Data Presented at the American Academy of Neurology's Annual Meeting Provide Update on Utilization and Safety of TYSABRI(R) in Patients with Multiple Sclerosis
Thursday May 3, 7:00 am ET
Additional Data From Extension Study Presented Show TYSABRI Benefit is Sustained Over Three Years
BOSTON--(BUSINESS WIRE)--Biogen Idec (NASDAQ: BIIB - News) and Elan Corporation, plc (NYSE: ELN - News) announced today that new data from the TOUCH Prescribing Program(TM) and TYGRIS safety study confirm the safety profile from previous clinical studies of TYSABRI® (natalizumab). Also presented at the 59th annual meeting of the American Academy of Neurology in Boston, MA were extension study data that showed that TYSABRI has a sustained treatment effect on clinical relapses and the risk of disability progression in multiple sclerosis (MS) patients treated for up to three years. The companies recently reported that as of mid-April 2007 approximately 12,500 patients have been prescribed TYSABRI worldwide. The companies estimate that in both commercial use and clinical trials, there are currently over 10,000 patients on TYSABRI therapy worldwide.
"The findings from the safety update combined with the data showing the sustained effect of TYSABRI in patients treated for up to three years, contribute to our evolving understanding of the utilization of this therapy as an important treatment option for people living with the debilitating effects of MS," said Paul O'Connor, MD, St. Michael's Hospital, Toronto, Ontario, Canada, lead investigator of the TYSABRI extension study.
TYSABRI Update
TYSABRI is available in the US through the TOUCH Prescribing Program. All prescribers, infusion sites and patients receiving TYSABRI are required to enroll in TOUCH. Safety information is also collected through ongoing clinical trials and registries, including STRATA, TYGRIS and the pregnancy registry. According to data available to the companies as of April 23, 2007, there have been no new reports of confirmed cases of progressive multifocal leukoencephalopathy (PML) or other serious opportunistic infections (OIs). The data confirm the safety profile from previous clinical studies of TYSABRI and will continue to expand the knowledge of the long-term safety and tolerability of TYSABRI.
The combination of TOUCH, TYGRIS and the pregnancy registry will be the largest long-term follow-up undertaken for an MS therapy, and the companies plan to continue to provide similar updates at upcoming medical meetings.
The companies recently announced that as of mid-April, approximately 12,500 patients have been prescribed TYSABRI worldwide. In both commercial use and clinical trials, there are currently over 10,000 patients on TYSABRI therapy worldwide.
In the US, approximately 6,600 patients are on TYSABRI therapy commercially. Approximately 10,000 patients have enrolled in the TOUCH program and 1,500 physicians have enrolled patients.
In the EU, approximately 2,500 patients internationally have received TYSABRI infusions commercially, mostly in Germany and the Nordic countries.
In clinical trial settings, over 1,000 patients are on TYSABRI therapy.
TYSABRI Efficacy Sustained through Three Years
Patients who participated in the Phase III TYSABRI program were eligible to enroll in an open-label extension study that evaluated the therapy's long-term effects. Included in this were patients from AFFIRM, a randomized, double-blind, placebo-controlled, two-year monotherapy study of TYSABRI that enrolled 942 patients (627 patients on TYSABRI, 315 on placebo). In AFFIRM, TYSABRI reduced the annualized relapse rate in patients with MS by 67% (p<0.001) and the risk of 12-week sustained disability progression by 42% (p<0.001) compared with placebo.
In the intent to treat analysis, the annualized relapse rate for patients treated with TYSABRI over the three-year period was 0.23, translating into an average of one relapse every 4.3 years. The relapse rate also continued to remain low over the three-year treatment period with TYSABRI: 0.27 during the first year; 0.20 during the second year; and 0.15 during the third year (based on 531 patients who entered the extension study, which includes approximately 250 patients with nearly three years of continuous therapy).
In addition, TYSABRI also decreased the cumulative probability of disability progression sustained for six months compared to placebo. The estimated proportion of patients who had 24-week sustained disability progression at two years was 11% in patients treated with TYSABRI compared to 23% in patients treated with placebo, a 54% relative reduction.
This effect was maintained in patients treated with TYSABRI for up to three years with 13% showing 24-week sustained disability progression.
About TOUCH and TYGRIS
Before initiating treatment, all US patients, prescribers and infusion sites must be enrolled in the TOUCH Prescribing Program (TYSABRI Outreach: Unified Commitment to Health). TOUCH is designed to determine the incidence of and risk factors for serious OIs, including PML, and to monitor patients for signs and symptoms of PML while promoting informed benefit/risk discussions prior to initiating TYSABRI treatment. Physicians report on PML, serious OIs, deaths and discontinuation of therapy on an ongoing basis.
TYGRIS (TYSABRI Global ObseRvation Program In Safety) is expected enroll 5,000 patients worldwide, including approximately 3,000 patients from TOUCH. Patients in TYGRIS are evaluated at baseline and every six months thereafter for five years. Researchers will evaluate data including medical/MS history; prior TYSABRI use; prior use of immunomodulatory, antineoplastic, or immunosuppressive agents; and all serious adverse events, including PML and other serious OIs, and malignancies.
The information provided here is derived from voluntary adverse event reporting. It is possible that not all reactions have been reported, or that some reactions are not reported to Biogen Idec or Elan in a timely manner.
About TYSABRI
In the US, TYSABRI is approved as a monotherapy treatment for relapsing forms of MS. TYSABRI increases the risk of PML, an opportunistic viral infection of the brain that usually leads to death or severe disability. Patients should be monitored at regular intervals for any new or worsening signs or symptoms suggestive of PML Because of the increased risk of PML, TYSABRI is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, alternate MS therapies. It is available in the US only through a restricted distribution program called the TOUCH Prescribing Program. According to product labeling, after two years, TYSABRI treatment led to a 67% relative reduction (p<0.001) in the annualized relapse rate compared to placebo and reduced the relative risk of disability progression by 42% (p<0.001). TYSABRI treatment also resulted in sustained and statistically significant reductions in brain lesion activity as measured by MRI. Changes in MRI findings often do not correlate with changes in the clinical status of patients (e.g., disability progression). The prognostic significance of the MRI findings in these studies has not been evaluated.
In the European Union, TYSABRI is indicated as a single disease-modifying therapy in highly active relapsing-remitting MS patients. Because of the increased risk of PML, it is for patients with high disease activity despite treatment with a beta-interferon or in patients with rapidly evolving severe relapsing-remitting MS.
According to product labeling in the EU, after two years, TYSABRI treatment led to a 68% relative reduction (p<0.001) in the annualized relapse rate compared to placebo and reduced the relative risk of disability progression by 42-54% (p<0.001).
Serious adverse events that occurred in TYSABRI-treated patients included hypersensitivity reactions (e.g., anaphylaxis), infections, depression and gallstones. In MS trials, the incidence and rate of other serious and common adverse events, including the overall incidence and rate of infections, were balanced between treatment groups. Herpes infections were slightly more common in patients treated with TYSABRI. Serious opportunistic and other atypical infections have been observed in TYSABRI-treated patients, some of whom were receiving concurrent immunosuppressants. Common adverse events reported in TYSABRI-treated patients include headache, fatigue, infusion reactions, urinary tract infections, joint and limb pain, lower respiratory infections, rash, gastroenteritis, abdominal discomfort, vaginitis, and diarrhea
http://biz.yahoo.com/bw/070503/20070503005129.html?.v=1
Data Presented at the American Academy of Neurology's Annual Meeting Provide Update on Utilization and Safety of TYSABRI(R) in Patients with Multiple Sclerosis
Thursday May 3, 7:00 am ET
Additional Data From Extension Study Presented Show TYSABRI Benefit is Sustained Over Three Years
BOSTON--(BUSINESS WIRE)--Biogen Idec (NASDAQ: BIIB - News) and Elan Corporation, plc (NYSE: ELN - News) announced today that new data from the TOUCH Prescribing Program(TM) and TYGRIS safety study confirm the safety profile from previous clinical studies of TYSABRI® (natalizumab). Also presented at the 59th annual meeting of the American Academy of Neurology in Boston, MA were extension study data that showed that TYSABRI has a sustained treatment effect on clinical relapses and the risk of disability progression in multiple sclerosis (MS) patients treated for up to three years. The companies recently reported that as of mid-April 2007 approximately 12,500 patients have been prescribed TYSABRI worldwide. The companies estimate that in both commercial use and clinical trials, there are currently over 10,000 patients on TYSABRI therapy worldwide.
"The findings from the safety update combined with the data showing the sustained effect of TYSABRI in patients treated for up to three years, contribute to our evolving understanding of the utilization of this therapy as an important treatment option for people living with the debilitating effects of MS," said Paul O'Connor, MD, St. Michael's Hospital, Toronto, Ontario, Canada, lead investigator of the TYSABRI extension study.
TYSABRI Update
TYSABRI is available in the US through the TOUCH Prescribing Program. All prescribers, infusion sites and patients receiving TYSABRI are required to enroll in TOUCH. Safety information is also collected through ongoing clinical trials and registries, including STRATA, TYGRIS and the pregnancy registry. According to data available to the companies as of April 23, 2007, there have been no new reports of confirmed cases of progressive multifocal leukoencephalopathy (PML) or other serious opportunistic infections (OIs). The data confirm the safety profile from previous clinical studies of TYSABRI and will continue to expand the knowledge of the long-term safety and tolerability of TYSABRI.
The combination of TOUCH, TYGRIS and the pregnancy registry will be the largest long-term follow-up undertaken for an MS therapy, and the companies plan to continue to provide similar updates at upcoming medical meetings.
The companies recently announced that as of mid-April, approximately 12,500 patients have been prescribed TYSABRI worldwide. In both commercial use and clinical trials, there are currently over 10,000 patients on TYSABRI therapy worldwide.
In the US, approximately 6,600 patients are on TYSABRI therapy commercially. Approximately 10,000 patients have enrolled in the TOUCH program and 1,500 physicians have enrolled patients.
In the EU, approximately 2,500 patients internationally have received TYSABRI infusions commercially, mostly in Germany and the Nordic countries.
In clinical trial settings, over 1,000 patients are on TYSABRI therapy.
TYSABRI Efficacy Sustained through Three Years
Patients who participated in the Phase III TYSABRI program were eligible to enroll in an open-label extension study that evaluated the therapy's long-term effects. Included in this were patients from AFFIRM, a randomized, double-blind, placebo-controlled, two-year monotherapy study of TYSABRI that enrolled 942 patients (627 patients on TYSABRI, 315 on placebo). In AFFIRM, TYSABRI reduced the annualized relapse rate in patients with MS by 67% (p<0.001) and the risk of 12-week sustained disability progression by 42% (p<0.001) compared with placebo.
In the intent to treat analysis, the annualized relapse rate for patients treated with TYSABRI over the three-year period was 0.23, translating into an average of one relapse every 4.3 years. The relapse rate also continued to remain low over the three-year treatment period with TYSABRI: 0.27 during the first year; 0.20 during the second year; and 0.15 during the third year (based on 531 patients who entered the extension study, which includes approximately 250 patients with nearly three years of continuous therapy).
In addition, TYSABRI also decreased the cumulative probability of disability progression sustained for six months compared to placebo. The estimated proportion of patients who had 24-week sustained disability progression at two years was 11% in patients treated with TYSABRI compared to 23% in patients treated with placebo, a 54% relative reduction.
This effect was maintained in patients treated with TYSABRI for up to three years with 13% showing 24-week sustained disability progression.
About TOUCH and TYGRIS
Before initiating treatment, all US patients, prescribers and infusion sites must be enrolled in the TOUCH Prescribing Program (TYSABRI Outreach: Unified Commitment to Health). TOUCH is designed to determine the incidence of and risk factors for serious OIs, including PML, and to monitor patients for signs and symptoms of PML while promoting informed benefit/risk discussions prior to initiating TYSABRI treatment. Physicians report on PML, serious OIs, deaths and discontinuation of therapy on an ongoing basis.
TYGRIS (TYSABRI Global ObseRvation Program In Safety) is expected enroll 5,000 patients worldwide, including approximately 3,000 patients from TOUCH. Patients in TYGRIS are evaluated at baseline and every six months thereafter for five years. Researchers will evaluate data including medical/MS history; prior TYSABRI use; prior use of immunomodulatory, antineoplastic, or immunosuppressive agents; and all serious adverse events, including PML and other serious OIs, and malignancies.
The information provided here is derived from voluntary adverse event reporting. It is possible that not all reactions have been reported, or that some reactions are not reported to Biogen Idec or Elan in a timely manner.
About TYSABRI
In the US, TYSABRI is approved as a monotherapy treatment for relapsing forms of MS. TYSABRI increases the risk of PML, an opportunistic viral infection of the brain that usually leads to death or severe disability. Patients should be monitored at regular intervals for any new or worsening signs or symptoms suggestive of PML Because of the increased risk of PML, TYSABRI is generally recommended for patients who have had an inadequate response to, or are unable to tolerate, alternate MS therapies. It is available in the US only through a restricted distribution program called the TOUCH Prescribing Program. According to product labeling, after two years, TYSABRI treatment led to a 67% relative reduction (p<0.001) in the annualized relapse rate compared to placebo and reduced the relative risk of disability progression by 42% (p<0.001). TYSABRI treatment also resulted in sustained and statistically significant reductions in brain lesion activity as measured by MRI. Changes in MRI findings often do not correlate with changes in the clinical status of patients (e.g., disability progression). The prognostic significance of the MRI findings in these studies has not been evaluated.
In the European Union, TYSABRI is indicated as a single disease-modifying therapy in highly active relapsing-remitting MS patients. Because of the increased risk of PML, it is for patients with high disease activity despite treatment with a beta-interferon or in patients with rapidly evolving severe relapsing-remitting MS.
According to product labeling in the EU, after two years, TYSABRI treatment led to a 68% relative reduction (p<0.001) in the annualized relapse rate compared to placebo and reduced the relative risk of disability progression by 42-54% (p<0.001).
Serious adverse events that occurred in TYSABRI-treated patients included hypersensitivity reactions (e.g., anaphylaxis), infections, depression and gallstones. In MS trials, the incidence and rate of other serious and common adverse events, including the overall incidence and rate of infections, were balanced between treatment groups. Herpes infections were slightly more common in patients treated with TYSABRI. Serious opportunistic and other atypical infections have been observed in TYSABRI-treated patients, some of whom were receiving concurrent immunosuppressants. Common adverse events reported in TYSABRI-treated patients include headache, fatigue, infusion reactions, urinary tract infections, joint and limb pain, lower respiratory infections, rash, gastroenteritis, abdominal discomfort, vaginitis, and diarrhea
http://biz.yahoo.com/bw/070503/20070503005129.html?.v=1
PRESS RELEASE
EMBARGOED FOR RELEASE UNTIL 4 PM ET, MAY 7, 2007
Media Contacts: Angela Babb, (651) 695-2789, ababb@aan.com or Robin Stinnett, (651) 695-2763, rstinnett@aan.com.
Brain Scans Show Early Alzheimer’s Disease in People with Memory Problems
ST. PAUL, Minn – Brain scans of people with mild cognitive impairment (MCI) show signs of early Alzheimer’s disease, according to a study published in the May 8, 2007, issue of Neurology®, the scientific journal of the American Academy of Neurology.
For the study, PET scans were performed on the brains of 13 elderly men and women with mild cognitive impairment (MCI) and 14 elderly people without memory problems. The scans were used to measure uptake of PIB, which is an imaging agent that allows doctors to see and measure abnormal protein aggregation growth, otherwise known as amyloid plaque, in the brain. Abnormal protein aggregation growth is a signature of Alzheimer’s disease. Until recently, Alzheimer’s disease couldn’t be officially diagnosed until after death with an autopsy.
The study found people with MCI had as much as 39 percent more PIB uptake in some parts of the brain than people without MCI. And about half of the MCI patients had PIB uptake in the Alzheimer’s disease range.
“This pattern of increased PIB in patients with MCI resembles what’s seen in Alzheimer’s disease and is suggestive of an early Alzheimer’s disease process,” said study author Juha O. Rinne, MD, PhD, with the University of Turku in Turku, Finland, and Fellow of the American Academy of Neurology. “Our findings are similar to what’s seen in post-mortem studies in which abnormal protein aggregation growth is found in people who had been diagnosed with probable Alzheimer’s disease.”
Rinne says larger studies and extended follow-up is needed since identifying people with MCI who have abnormal protein aggregation growth will become increasingly important as treatments affecting such plaque amyloid accumulation become available.
The study was supported by Turku University Hospital, the Finnish Medical Society Duodecim, the Finnish Cultural Foundation, the Research Foundation of Orion Corporation, the Research Council for Health of the Academy of Finland, and the Sigrid Juselius Foundation.
The American Academy of Neurology, an association of more than 20,000 neurologists and neuroscience professionals, is dedicated to improving patient care through education and research. A neurologist is a doctor with specialized training in diagnosing, treating and managing disorders of the brain and nervous system such as epilepsy, dystonia, migraine, Huntington’s disease, and dementia. For more information about the American Academy of Neurology, visit www.aan.com.
http://www.investorvillage.com/smbd.asp?mb=160&mn=105851&pt=…
EMBARGOED FOR RELEASE UNTIL 4 PM ET, MAY 7, 2007
Media Contacts: Angela Babb, (651) 695-2789, ababb@aan.com or Robin Stinnett, (651) 695-2763, rstinnett@aan.com.
Brain Scans Show Early Alzheimer’s Disease in People with Memory Problems
ST. PAUL, Minn – Brain scans of people with mild cognitive impairment (MCI) show signs of early Alzheimer’s disease, according to a study published in the May 8, 2007, issue of Neurology®, the scientific journal of the American Academy of Neurology.
For the study, PET scans were performed on the brains of 13 elderly men and women with mild cognitive impairment (MCI) and 14 elderly people without memory problems. The scans were used to measure uptake of PIB, which is an imaging agent that allows doctors to see and measure abnormal protein aggregation growth, otherwise known as amyloid plaque, in the brain. Abnormal protein aggregation growth is a signature of Alzheimer’s disease. Until recently, Alzheimer’s disease couldn’t be officially diagnosed until after death with an autopsy.
The study found people with MCI had as much as 39 percent more PIB uptake in some parts of the brain than people without MCI. And about half of the MCI patients had PIB uptake in the Alzheimer’s disease range.
“This pattern of increased PIB in patients with MCI resembles what’s seen in Alzheimer’s disease and is suggestive of an early Alzheimer’s disease process,” said study author Juha O. Rinne, MD, PhD, with the University of Turku in Turku, Finland, and Fellow of the American Academy of Neurology. “Our findings are similar to what’s seen in post-mortem studies in which abnormal protein aggregation growth is found in people who had been diagnosed with probable Alzheimer’s disease.”
Rinne says larger studies and extended follow-up is needed since identifying people with MCI who have abnormal protein aggregation growth will become increasingly important as treatments affecting such plaque amyloid accumulation become available.
The study was supported by Turku University Hospital, the Finnish Medical Society Duodecim, the Finnish Cultural Foundation, the Research Foundation of Orion Corporation, the Research Council for Health of the Academy of Finland, and the Sigrid Juselius Foundation.
The American Academy of Neurology, an association of more than 20,000 neurologists and neuroscience professionals, is dedicated to improving patient care through education and research. A neurologist is a doctor with specialized training in diagnosing, treating and managing disorders of the brain and nervous system such as epilepsy, dystonia, migraine, Huntington’s disease, and dementia. For more information about the American Academy of Neurology, visit www.aan.com.
http://www.investorvillage.com/smbd.asp?mb=160&mn=105851&pt=…

Tysabri abstracts to be presented on May 22 at DDW
Efficacy of Natalizumab in Crohn's Patients With Disease Duration Less Than Three Years
S. Schreiber1; S. R. Targan2
1. Christian Albrechts University, Kiel, Germany.
2. Cedars Sinai, Los Angeles, CA, USA.
Purpose: The phase 3 ENCORE and ENACT-2 trials demonstrated that natalizumab was an effective induction and maintenance therapy for Crohn’s disease (CD) patients. More than half of the patients in either trial had a disease duration >7 years. This post-hoc analysis examined remission rates with natalizumab in patients with disease duration ?3 years in these trials. Methods: In the ENCORE trial, patients (N=509) with Crohn’s Disease Activity Index (CDAI) scores ?220 and ?450 and C-reactive protein levels >2.87 mg/L were randomized 1:1 and received natalizumab (300 mg; n=259) or placebo (n=250) infusions at Weeks 0, 4, and 8. Twenty-four percent (120/509) of ENCORE patients had a mean disease duration ?3 years. In the ENACT-2 maintenance trial, adult patients with CD who responded (?70-point reduction in baseline CDAI score and CDAI score <220) to natalizumab induction therapy in the ENACT-1 trial were re-randomized 1:1 to natalizumab (300 mg; n=168) or placebo (n=171) and received up to 12 monthly infusions. Nearly 20% (67/339) of ENACT-2 patients had a mean disease duration ?3 years. Remission rates were analyzed at the various endpoints of both trials (Table). Results: Natalizumab was significantly superior to placebo (p<0.05) at all assessments in both groups studied (1 exception noted). Remission induction rates with natalizumab were consistently higher for patients with short disease duration than in the overall population of the ENCORE study, while placebo rates were similar. Natalizumab was equally efficacious for long-term maintenance of remission in both groups. Conclusions: Natalizumab was effective in inducing and sustaining remission in CD patients with short disease duration (?3 years) and in the overall population, despite the fact that a large proportion of patients in natalizumab phase 3 trials had extended disease duration. There were trends for higher induction remission rates for patients with short disease duration compared to the overall population, while maintenance therapy with natalizumab was efficacious irrespective of disease duration.
------------------------------------------------------------------------------
Natalizumab Induces Sustained Response and Remission in Patients With Crohn’s Disease Activity Index Scores ?330: Results From the ENCORE Trial
R. Panaccione1; B. G. Feagan2; R. Fedorak3; B. Lashner4; D. H. Present5; P. Rutgeerts6; W. J. Sandborn7; M. E. Spehlmann8; Z. Tulassay9; M. Volfova10; D. C. Wolf11; S. R. Targan12
1. University of Calgary, Calgary, AB, Canada.
2. Robarts Research Institute, University of Western Ontario, London, ON, Canada.
3. University of Alberta, Edmonton, AB, Canada.
4. Cleveland Clinic, Cleveland, OH, USA.
5. Mount Sinai School of Medicine, New York, NY, USA.
6. University Hospital Gasthuisberg, Leuven, Belgium.
7. Mayo Clinic, Rochester, MN, USA.
8. Asklepios Westklinikum, Hamburg, Germany.
9. Semmelweis University, Budapest, Hungary.
10. Hepato-Gastroenterology, Hradec Králové, Czech Republic.
11. Atlanta Gastroenterology Associates, Atlanta, GA, USA.
12. Cedars Sinai, Los Angeles, CA, USA.
Purpose: Natalizumab has been shown to be effective in inducing response and remission (by Week 8 and through Week 12) in patients with moderate to severely active Crohn’s disease in a large phase 3 randomized controlled trial: ENCORE (Efficacy of Natalizumab in Crohn's Disease Response and Remission).1 Approximately 30% (155/509) of these patients entered the ENCORE trial with a baseline Crohn’s Disease Activity Index (CDAI) score ? 330; this post-hoc analysis assessed the efficacy of natalizumab in this subpopulation. Methods: Patients (N=509) with CDAI scores ?220 and ?450 and CRP >2.87 mg/L were randomized 1:1 and received natalizumab (300 mg; n=259) or placebo (n=250) infusions at Weeks 0, 4, and 8. Efficacy and safety were assessed at Weeks 4, 8 and 12. The primary endpoint was the ability of natalizumab to induce a clinical response (?70 point decrease in baseline CDAI score) by Week 8 that was sustained through Week 12. Results: Of the patients who entered the trial with a baseline CDAI score ? 330 (n=155), 51% had a sustained response to natalizumab through Weeks 8 and 12 compared with 27% of patients treated with placebo (p=0.002). Approximately 60% of patients with baseline CDAI ?330 were in response at either time point (62% at Week 8 and 60% at Week 12, p?0.005 for both comparisons with placebo). The response to natalizumab was demonstrated early, with 57% responding after the first infusion of natalizumab (Week 4) compared with 39% in the placebo group (p=0.029). Remission was sustained through Weeks 8 and 12 in 14% of patients treated with natalizumab compared with 3% of patients in the placebo group (p=0.025); remission rates at the Week 8 and 12 time-points were 21% and 23% in the natalizumab-treated patients, compared with 7% and 10% in placebo-treated patients (p?0.039 for both comparisons with placebo). The mean change in CDAI score from baseline exceeded 100 points at each time point in the natalizumab group and was statistically significant compared with placebo (-103 vs -55 at Week 4, p=0.002; -135 vs -69 at Week 8, p<0.001; -146 vs -66 at Week 12, p<0.001). Conclusions: In patients whose baseline CDAI score was ?330 natalizumab induced rapid, statistically significant, and durable response and remission (at Weeks 8 and 12) compared with placebo. Durable remission in this patient population is particularly impressive due to the magnitude of the decrease in CDAI scores necessary to achieve and sustain this endpoint.
-----------------------------------------------------------------
Natalizumab Induces Sustained Response and Remission in Crohn’s Patients After Previous Infliximab Failure: Results From the ENCORE Trial
D. H. Present1; B. G. Feagan2; R. N. Fedorak3; B. Lashner4; R. Panaccione5; P. Rutgeerts6; W. J. Sandborn7; M. E. Spehlmann8; Z. Tulassay9; M. Volfova10; D. C. Wolf11; S. R. Targan12
1. Mount Sinai School of Medicine, New York , NY, USA.
2. Robarts Research Institute, University of Western Ontario, London , ON, Canada.
3. University of Alberta, Edmonton, AB, Canada.
4. Cleveland Clinic, Cleveland, OH, USA.
5. University of Calgary, Calgary , AB, Canada.
6. University Hospital Gasthuisberg, Leuven, Belgium.
7. Mayo Clinic, Rochester, MN, USA.
8. Asklepios Westklinikum, Hamburg, Germany.
9. Semmelweis University, Budapest, Hungary.
10. Hepato-Gastroenterology, Hradec Králové, Czech Republic.
11. Atlanta Gastroenterology Associates, Atlanta, GA, USA.
12. Cedars Sinai, Los Angeles, CA, USA.
Purpose: The phase 3 ENCORE (Efficacy of Natalizumab in Crohn's Disease Response and Remission) trial demonstrated that natalizumab was effective in inducing durable response and remission in Crohn’s patients with active disease and evidence of inflammation. Forty-eight percent of patients sustained response through Weeks 8 and 12, and 26% sustained remission compared with 32% and 16% in the placebo groups (p?0.002). Nearly 50% (242/509) of patients in the ENCORE trial had been previously exposed to infliximab, and over two-thirds of these patients (71%; 172/242) were considered by the study investigators to have failed this anti-TNF therapy. This post-hoc analysis assessed the efficacy of natalizumab in the ENCORE subpopulation of patients with prior infliximab failure. Methods: Patients (N=509) with Crohn’s Disease Activity Index (CDAI) scores ?220 and ?450 and C-reactive protein leves >2.87 mg/L were randomized 1:1 and received natalizumab (300 mg; n=259) or placebo (n=250) infusions at Weeks 0, 4, and 8. Efficacy and safety were assessed at Weeks 4, 8 and 12. The primary endpoint was the ability of natalizumab to induce a clinical response (?70 point decrease in baseline CDAI score) by Week 8 that was sustained through Week 12. Results: Of the patients who had previously failed therapy with infliximab (N=172), 38% had a sustained response to natalizumab through Weeks 8 and 12 compared with 15% of patients treated with placebo (p<0.001). Approximately 50% of natalizumab-treated patients with prior infliximab failure were in response at either time point (52% at Week 8 and 49% at Week 12) compared with 22% and 29% in the placebo group (p?0.006). Patients treated with natalizumab also had higher sustained remission rates. Remission was sustained through Weeks 8 and 12 in 17% of patients treated with natalizumab compared with 5% of patients in the placebo group (p=0.012). The incidence and types of adverse events were similar between groups and were comparable to the overall treatment population. Conclusions: Natalizumab induced durable response and remission in patients who had previously failed infliximab therapy. A statistically greater proportion of natalizumab-treated patients achieved response or remission by Week 8 that was sustained through Week 12 compared with patients receiving placebo. Natalizumab was well tolerated in this sub-population of patients, with adverse events not significantly different than placebo or the overall ENCORE population
http://www.investorvillage.com/smbd.asp?mb=160&mn=105858&pt=…
Business Wire
Auf der Jahresversammlung der American Academy of Neurology präsentierte Daten liefern neue Informationen zur Anwendung und Verträglichkeit von TYSABR
Mittwoch 9. Mai 2007, 17:46 Uhr
BOSTON Biogen Idec (NASDAQ: BIIB) und die Elan Corporation PLC (NYSE: ELN) teilten heute mit, dass neue Daten des TOUCH Prescribing Program(TM) und der Sicherheitsstudie TYGRIS das Sicherheitsprofil aus früheren klinischen Studien zu TYSABRI(R) (natalizumab) bestätigen. Zudem wurden auf der 59. Jahresversammlung der American Academy of Neurology in Boston, Massachusetts, Daten einer Anschlussstudie präsentiert, die zeigen, dass TYSABRI einen anhaltenden Behandlungseffekt auf klinische Rückfälle und das Risiko der Behinderungsprogression bei Patienten mit multipler Sklerose (MS) hat, die bis zu drei Jahre lang behandelt werden. Die Unternehmen meldeten vor kurzem, dass mit Stand Mitte April 2007 ca. 12.500 Patienten weltweit TYSABRI verschrieben wurde. Schätzungen der beiden Unternehmen zufolge wenden gegenwärtig mehr als 10.000 Patienten TYSABRI in klinischen Studien und im kommerziellen Rahmen an.
,,Die neuen Ergebnisse hinsichtlich der Sicherheit des Mittels zusammen mit den Daten, die den anhaltenden Behandlungseffekt bei Patienten zeigen, die bis zu drei Jahre lang mit TYSABRI behandelt wurden, liefern aufschlussreiche Informationen darüber, wie diese Therapie als Behandlungsmöglichkeit für Patienten eingesetzt werden kann, die mit den Folgen multipler Sklerose leben müssen", sagte Dr. med. Paul O'Connor, St. Michael's Hospital, Toronto, Ontario, Kanada, Hauptversuchsleiter der TYSABRI-Anschlussstudie.
Aktuelle Informationen zu TYSABRI
TYSABRI ist in den USA über das TOUCH Prescribing Program erhältlich. Alle Verordner, Infusionsstellen und Patienten, die TYSABRI erhalten, müssen sich für das TOUCH-Programm registrieren. Informationen über die Sicherheit des Mittels werden zudem anhand von laufenden klinischen Studien und Registern zusammengetragen, darunter STRATA, TYGRIS und das Schwangerschaftsregister. Laut den Daten, die den Unternehmen zum 23. April 2007 vorlagen, gab es keine neuen Berichte über bestätigte Fälle von progressiver multifokaler Leukoenzephalopathie (PML) oder andere schwere opportunistische Infektionen (OIs). Die Daten bestätigen das Sicherheitsprofil aus früheren klinischen Studien zu TYSABRI und werden die Kenntnisse in Bezug auf die langfristige Sicherheit und Verträglichkeit von TYSABRI erweitern.
TOUCH, TYGRIS und das Schwangerschaftsregister bilden das umfassendste jemals durchgeführte Programm zur langfristigen Nachkontrolle einer MS-Therapie und die Unternehmen planen, auf kommenden medizinischen Veranstaltungen weiterhin aktuelle Informationen dieser Art bereit zu stellen.
Den Unternehmen zufolge wurde mit Stand Mitte April ca. 12.500 Patienten weltweit TSBARI verschrieben. Gegenwärtig werden mehr als 10.000 Patienten weltweit sowohl im kommerziellen Rahmen als auch in klinischen Studien mit TYSABRI behandelt.
-- In den USA wenden gegenwärtig ca. 6.600 Patienten TYSABRI im kommerziellen Rahmen an. Ungefähr 10.000 Patienten haben sich für das TOUCH-Programm registriert und 1.500 Mediziner haben registrierte Patienten.
-- In der EU haben etwa 2.500 Patienten TYSABRI-Infusionen im kommerziellen Rahmen erhalten, hauptsächlich in Deutschland und den nordischen Ländern.
-- Mehr als 1.000 Patienten unterziehen sich im Rahmen klinischer Studien einer TYSABRI-Therapie.
Anhaltende Wirksamkeit von TYSABRI über drei Jahre
Patienten, die an dem Phase-III-TYSABRI-Programm teilgenommen hatten, waren zur Anmeldung für eine Open-Label-Anschlussstudie berechtigt, in deren Rahmen die Langzeitwirkung der Behandlung evaluiert wurde. Dazu gehörten Patienten von AFFIRM, einer randomisierten, doppelblinden, Placebo-kontrollierten, zweijährigen Monotherapiestudie zu TYSABRI, an der 942 Patienten teilnahmen (von denen 627 Patienten TYSABRI und 315 ein Placebo erhielten). In der AFFIRM-Studie reduzierte TYSABRI im Vergleich zur Placebogruppe die auf das Jahr bezogene Rückfallrate bei MS-Patienten um 67 % (p<0,001) und das Risiko der über 12 Wochen anhaltenden Behinderungsprogression um 42 % (p<0,001).
In der Intent-to-Treat-Analyse lag die auf das Jahr bezogene Rückfallrate für mit TYSABRI behandelte Patienten über den Zeitraum von drei Jahren bei 0,23, was im Durchschnitt einem Rückfall aller 4,3 Jahre entspricht. Die Rückfallrate blieb während der dreijährigen Behandlung mit TYSABRI zudem weiterhin niedrig: 0,27 im ersten Jahr; 0,20 im zweiten Jahr und 0,15 im dritten Jahr (basierend auf 531 Patienten, die an der Anschlussstudie teilnahmen, darunter ca. 250 Patienten mit fast drei Jahren fortlaufender Therapie).
Des Weiteren reduzierte TYSABRI die kumulative Wahrscheinlichkeit der über sechs Monate anhaltenden Behinderungsprogression im Vergleich zur Placebogruppe. Der geschätzte Anteil von Patienten mit einer über 24 Wochen anhaltenden Behinderungsprogression lag bei mit TYSABRI behandelten Patienten nach zwei Jahren bei 11 % gegenüber 23 % bei Patienten, die ein Placebo erhielten. Dies entspricht einer relativen Reduktion von 54 %.
Dieser Effekt wurde bei mit TYSABRI behandelten Patienten bis zu drei Jahre lang aufrechterhalten. 13 % von ihnen zeigten eine über 24 Wochen andauernde Behinderungsprogression.
Über TOUCH und TYGRIS
Vor Behandlungsbeginn müssen alle Patienten, Verordner und Infusionsstellen beim TOUCH Prescribing Program (Abk. f. TYSABRI Outreach: Unified Commitment to Health) registriert sein. TOUCH dient der Bestimmung des Auftretens von schweren OIs sowie von Risikofaktoren für diese, darunter PML, sowie der Überwachung von Patienten, um PML-Symptome zu erkennen, und fördert sachkundige Nutzen-/Risiko-Diskussionen vor dem Beginn einer Behandlung mit TYSABRI. Ärzte berichten auf fortlaufender Basis über PML, schwere OIs, Todesfälle und Therapieabbrüche.
Für TYGRIS (Abk. f. TYSABRI Global ObseRvation Program In Safety) werden sich voraussichtlich 5.000 Patienten weltweit anmelden, darunter ca. 3.000 Patienten von TOUCH. Patienten, die an TYGRIS teilnehmen, werden zu Beginn der Studie und danach fünf Jahre lang alle sechs Monate untersucht. Die Forscher werden u. a. folgende Daten auswerten: medizinische/MS-Vorgeschichte; frühere Anwendung von TYSABRI; frühere Anwendung von immunmodulatorischen, antineoplastischen oder immunsuppressiven Wirkstoffen und sämtliche schwerwiegende unerwünschte Ereignisse, darunter PML und andere schwere OIs und bösartige Tumore.
Die hier bereitgestellten Informationen beruhen auf freiwilligen Meldungen unerwünschter Ereignisse. Es besteht die Möglichkeit, dass nicht alle Reaktionen gemeldet wurden oder dass einige Reaktionen Biogen Idec oder Elan nicht rechtzeitig gemeldet werden.
Über TYSABRI
In den USA ist TYSABRI als Monotherapie für die Behandlung der in Schüben verlaufenden multiplen Sklerose zugelassen. TYSABRI erhöht das Risiko einer PML, einer opportunistischen Virusinfektion des Gehirns, die in der Regel tödlich verläuft oder zu schweren Behinderungen führt. Die Patienten müssen daher regelmäßig auf neue bzw. sich verschlechternde Symptome untersucht werden, die eine PML vermuten lassen. Wegen des erhöhten PML-Risikos empfiehlt sich TYSABRI nur für Patienten, die auf alternative MS-Therapien nicht ansprechen oder diese nicht vertragen. TYSABRI unterliegt in den USA strengen Auflagen und ist nur über das sogenannte TOUCH Prescribing Program erhältlich. Laut Produktbeschriftung führte die Behandlung mit TYSABRI gegenüber der Vergleichstherapie mit einem Placebo zu einer relativen Verminderung der auf das Jahr bezogenen Rückfallrate um 67 % (p<0,001) und reduzierte das relative Risiko der Behinderungsprogression um 42 % (p<0,001). Die Behandlung mit TYSABRI resultierte zudem in einer anhaltenden und statistisch signifikanten Reduzierung der mittels MRT gemessenen Hirnverletzungsaktivität. Veränderungen der MRT-Ergebnisse korrelieren oft nicht mit den Veränderungen des klinischen Status von Patienten (z. B. Behinderungsprogression). Die prognostische Bedeutung der MRT-Ergebnisse in diesen Studien wurde nicht evaluiert.
In der Europäischen Union wird TYSABRI als krankheitsmodifizierende Einzeltherapie bei Patienten mit hochaktiver schubförmig-remittierender MS eingesetzt. Wegen des erhöhten Risikos einer PML wird TYSABRI nur bei hoher Krankheitsaktivität trotz Behandlung mit einem Beta-Interferon oder bei schnell fortschreitender schwerer schubförmig-remittierender MS verabreicht.
Laut Produktbeschriftung in der EU führte die Behandlung mit TYSABRI gegenüber der Vergleichstherapie mit einem Placebo zu einer relativen Verminderung der auf das Jahr bezogenen Rückfallrate um 68 % (p<0,001) und reduzierte das relative Risiko der Behinderungsprogression um 42-54 % (p<0,001).
Zu den schweren Nebenwirkungen, die bei mit TYSABRI behandelten MS-Patienten auftraten, zählen u. a. Überempfindlichkeitsreaktionen (z. B. allergische Reaktionen), Infektionen, Depressionen und Gallensteine. Das Auftreten anderer schwerer oder häufiger Nebenwirkungen wie auch die Gesamtrate der Infektionen war bei den Behandlungsgruppen ausgeglichen. Bei den Patienten, die mit TYSABRI behandelt wurden, kam es etwas häufiger zu Herpesinfektionen. Schwere opportunistische und andere atypische Infektionen wurden bei mit TYSABRI behandelten Patienten beobachtet, von denen einige gleichzeitig Immunsuppressiva erhielten. Häufige Nebenwirkungen, über die von Patienten berichtet wurde, die mit TYSABRI behandelt wurden, sind Kopfschmerzen, Müdigkeit, Infusionsreaktionen, Harnwegsinfektionen, Gelenk- und Gliederschmerzen, Infektionen der unteren Atemwege, Ausschlag, Gastroenteritis, Vaginitis, Depressionen, Bauchbeschwerden und Diarrhö.
Weitere Informationen zu TYSABRI erhalten Sie unter www.tysabri.com, www.biogenidec.com oder www.elan.com oder telefonisch unter +1-800-456-2255.
Über Biogen Idec
Biogen Idec setzt in therapeutischen Bereichen mit erheblichen medizinischen Versorgungslücken neue Standards. Das Unternehmen wurde 1978 gegründet und ist bei der Entwicklung, Herstellung und Kommerzialisierung innovativer Therapien weltweit führend. Patienten in mehr als 90 Ländern profitieren von Biogen Idecs leistungsfähigen Produkten für die Behandlung von Lymphknotenerkrankungen und Krankheiten wie multipler Sklerose und Gelenkrheumatismus. Produktinformationen, Pressemitteilungen und zusätzliche Informationen über das Unternehmen finden Sie unter http://www.biogenidec.com.
Über Elan
Die Elan Corporation, plc ist ein Biotechnologie-Unternehmen mit neurowissenschaftlicher Ausrichtung und strebt danach, das Leben der Patienten und ihrer Familien zu verbessern. Das Unternehmen setzt sich dafür ein, wissenschaftliche Innovationen für ernste, nicht gelöste medizinische Probleme nutzbar zu machen, da diese nach wie vor weltweit anzutreffen sind. Die Aktien von Elan werden an den Börsen in New York, London und Dublin gehandelt. Weitere Informationen über das Unternehmen sind unter www.elan.com abrufbar.
Safe-Harbor/Zukunftsbezogene Aussagen
Diese Pressemitteilung enthält zukunftsbezogene Aussagen in Bezug auf TYSABRI. Diese Aussagen beruhen auf den gegenwärtigen Überzeugungen und Erwartungen der Unternehmen. Das kommerzielle Potential von TYSABRI ist einer Reihe von Risiken und Unsicherheiten ausgesetzt. Zu den Faktoren, die dazu führen können, dass die tatsächlichen Ergebnisse erheblich von den derzeitigen Erwartungen der Unternehmen abweichen, gehört das Risiko, dass wir möglicherweise nicht in der Lage sind, in angemessenen Maße auf die Bedenken oder Fragen der FDA oder anderer Zulassungsbehörden zu reagieren, dass sich aus den zusätzlichen Daten Bedenken ergeben könnten, dass das Auftreten und/oder das Risiko von PML oder anderer opportunistischer Infektionen bei mit TYSABRI behandelten Patienten häufiger bzw. größer ist als in klinischen Studien beobachtet wurde, oder dass die Unternehmen auf andere unerwartete Hindernisse stoßen. Die Entwicklung und Vermarktung von Medikamenten ist mit zahlreichen Risiken behaftet.
Ausführlichere Informationen über die Risiken und Unsicherheiten in Verbindung mit der Medikamentenentwicklung und anderen Aktivitäten der Unternehmen sind in den regelmäßigen und aktuellen Berichten enthalten, die Biogen Idec und Elan bei der US-Börsenaufsichtsbehörde SEC eingereicht haben. Die Unternehmen übernehmen keine Verpflichtung, zukunftsbezogene Aussagen zu aktualisieren, sollten sich neue Informationen, Ereignisse o. ä. ergeben.
Kontakt
Ansprechpartner Medien:
Biogen Idec
Amy Brockelman, 617 914 6524
oder
Elan
Matt Dallas, 212 850 5664
Elizabeth Headon, 353 1 498 0300
oder
Ansprechpartner Investoren:
Biogen Idec
Eric Hoffman, 617 679 2812
oder
Elan
Chris Burns, 353 1 709 4444
800 252 3526
http://de.biz.yahoo.com/09052007/240/jahresversammlung-ameri…
Auf der Jahresversammlung der American Academy of Neurology präsentierte Daten liefern neue Informationen zur Anwendung und Verträglichkeit von TYSABR
Mittwoch 9. Mai 2007, 17:46 Uhr
BOSTON Biogen Idec (NASDAQ: BIIB) und die Elan Corporation PLC (NYSE: ELN) teilten heute mit, dass neue Daten des TOUCH Prescribing Program(TM) und der Sicherheitsstudie TYGRIS das Sicherheitsprofil aus früheren klinischen Studien zu TYSABRI(R) (natalizumab) bestätigen. Zudem wurden auf der 59. Jahresversammlung der American Academy of Neurology in Boston, Massachusetts, Daten einer Anschlussstudie präsentiert, die zeigen, dass TYSABRI einen anhaltenden Behandlungseffekt auf klinische Rückfälle und das Risiko der Behinderungsprogression bei Patienten mit multipler Sklerose (MS) hat, die bis zu drei Jahre lang behandelt werden. Die Unternehmen meldeten vor kurzem, dass mit Stand Mitte April 2007 ca. 12.500 Patienten weltweit TYSABRI verschrieben wurde. Schätzungen der beiden Unternehmen zufolge wenden gegenwärtig mehr als 10.000 Patienten TYSABRI in klinischen Studien und im kommerziellen Rahmen an.
,,Die neuen Ergebnisse hinsichtlich der Sicherheit des Mittels zusammen mit den Daten, die den anhaltenden Behandlungseffekt bei Patienten zeigen, die bis zu drei Jahre lang mit TYSABRI behandelt wurden, liefern aufschlussreiche Informationen darüber, wie diese Therapie als Behandlungsmöglichkeit für Patienten eingesetzt werden kann, die mit den Folgen multipler Sklerose leben müssen", sagte Dr. med. Paul O'Connor, St. Michael's Hospital, Toronto, Ontario, Kanada, Hauptversuchsleiter der TYSABRI-Anschlussstudie.
Aktuelle Informationen zu TYSABRI
TYSABRI ist in den USA über das TOUCH Prescribing Program erhältlich. Alle Verordner, Infusionsstellen und Patienten, die TYSABRI erhalten, müssen sich für das TOUCH-Programm registrieren. Informationen über die Sicherheit des Mittels werden zudem anhand von laufenden klinischen Studien und Registern zusammengetragen, darunter STRATA, TYGRIS und das Schwangerschaftsregister. Laut den Daten, die den Unternehmen zum 23. April 2007 vorlagen, gab es keine neuen Berichte über bestätigte Fälle von progressiver multifokaler Leukoenzephalopathie (PML) oder andere schwere opportunistische Infektionen (OIs). Die Daten bestätigen das Sicherheitsprofil aus früheren klinischen Studien zu TYSABRI und werden die Kenntnisse in Bezug auf die langfristige Sicherheit und Verträglichkeit von TYSABRI erweitern.
TOUCH, TYGRIS und das Schwangerschaftsregister bilden das umfassendste jemals durchgeführte Programm zur langfristigen Nachkontrolle einer MS-Therapie und die Unternehmen planen, auf kommenden medizinischen Veranstaltungen weiterhin aktuelle Informationen dieser Art bereit zu stellen.
Den Unternehmen zufolge wurde mit Stand Mitte April ca. 12.500 Patienten weltweit TSBARI verschrieben. Gegenwärtig werden mehr als 10.000 Patienten weltweit sowohl im kommerziellen Rahmen als auch in klinischen Studien mit TYSABRI behandelt.
-- In den USA wenden gegenwärtig ca. 6.600 Patienten TYSABRI im kommerziellen Rahmen an. Ungefähr 10.000 Patienten haben sich für das TOUCH-Programm registriert und 1.500 Mediziner haben registrierte Patienten.
-- In der EU haben etwa 2.500 Patienten TYSABRI-Infusionen im kommerziellen Rahmen erhalten, hauptsächlich in Deutschland und den nordischen Ländern.
-- Mehr als 1.000 Patienten unterziehen sich im Rahmen klinischer Studien einer TYSABRI-Therapie.
Anhaltende Wirksamkeit von TYSABRI über drei Jahre
Patienten, die an dem Phase-III-TYSABRI-Programm teilgenommen hatten, waren zur Anmeldung für eine Open-Label-Anschlussstudie berechtigt, in deren Rahmen die Langzeitwirkung der Behandlung evaluiert wurde. Dazu gehörten Patienten von AFFIRM, einer randomisierten, doppelblinden, Placebo-kontrollierten, zweijährigen Monotherapiestudie zu TYSABRI, an der 942 Patienten teilnahmen (von denen 627 Patienten TYSABRI und 315 ein Placebo erhielten). In der AFFIRM-Studie reduzierte TYSABRI im Vergleich zur Placebogruppe die auf das Jahr bezogene Rückfallrate bei MS-Patienten um 67 % (p<0,001) und das Risiko der über 12 Wochen anhaltenden Behinderungsprogression um 42 % (p<0,001).
In der Intent-to-Treat-Analyse lag die auf das Jahr bezogene Rückfallrate für mit TYSABRI behandelte Patienten über den Zeitraum von drei Jahren bei 0,23, was im Durchschnitt einem Rückfall aller 4,3 Jahre entspricht. Die Rückfallrate blieb während der dreijährigen Behandlung mit TYSABRI zudem weiterhin niedrig: 0,27 im ersten Jahr; 0,20 im zweiten Jahr und 0,15 im dritten Jahr (basierend auf 531 Patienten, die an der Anschlussstudie teilnahmen, darunter ca. 250 Patienten mit fast drei Jahren fortlaufender Therapie).
Des Weiteren reduzierte TYSABRI die kumulative Wahrscheinlichkeit der über sechs Monate anhaltenden Behinderungsprogression im Vergleich zur Placebogruppe. Der geschätzte Anteil von Patienten mit einer über 24 Wochen anhaltenden Behinderungsprogression lag bei mit TYSABRI behandelten Patienten nach zwei Jahren bei 11 % gegenüber 23 % bei Patienten, die ein Placebo erhielten. Dies entspricht einer relativen Reduktion von 54 %.
Dieser Effekt wurde bei mit TYSABRI behandelten Patienten bis zu drei Jahre lang aufrechterhalten. 13 % von ihnen zeigten eine über 24 Wochen andauernde Behinderungsprogression.
Über TOUCH und TYGRIS
Vor Behandlungsbeginn müssen alle Patienten, Verordner und Infusionsstellen beim TOUCH Prescribing Program (Abk. f. TYSABRI Outreach: Unified Commitment to Health) registriert sein. TOUCH dient der Bestimmung des Auftretens von schweren OIs sowie von Risikofaktoren für diese, darunter PML, sowie der Überwachung von Patienten, um PML-Symptome zu erkennen, und fördert sachkundige Nutzen-/Risiko-Diskussionen vor dem Beginn einer Behandlung mit TYSABRI. Ärzte berichten auf fortlaufender Basis über PML, schwere OIs, Todesfälle und Therapieabbrüche.
Für TYGRIS (Abk. f. TYSABRI Global ObseRvation Program In Safety) werden sich voraussichtlich 5.000 Patienten weltweit anmelden, darunter ca. 3.000 Patienten von TOUCH. Patienten, die an TYGRIS teilnehmen, werden zu Beginn der Studie und danach fünf Jahre lang alle sechs Monate untersucht. Die Forscher werden u. a. folgende Daten auswerten: medizinische/MS-Vorgeschichte; frühere Anwendung von TYSABRI; frühere Anwendung von immunmodulatorischen, antineoplastischen oder immunsuppressiven Wirkstoffen und sämtliche schwerwiegende unerwünschte Ereignisse, darunter PML und andere schwere OIs und bösartige Tumore.
Die hier bereitgestellten Informationen beruhen auf freiwilligen Meldungen unerwünschter Ereignisse. Es besteht die Möglichkeit, dass nicht alle Reaktionen gemeldet wurden oder dass einige Reaktionen Biogen Idec oder Elan nicht rechtzeitig gemeldet werden.
Über TYSABRI
In den USA ist TYSABRI als Monotherapie für die Behandlung der in Schüben verlaufenden multiplen Sklerose zugelassen. TYSABRI erhöht das Risiko einer PML, einer opportunistischen Virusinfektion des Gehirns, die in der Regel tödlich verläuft oder zu schweren Behinderungen führt. Die Patienten müssen daher regelmäßig auf neue bzw. sich verschlechternde Symptome untersucht werden, die eine PML vermuten lassen. Wegen des erhöhten PML-Risikos empfiehlt sich TYSABRI nur für Patienten, die auf alternative MS-Therapien nicht ansprechen oder diese nicht vertragen. TYSABRI unterliegt in den USA strengen Auflagen und ist nur über das sogenannte TOUCH Prescribing Program erhältlich. Laut Produktbeschriftung führte die Behandlung mit TYSABRI gegenüber der Vergleichstherapie mit einem Placebo zu einer relativen Verminderung der auf das Jahr bezogenen Rückfallrate um 67 % (p<0,001) und reduzierte das relative Risiko der Behinderungsprogression um 42 % (p<0,001). Die Behandlung mit TYSABRI resultierte zudem in einer anhaltenden und statistisch signifikanten Reduzierung der mittels MRT gemessenen Hirnverletzungsaktivität. Veränderungen der MRT-Ergebnisse korrelieren oft nicht mit den Veränderungen des klinischen Status von Patienten (z. B. Behinderungsprogression). Die prognostische Bedeutung der MRT-Ergebnisse in diesen Studien wurde nicht evaluiert.
In der Europäischen Union wird TYSABRI als krankheitsmodifizierende Einzeltherapie bei Patienten mit hochaktiver schubförmig-remittierender MS eingesetzt. Wegen des erhöhten Risikos einer PML wird TYSABRI nur bei hoher Krankheitsaktivität trotz Behandlung mit einem Beta-Interferon oder bei schnell fortschreitender schwerer schubförmig-remittierender MS verabreicht.
Laut Produktbeschriftung in der EU führte die Behandlung mit TYSABRI gegenüber der Vergleichstherapie mit einem Placebo zu einer relativen Verminderung der auf das Jahr bezogenen Rückfallrate um 68 % (p<0,001) und reduzierte das relative Risiko der Behinderungsprogression um 42-54 % (p<0,001).
Zu den schweren Nebenwirkungen, die bei mit TYSABRI behandelten MS-Patienten auftraten, zählen u. a. Überempfindlichkeitsreaktionen (z. B. allergische Reaktionen), Infektionen, Depressionen und Gallensteine. Das Auftreten anderer schwerer oder häufiger Nebenwirkungen wie auch die Gesamtrate der Infektionen war bei den Behandlungsgruppen ausgeglichen. Bei den Patienten, die mit TYSABRI behandelt wurden, kam es etwas häufiger zu Herpesinfektionen. Schwere opportunistische und andere atypische Infektionen wurden bei mit TYSABRI behandelten Patienten beobachtet, von denen einige gleichzeitig Immunsuppressiva erhielten. Häufige Nebenwirkungen, über die von Patienten berichtet wurde, die mit TYSABRI behandelt wurden, sind Kopfschmerzen, Müdigkeit, Infusionsreaktionen, Harnwegsinfektionen, Gelenk- und Gliederschmerzen, Infektionen der unteren Atemwege, Ausschlag, Gastroenteritis, Vaginitis, Depressionen, Bauchbeschwerden und Diarrhö.
Weitere Informationen zu TYSABRI erhalten Sie unter www.tysabri.com, www.biogenidec.com oder www.elan.com oder telefonisch unter +1-800-456-2255.
Über Biogen Idec
Biogen Idec setzt in therapeutischen Bereichen mit erheblichen medizinischen Versorgungslücken neue Standards. Das Unternehmen wurde 1978 gegründet und ist bei der Entwicklung, Herstellung und Kommerzialisierung innovativer Therapien weltweit führend. Patienten in mehr als 90 Ländern profitieren von Biogen Idecs leistungsfähigen Produkten für die Behandlung von Lymphknotenerkrankungen und Krankheiten wie multipler Sklerose und Gelenkrheumatismus. Produktinformationen, Pressemitteilungen und zusätzliche Informationen über das Unternehmen finden Sie unter http://www.biogenidec.com.
Über Elan
Die Elan Corporation, plc ist ein Biotechnologie-Unternehmen mit neurowissenschaftlicher Ausrichtung und strebt danach, das Leben der Patienten und ihrer Familien zu verbessern. Das Unternehmen setzt sich dafür ein, wissenschaftliche Innovationen für ernste, nicht gelöste medizinische Probleme nutzbar zu machen, da diese nach wie vor weltweit anzutreffen sind. Die Aktien von Elan werden an den Börsen in New York, London und Dublin gehandelt. Weitere Informationen über das Unternehmen sind unter www.elan.com abrufbar.
Safe-Harbor/Zukunftsbezogene Aussagen
Diese Pressemitteilung enthält zukunftsbezogene Aussagen in Bezug auf TYSABRI. Diese Aussagen beruhen auf den gegenwärtigen Überzeugungen und Erwartungen der Unternehmen. Das kommerzielle Potential von TYSABRI ist einer Reihe von Risiken und Unsicherheiten ausgesetzt. Zu den Faktoren, die dazu führen können, dass die tatsächlichen Ergebnisse erheblich von den derzeitigen Erwartungen der Unternehmen abweichen, gehört das Risiko, dass wir möglicherweise nicht in der Lage sind, in angemessenen Maße auf die Bedenken oder Fragen der FDA oder anderer Zulassungsbehörden zu reagieren, dass sich aus den zusätzlichen Daten Bedenken ergeben könnten, dass das Auftreten und/oder das Risiko von PML oder anderer opportunistischer Infektionen bei mit TYSABRI behandelten Patienten häufiger bzw. größer ist als in klinischen Studien beobachtet wurde, oder dass die Unternehmen auf andere unerwartete Hindernisse stoßen. Die Entwicklung und Vermarktung von Medikamenten ist mit zahlreichen Risiken behaftet.
Ausführlichere Informationen über die Risiken und Unsicherheiten in Verbindung mit der Medikamentenentwicklung und anderen Aktivitäten der Unternehmen sind in den regelmäßigen und aktuellen Berichten enthalten, die Biogen Idec und Elan bei der US-Börsenaufsichtsbehörde SEC eingereicht haben. Die Unternehmen übernehmen keine Verpflichtung, zukunftsbezogene Aussagen zu aktualisieren, sollten sich neue Informationen, Ereignisse o. ä. ergeben.
Kontakt
Ansprechpartner Medien:
Biogen Idec
Amy Brockelman, 617 914 6524
oder
Elan
Matt Dallas, 212 850 5664
Elizabeth Headon, 353 1 498 0300
oder
Ansprechpartner Investoren:
Biogen Idec
Eric Hoffman, 617 679 2812
oder
Elan
Chris Burns, 353 1 709 4444
800 252 3526
http://de.biz.yahoo.com/09052007/240/jahresversammlung-ameri…
Tysabri nun auch in der Schweiz:
Das Arzneimittel Tysabri® zur Behandlung der schubförmig-remittierenden Multiplen Sklerose wurde am 8. Mai vom schweizerischen Heilmittelinstitut Swissmedic zugelassen, wie die Herstellerin Biogen-Dompé AG meldet. Die Zulassung basierte auf der Einreichung eines Dossiers, das die Zwei-Jahres-Daten der klinischen Phase III Studien sowie die Ergebnisse der ausführlichen Sicherheitsevaluierung umfasste.
Tysabri® wurde für die Monotherapie bei hochaktiver, schubförmig remittierend verlaufender MS zugelassen. Die Behandlung ist für Patienten indiziert, die ungenügend auf eine Interferon-beta-Therapie ansprechen oder eine rasch fortschreitende schubförmig remittierende MS aufweisen.
Das Arzneimittel Tysabri® zur Behandlung der schubförmig-remittierenden Multiplen Sklerose wurde am 8. Mai vom schweizerischen Heilmittelinstitut Swissmedic zugelassen, wie die Herstellerin Biogen-Dompé AG meldet. Die Zulassung basierte auf der Einreichung eines Dossiers, das die Zwei-Jahres-Daten der klinischen Phase III Studien sowie die Ergebnisse der ausführlichen Sicherheitsevaluierung umfasste.
Tysabri® wurde für die Monotherapie bei hochaktiver, schubförmig remittierend verlaufender MS zugelassen. Die Behandlung ist für Patienten indiziert, die ungenügend auf eine Interferon-beta-Therapie ansprechen oder eine rasch fortschreitende schubförmig remittierende MS aufweisen.
Antwort auf Beitrag Nr.: 29.322.535 von Cyberhexe am 15.05.07 12:10:11http://www.msgesellschaft.org/wDeutsch/ms_forschung/forschun…
...back to the roots - bis auf 1000 dndn-Erinnerungsstücke (nach dem Motto, ich war vom 29.3. bis 9.5. mit dabei) bin ich wieder "all in one, all in Elan" investiert. Und nun gibt es einiges aus dieser Zeit aufzuarbeiten. Elan hat erfreulicherweise im 4.Quartal 06 und im 1. Q 07 eine Vielzahl von Patenten angemeldet und zwar für etliche nanoformulierte Wirkstoffe - insgesamt 17 neue Patente), beispielsweise für nanoformuliertes Benipidine zur behandlung von Bluthochdruck.
Building IP : Patent Application re Nanoparticulate Benipidine Compositions United States Patent Application 20070042049
Kind Code A1
Liversidge; Gary G. ; et al. February 22, 2007
--------------------------------------------------------------------------------
Nanoparticulate benidipine compositions
Abstract
The present invention relates to nanoparticulate benidipine compositions having improved bioavailability. The compositions comprise benidipine particles having an effective average particle size of less than about 2000 nm and may be useful in the prevention and treatment of hypertension, renal parenchymal hypertension and angina pectoris.
--------------------------------------------------------------------------------
Inventors: Liversidge; Gary G.; (West Chester, PA) ; Jenkins; Scott; (Downingtown, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name anA. Background Regarding Benidipine
[0003] Hypertension, or high blood pressure, is known as "the silent killer" for two reasons. First, there are no specific symptoms. Estimates suggest that about one in three Americans has high blood pressure but is unaware. Second high blood pressure can lead to serious medical conditions that kill. Such medical conditions include heart arrhythmias, heart attack, stroke and organ failure.
[0004] Unfortunately, the precise cause of hypertension in more than 90 percent of cases is unknown. Factors often associated with this type of "primary" or "essential" hypertension have been shown to include race, heredity, sex, age, obesity, drug use, physical activity and diet. Occasionally, (e.g., in the remaining 10 percent of cases), hypertension is caused by some other physical problem such as atherosclerosis or cancer. This is termed "secondary" hypertension. Blood pressure may be restored to non-hypertensive levels if the primary problem is treated.
[0005] There are numerous classes of medication used to treat high blood pressure including centrally acting drugs, diuretics, angtiotensin converting enzyme ("ACE") inhibitors, beta-blockers and calcium channel blockers ("CCBs"). CCBs work by blocking calcium channels and inhibiting the entry of calcium into the blood vessels and the heart tissue. The lowered calcium levels in the blood vessels and heart cause the blood vessels to dilate and the heart to beat more slowly, thereby lowering blood pressure. Some commercially available calcium channel blockers include Verapamil, Diltiazem, Nifedipine, Nicardipine, Bepridil, and Mibefradil.
[0006] Another calcium channel blocker, benidipine, chemically known as 3-(1-benzyl-3-piperidinyloxycarbonyl)-2,6-dimethyl-5-methoxycarbonyl-4-(3- -nitrophenyl)-1,4-dihydropyridine or its hydrochloride salt being (.+-.)-(R*)-3-[(R*)-1-benzyl-3-piperidinyl]methyl-4-(m-nitrophenyl)-3,5-p- yridinedicarboxylate hydrochloride, has the empirical formula of C.sub.28H.sub.31N.sub.3O.sub.6.HCl and molecular weight of 542.03. The structural formula of benidipine is:
[0007] Benidipine is a calcium channel blockers useful for treatment of hypertension, renal parenchymal hypertension and angina pectoris. Benidipine is commercially available under the tradename Coniel.RTM. Tablets as the hydrochloride salt of benidipine in strengths of 2 mg, 4 mg and 8 mg, and generally prescribed in regimes of once or twice daily.
[0008] Benidipine hydrochloride is an odorless yellow crystalline powder. It is very soluble in formic acid, freely soluble in dimethylformamide, soluble in methanol or ethanol, slightly soluble in acetic anhydride and relatively insoluble in water.
[0009] Benidipine has been disclosed, for example, in U.S. Pat. No. 4,501,748 for "1,4-dihydropyridine derivatives" and U.S. Patent Application No. 2003/0073670 for "Stabilized pharmaceutical composition containing a calcium channel blocker".
[0010] Benidipine has high therapeutic value for the treatment of patients suffering from hypertension, renal parenchymal hypertension and angina pectoris. However, given the need to take benidipine daily after meals, such as breakfast, strict patient compliance is a critical factor in the efficacy of benidipine in the treatment of hypertension, renal parenchymal hypertension and angina pectoris. Moreover, it is desirable for increased dissolution rate for faster onset of the benidipine. Thus, there is a need in the art for benidipine compositions which overcome these and other problems associated with their use in the treatment of hypertension, renal parenchymal hypertension and angina pectoris.
[0011] The present invention fulfills such a need by providing nanoparticulate benidipine compositions which overcome the poor bioavailability of benidipine and eliminate the requirement to take the product with food.
B. Background Regarding Nanoparticulate Compositions
etc.
Building IP : Patent Application re Nanoparticulate Benipidine Compositions United States Patent Application 20070042049
Kind Code A1
Liversidge; Gary G. ; et al. February 22, 2007
--------------------------------------------------------------------------------
Nanoparticulate benidipine compositions
Abstract
The present invention relates to nanoparticulate benidipine compositions having improved bioavailability. The compositions comprise benidipine particles having an effective average particle size of less than about 2000 nm and may be useful in the prevention and treatment of hypertension, renal parenchymal hypertension and angina pectoris.
--------------------------------------------------------------------------------
Inventors: Liversidge; Gary G.; (West Chester, PA) ; Jenkins; Scott; (Downingtown, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name anA. Background Regarding Benidipine
[0003] Hypertension, or high blood pressure, is known as "the silent killer" for two reasons. First, there are no specific symptoms. Estimates suggest that about one in three Americans has high blood pressure but is unaware. Second high blood pressure can lead to serious medical conditions that kill. Such medical conditions include heart arrhythmias, heart attack, stroke and organ failure.
[0004] Unfortunately, the precise cause of hypertension in more than 90 percent of cases is unknown. Factors often associated with this type of "primary" or "essential" hypertension have been shown to include race, heredity, sex, age, obesity, drug use, physical activity and diet. Occasionally, (e.g., in the remaining 10 percent of cases), hypertension is caused by some other physical problem such as atherosclerosis or cancer. This is termed "secondary" hypertension. Blood pressure may be restored to non-hypertensive levels if the primary problem is treated.
[0005] There are numerous classes of medication used to treat high blood pressure including centrally acting drugs, diuretics, angtiotensin converting enzyme ("ACE") inhibitors, beta-blockers and calcium channel blockers ("CCBs"). CCBs work by blocking calcium channels and inhibiting the entry of calcium into the blood vessels and the heart tissue. The lowered calcium levels in the blood vessels and heart cause the blood vessels to dilate and the heart to beat more slowly, thereby lowering blood pressure. Some commercially available calcium channel blockers include Verapamil, Diltiazem, Nifedipine, Nicardipine, Bepridil, and Mibefradil.
[0006] Another calcium channel blocker, benidipine, chemically known as 3-(1-benzyl-3-piperidinyloxycarbonyl)-2,6-dimethyl-5-methoxycarbonyl-4-(3- -nitrophenyl)-1,4-dihydropyridine or its hydrochloride salt being (.+-.)-(R*)-3-[(R*)-1-benzyl-3-piperidinyl]methyl-4-(m-nitrophenyl)-3,5-p- yridinedicarboxylate hydrochloride, has the empirical formula of C.sub.28H.sub.31N.sub.3O.sub.6.HCl and molecular weight of 542.03. The structural formula of benidipine is:
[0007] Benidipine is a calcium channel blockers useful for treatment of hypertension, renal parenchymal hypertension and angina pectoris. Benidipine is commercially available under the tradename Coniel.RTM. Tablets as the hydrochloride salt of benidipine in strengths of 2 mg, 4 mg and 8 mg, and generally prescribed in regimes of once or twice daily.
[0008] Benidipine hydrochloride is an odorless yellow crystalline powder. It is very soluble in formic acid, freely soluble in dimethylformamide, soluble in methanol or ethanol, slightly soluble in acetic anhydride and relatively insoluble in water.
[0009] Benidipine has been disclosed, for example, in U.S. Pat. No. 4,501,748 for "1,4-dihydropyridine derivatives" and U.S. Patent Application No. 2003/0073670 for "Stabilized pharmaceutical composition containing a calcium channel blocker".
[0010] Benidipine has high therapeutic value for the treatment of patients suffering from hypertension, renal parenchymal hypertension and angina pectoris. However, given the need to take benidipine daily after meals, such as breakfast, strict patient compliance is a critical factor in the efficacy of benidipine in the treatment of hypertension, renal parenchymal hypertension and angina pectoris. Moreover, it is desirable for increased dissolution rate for faster onset of the benidipine. Thus, there is a need in the art for benidipine compositions which overcome these and other problems associated with their use in the treatment of hypertension, renal parenchymal hypertension and angina pectoris.
[0011] The present invention fulfills such a need by providing nanoparticulate benidipine compositions which overcome the poor bioavailability of benidipine and eliminate the requirement to take the product with food.
B. Background Regarding Nanoparticulate Compositions
etc.
...oder beispielweise im Feld der Blutgerinnungshemmer
folgendes Patent für nanoformuliertes Clopidogrel in Kombination mit Aspirin:
Building IP: Patent Application re Nanoparticulate Clopidogrel and Aspirin Combo Formulations
United States Patent Application 20070003615
Kind Code A1
Jenkins; Scott ; et al. January 4, 2007
Nanoparticulate clopidogrel and aspirin combination formulations
Abstract
The present invention is directed to compositions comprising a nanoparticulate clopidogrel and aspirin combination, or salts or derivatives thereof, having improved clopidogrel bioavailability. The nanoparticulate clopidogrel particles, and optionally the nanoparticulate aspirin particles, of the composition have an effective average particle size of less than about 2000 nm and are useful in the prevention and treatment of pathologies induced by platelet aggregation. The clopidogrel and aspirin particles may also be formulated as a controlled release polymeric coating or matrix drug delivery system.
Inventors: Jenkins; Scott; (Downingtown, PA) ; Liversidge; Gary G.; (West Chester, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name and Adress: Elan Pharma International Limited
Serial No.: 450890
Series Code: 11
Filed: June 12, 2006
FIELD OF INVENTION
[0002] The present invention relates generally to compounds and compositions useful in the prevention and treatment of pathological states induced by platelet aggregation. More specifically, the invention relates to nanoparticulate clopidogrel combined with aspirin, optionally in a nanoparticulate form, or salts or derivatives thereof (referred to herein as "nanoparticulate clopidogrel and aspirin combination"), and compositions comprising the same. The nanoparticulate clopidogrel, and optionally the aspirin, within the combination compositions have an effective average particle size of less than about 2000 nm. The clopidogrel and/or aspirin particles may also be coated with any one of a number of polymeric materials for a controlled and/or delayed release formulation.
BACKGROUND OF INVENTION
A. Background Regarding Clopidogrel
[0003] Clopidogrel is an inhibitor of platelet aggregation. Clopidogrel inhibits ADP-induced platelet aggregation by direct inhibition of adenosine diphosphate (ADP) binding to its receptor and of the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIa complex. Clopidogrel also inhibits platelet aggregation induced by agonists other than ADP by blocking the amplification of platelet activation by released ADP.
[0004] The chemical name for clopidogrel bisulfate is methyl (+)-(S)-.alpha.-(2-chorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-ac- etate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C.sub.16H.sub.16ClNO.sub.2S.H.sub.2SO.sub.4 and its molecular weight is 419.9. The structural formula is as follows:
[0005] Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but is freely soluble at pH 1.0. It also dissolves freely in methanol, it dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether.
[0006] Clopidogrel bisulfate is commercially available under the trade name PLAVIX.RTM. by Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership (New York, N.Y.). PLAVIX.RTM. is administered as an oral tablet at a recommended dose of 75 mg once daily. PLAVIX.RTM. is provided as pink, round, biconvex, debossed film-coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base.
[0007] Clopidogrel bisulfate is indicated for the reduction of thrombotic events such as recent myocardial infarction (MI), recent stroke, or established arterial disease, and has been shown to reduce the rate of a combined end point of new ischemic stroke, new MI, and other vascular death. For patients with acute coronary syndrome, clopidogrel bisulfate has been shown to decrease the rate of a combined end point of cardiovascular death, MI, or stroke as well as the rate of a combined end point of cardiovascular death, MI, stroke, or refractory ischemia.
[0008] Clopidogrel has been described, for example, in U.S. Pat. Nos. 4,847,265 for "Dextro-Rotatory Enantiomer of Methyl Alpha-5 (4,5,6,7-Tetrahydro (3,2-c) Thieno Pyridyl) (2-Chlorophenyl)-Acetate and the Pharmaceutical Compositions Containing It", 5,576,328 for "Method for the Secondary Prevention of Ischemic Events", 5,989,578 for "Associations of Active Principles Containing Clopidogrel and an Anti-thrombotic Agent", 6,429,210 and 6,504,030 both for "Polymorphic Clopidogrel Hydrogen Sulphate Form", 6,635,763 for "Process to Prepare Clopidogrel", 6,737,411 and 6,800,759 both for "Racemization and Enantiomer Separation of Clopidogrel", and 6,858,734 for "Preparation of (S)-Clopidogrel and Related Compounds" These patents are hereby incorporated by reference.
[0009] Aspirin, also known as acetylsalicylic acid, is often used as an analgesic (against minor pains and aches), antipyretic (against fever), and anti-inflammatory. It has also an anticoagulant (blood thinning) effect and is used in long-term low-doses to prevent heart attacks.
[0010] Aspirin, CAS Number: 50-78-2, is chemically known as 2-acetoxybenzoic acid. Aspirin has a molecular formula of C.sub.9H.sub.8O.sub.4 and a molecular weight of 180.16. The chemical structure of aspirin is shown below:
[0011] Aspirin is a colorless or white crystals or white crystalline powder or granule. It is odorless or almost odorless with a slight acid taste. Aspirin has a melting point of 136.degree. C. (277.degree. F.) and boiling point of 140.degree. C. (284.degree. F.). It is soluble 1 gm. in 300 of water, 1 in 5-7 gm./ml. in alcohol, 1 in 17 gm./ml. of chloroform and 1 in 20 gm./ml. of ether; soluble in solutions of acetates and citrates and, with decomposition, in solutions of alkali hydroxides and carbonates. It is incompatible with free acids, acetanilide, aminopyrine, phenazone, hexamine, iron salts, phenobarbitone sodium, quinine salts, potassium and sodium iodides, and alkali hydroxides, carbonates, and stearates. Acetylsalicylic acid is stable in dry air, but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis, the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate. Acetylsalicylic acid decomposes rapidly in solutions of ammonium acetate or of the acetates, carbonates, citrates or hydroxides of the alkali metals.
[0012] Aspirin is indicated as an analgesic for the treatment of mild to moderate pain, as an anti-inflammatory agent for the treatment of soft tissue and joint inflammation, and as an antipyretic drug. Aspirin is generally dosed in adults for pain and fever in amounts of 300-1000 mg every 4 hour for a maximum of 4 gram per day. For acute polyarthritis rheumatica, dosing is generally 1 gram given 6 times a day for a maximum of 8 grams a day. For rheumatoid arthritis, dosing is generally 0.5 grams to 1 gram given 6 times a day for a maximum of 8 grams a day. For prevention of transient ischaemic attacks and prevention of arterial thrombosis, dosing is generally 300 mg to 1200 mg a day in 2 or 3 doses.
[0013] Aspirin is used to lessen the chance of heart attack, stroke, or other problems that may occur when a blood vessel is blocked by blood clots. Aspirin helps prevent dangerous blood clots from forming. Low-dose long-term aspirin irreversibly blocks formation of thromboxane A2 in platelets, producing an inhibitory affect on platelet aggregation, and this blood thinning property makes it useful for reducing the incidence of heart attacks. Aspirin produced for this purpose often has strengths of 75 mg, 81 mg or 325 mg enteric coated tablets. High doses of aspirin are also given immediately after an acute heart attack.
[0014] Various brands of aspirin sold in the United States that include, for example, Acuprin 81, Amigesic, Anacin, Caplets, Anacin Maximum Strength, Anacin Tablets, Anaflex 750, Arthritis Pain Ascriptin, Arthritis Pain Formula, Arthritis Strength Bufferin, Arthropan, Aspergum, Aspirin Regimen Bayer Adult Low Dose, Aspirin Regimen Bayer Regular Strength Caplets, Aspir-Low, Aspirtab, Aspirtab-Max, Backache Caplets, Bayer Children's Aspirin, Bayer Select Maximum Strength Backache Pain Relief Formula, Bufferin Caplets, Bufferin Tablets, Buffex, Buffinol, Buffinol Extra, Cama Arthritis Pain Reliever, CMT, Cope, Disalcid, Doan's Regular Strength Tablets, Easprin, Ecotrin Caplets, Ecotrin Tablets, Empirin, Extended-release Bayer 8-Hour, Extra Strength Bayer Arthritis Pain Formula Caplets, Extra Strength Bayer Aspirin Caplets, Extra Strength Bayer Aspirin Tablets, Extra Strength Bayer Plus Caplets, Gensan, Genuine Bayer Aspirin Caplets, Genuine Bayer Aspirin Tablets, Halfprin, Healthprin Adult Low Strength, Healthprin Full Strength, Healthprin Half-Dose, Magan, Magnaprin, Marthritic, Maximum Strength Arthritis. Foundation Safety Coated Aspirin, Maximum Strength Ascriptin, Maximum Strength Doan's Analgesic Caplets, Mobidin. Mono-Gesic, Norwich Aspirin, P-A-C Revised Formula, Regular Strength Ascriptin, Salflex, Salsitab, Sloprin, St. Joseph Adult Chewable Aspirin, Tricosal, Trilisate, and ZORprin.
[0015] Aspirin has been described in numerous patents such as, for example, in U.S. Pat. No. 4,520,09 to Dunn for "Sustained Released Aspirin Formulation"; U.S. Pat. No. 4,716,042 to Blank et al. for "Stabilized Coated Aspirin Tablets"; U.S. Pat. No. 5,157,030 to Galat for "Rapidly Soluble Aspirin Compositions and Method"; U.S. Pat. No. 5,723,453 to Phykitt for "Stabilized, Water-Soluble Aspirin Composition"; and U.S. Reissued Pat. No. RE38,576 to Blahut for "Stabilized Aspirin Compositions and Method of Preparation for Oral and Topical Use".
B. Background Regarding Nanoparticulate Active Agent Compositions
[0016] Nanoparticulate active agent compositions, first described in U.S. Pat. No. 5,145,684 ("the '684 patent"), are particles consisting of a poorly soluble therapeutic or diagnostic agent having adsorbed onto the surface thereof a non-crosslinked surface stabilizer. The '684 patent does not describe nanoparticulate compositions of clopidogrel and aspirin combination.
[0017] Methods of making nanoparticulate active agent compositions are described in, for example, U.S. Pat. Nos. 5,518,187 and 5,862,999, both for "Method of Grinding Pharmaceutical Substances;" U.S. Pat. No. 5,718,388, for "Continuous Method of Grinding Pharmaceutical Substances;" and U.S. Pat. No. 5,510,118 for "Process of Preparing Therapeutic Compositions Containing Nanoparticles."
folgendes Patent für nanoformuliertes Clopidogrel in Kombination mit Aspirin:
Building IP: Patent Application re Nanoparticulate Clopidogrel and Aspirin Combo Formulations
United States Patent Application 20070003615
Kind Code A1
Jenkins; Scott ; et al. January 4, 2007
Nanoparticulate clopidogrel and aspirin combination formulations
Abstract
The present invention is directed to compositions comprising a nanoparticulate clopidogrel and aspirin combination, or salts or derivatives thereof, having improved clopidogrel bioavailability. The nanoparticulate clopidogrel particles, and optionally the nanoparticulate aspirin particles, of the composition have an effective average particle size of less than about 2000 nm and are useful in the prevention and treatment of pathologies induced by platelet aggregation. The clopidogrel and aspirin particles may also be formulated as a controlled release polymeric coating or matrix drug delivery system.
Inventors: Jenkins; Scott; (Downingtown, PA) ; Liversidge; Gary G.; (West Chester, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name and Adress: Elan Pharma International Limited
Serial No.: 450890
Series Code: 11
Filed: June 12, 2006
FIELD OF INVENTION
[0002] The present invention relates generally to compounds and compositions useful in the prevention and treatment of pathological states induced by platelet aggregation. More specifically, the invention relates to nanoparticulate clopidogrel combined with aspirin, optionally in a nanoparticulate form, or salts or derivatives thereof (referred to herein as "nanoparticulate clopidogrel and aspirin combination"), and compositions comprising the same. The nanoparticulate clopidogrel, and optionally the aspirin, within the combination compositions have an effective average particle size of less than about 2000 nm. The clopidogrel and/or aspirin particles may also be coated with any one of a number of polymeric materials for a controlled and/or delayed release formulation.
BACKGROUND OF INVENTION
A. Background Regarding Clopidogrel
[0003] Clopidogrel is an inhibitor of platelet aggregation. Clopidogrel inhibits ADP-induced platelet aggregation by direct inhibition of adenosine diphosphate (ADP) binding to its receptor and of the subsequent ADP-mediated activation of the glycoprotein GPIIb/IIa complex. Clopidogrel also inhibits platelet aggregation induced by agonists other than ADP by blocking the amplification of platelet activation by released ADP.
[0004] The chemical name for clopidogrel bisulfate is methyl (+)-(S)-.alpha.-(2-chorophenyl)-6,7-dihydrothieno[3,2-c]pyridine-5(4H)-ac- etate sulfate (1:1). The empirical formula of clopidogrel bisulfate is C.sub.16H.sub.16ClNO.sub.2S.H.sub.2SO.sub.4 and its molecular weight is 419.9. The structural formula is as follows:
[0005] Clopidogrel bisulfate is a white to off-white powder. It is practically insoluble in water at neutral pH but is freely soluble at pH 1.0. It also dissolves freely in methanol, it dissolves sparingly in methylene chloride, and is practically insoluble in ethyl ether.
[0006] Clopidogrel bisulfate is commercially available under the trade name PLAVIX.RTM. by Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership (New York, N.Y.). PLAVIX.RTM. is administered as an oral tablet at a recommended dose of 75 mg once daily. PLAVIX.RTM. is provided as pink, round, biconvex, debossed film-coated tablets containing 97.875 mg of clopidogrel bisulfate which is the molar equivalent of 75 mg of clopidogrel base.
[0007] Clopidogrel bisulfate is indicated for the reduction of thrombotic events such as recent myocardial infarction (MI), recent stroke, or established arterial disease, and has been shown to reduce the rate of a combined end point of new ischemic stroke, new MI, and other vascular death. For patients with acute coronary syndrome, clopidogrel bisulfate has been shown to decrease the rate of a combined end point of cardiovascular death, MI, or stroke as well as the rate of a combined end point of cardiovascular death, MI, stroke, or refractory ischemia.
[0008] Clopidogrel has been described, for example, in U.S. Pat. Nos. 4,847,265 for "Dextro-Rotatory Enantiomer of Methyl Alpha-5 (4,5,6,7-Tetrahydro (3,2-c) Thieno Pyridyl) (2-Chlorophenyl)-Acetate and the Pharmaceutical Compositions Containing It", 5,576,328 for "Method for the Secondary Prevention of Ischemic Events", 5,989,578 for "Associations of Active Principles Containing Clopidogrel and an Anti-thrombotic Agent", 6,429,210 and 6,504,030 both for "Polymorphic Clopidogrel Hydrogen Sulphate Form", 6,635,763 for "Process to Prepare Clopidogrel", 6,737,411 and 6,800,759 both for "Racemization and Enantiomer Separation of Clopidogrel", and 6,858,734 for "Preparation of (S)-Clopidogrel and Related Compounds" These patents are hereby incorporated by reference.
[0009] Aspirin, also known as acetylsalicylic acid, is often used as an analgesic (against minor pains and aches), antipyretic (against fever), and anti-inflammatory. It has also an anticoagulant (blood thinning) effect and is used in long-term low-doses to prevent heart attacks.
[0010] Aspirin, CAS Number: 50-78-2, is chemically known as 2-acetoxybenzoic acid. Aspirin has a molecular formula of C.sub.9H.sub.8O.sub.4 and a molecular weight of 180.16. The chemical structure of aspirin is shown below:
[0011] Aspirin is a colorless or white crystals or white crystalline powder or granule. It is odorless or almost odorless with a slight acid taste. Aspirin has a melting point of 136.degree. C. (277.degree. F.) and boiling point of 140.degree. C. (284.degree. F.). It is soluble 1 gm. in 300 of water, 1 in 5-7 gm./ml. in alcohol, 1 in 17 gm./ml. of chloroform and 1 in 20 gm./ml. of ether; soluble in solutions of acetates and citrates and, with decomposition, in solutions of alkali hydroxides and carbonates. It is incompatible with free acids, acetanilide, aminopyrine, phenazone, hexamine, iron salts, phenobarbitone sodium, quinine salts, potassium and sodium iodides, and alkali hydroxides, carbonates, and stearates. Acetylsalicylic acid is stable in dry air, but gradually hydrolyses in contact with moisture to acetic and salicylic acids. In solution with alkalis, the hydrolysis proceeds rapidly and the clear solutions formed may consist entirely of acetate and salicylate. Acetylsalicylic acid decomposes rapidly in solutions of ammonium acetate or of the acetates, carbonates, citrates or hydroxides of the alkali metals.
[0012] Aspirin is indicated as an analgesic for the treatment of mild to moderate pain, as an anti-inflammatory agent for the treatment of soft tissue and joint inflammation, and as an antipyretic drug. Aspirin is generally dosed in adults for pain and fever in amounts of 300-1000 mg every 4 hour for a maximum of 4 gram per day. For acute polyarthritis rheumatica, dosing is generally 1 gram given 6 times a day for a maximum of 8 grams a day. For rheumatoid arthritis, dosing is generally 0.5 grams to 1 gram given 6 times a day for a maximum of 8 grams a day. For prevention of transient ischaemic attacks and prevention of arterial thrombosis, dosing is generally 300 mg to 1200 mg a day in 2 or 3 doses.
[0013] Aspirin is used to lessen the chance of heart attack, stroke, or other problems that may occur when a blood vessel is blocked by blood clots. Aspirin helps prevent dangerous blood clots from forming. Low-dose long-term aspirin irreversibly blocks formation of thromboxane A2 in platelets, producing an inhibitory affect on platelet aggregation, and this blood thinning property makes it useful for reducing the incidence of heart attacks. Aspirin produced for this purpose often has strengths of 75 mg, 81 mg or 325 mg enteric coated tablets. High doses of aspirin are also given immediately after an acute heart attack.
[0014] Various brands of aspirin sold in the United States that include, for example, Acuprin 81, Amigesic, Anacin, Caplets, Anacin Maximum Strength, Anacin Tablets, Anaflex 750, Arthritis Pain Ascriptin, Arthritis Pain Formula, Arthritis Strength Bufferin, Arthropan, Aspergum, Aspirin Regimen Bayer Adult Low Dose, Aspirin Regimen Bayer Regular Strength Caplets, Aspir-Low, Aspirtab, Aspirtab-Max, Backache Caplets, Bayer Children's Aspirin, Bayer Select Maximum Strength Backache Pain Relief Formula, Bufferin Caplets, Bufferin Tablets, Buffex, Buffinol, Buffinol Extra, Cama Arthritis Pain Reliever, CMT, Cope, Disalcid, Doan's Regular Strength Tablets, Easprin, Ecotrin Caplets, Ecotrin Tablets, Empirin, Extended-release Bayer 8-Hour, Extra Strength Bayer Arthritis Pain Formula Caplets, Extra Strength Bayer Aspirin Caplets, Extra Strength Bayer Aspirin Tablets, Extra Strength Bayer Plus Caplets, Gensan, Genuine Bayer Aspirin Caplets, Genuine Bayer Aspirin Tablets, Halfprin, Healthprin Adult Low Strength, Healthprin Full Strength, Healthprin Half-Dose, Magan, Magnaprin, Marthritic, Maximum Strength Arthritis. Foundation Safety Coated Aspirin, Maximum Strength Ascriptin, Maximum Strength Doan's Analgesic Caplets, Mobidin. Mono-Gesic, Norwich Aspirin, P-A-C Revised Formula, Regular Strength Ascriptin, Salflex, Salsitab, Sloprin, St. Joseph Adult Chewable Aspirin, Tricosal, Trilisate, and ZORprin.
[0015] Aspirin has been described in numerous patents such as, for example, in U.S. Pat. No. 4,520,09 to Dunn for "Sustained Released Aspirin Formulation"; U.S. Pat. No. 4,716,042 to Blank et al. for "Stabilized Coated Aspirin Tablets"; U.S. Pat. No. 5,157,030 to Galat for "Rapidly Soluble Aspirin Compositions and Method"; U.S. Pat. No. 5,723,453 to Phykitt for "Stabilized, Water-Soluble Aspirin Composition"; and U.S. Reissued Pat. No. RE38,576 to Blahut for "Stabilized Aspirin Compositions and Method of Preparation for Oral and Topical Use".
B. Background Regarding Nanoparticulate Active Agent Compositions
[0016] Nanoparticulate active agent compositions, first described in U.S. Pat. No. 5,145,684 ("the '684 patent"), are particles consisting of a poorly soluble therapeutic or diagnostic agent having adsorbed onto the surface thereof a non-crosslinked surface stabilizer. The '684 patent does not describe nanoparticulate compositions of clopidogrel and aspirin combination.
[0017] Methods of making nanoparticulate active agent compositions are described in, for example, U.S. Pat. Nos. 5,518,187 and 5,862,999, both for "Method of Grinding Pharmaceutical Substances;" U.S. Pat. No. 5,718,388, for "Continuous Method of Grinding Pharmaceutical Substances;" and U.S. Pat. No. 5,510,118 for "Process of Preparing Therapeutic Compositions Containing Nanoparticles."
...und das ist ebenfalls der "Hammer", das Patent für einen nanoformulierten Lipasehemmer, wobei in der patentschrift ein Bezug zu Tetrahydrolipstatin hergestellt wird. Tetrahydrolipstatin ist der Lipashemmer von Roche (generic name Orlistat, brand name Xenical) und generiert aktuell pro Quartal ca. 170 Mio CHF mit diesem Präparat. Zudem wurde eine nichtrezeptpflichtige Formulierung mit 60 mg Orlistat (120 mg/Tablette ist eine rezeptpoflichtige Formulierung) an GlaxoSmithKline Consumer Healthcare auslizenziert.
Building IP: Patent Application re Nanoparticulate Lipase Inhibitor Formulations
United States Patent Application 20060246141
Kind Code A1
Liversidge; Gary G. ; et al. November 2, 2006
Nanoparticulate lipase inhibitor formulations
Abstract
The invention relates to nanoparticulate lipase inhibitor compositions having improved pharmacokinetic profiles. The nanoparticulate lipase inhibitor compositions have an effective average particle size of less than about 2000 nm and are useful in the treatment of obesity and related diseases.
Inventors: Liversidge; Gary G.; (West Chester, PA) ; Jenkins; Scott; (Downingtown, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name and Adress: Elan Pharma International, Limited
FIELD OF THE INVENTION
[0002] The invention relates to nanoparticulate lipase inhibitor compositions, including nanoparticulate orlistat formulations, with an average effective particle size of less than about 2000 nm. The invention also relates to methods of preparing nanoparticulate lipase inhibitors as well as to methods of treating patients using the nanoparticulate lipase inhibitor compositions.
BACKGROUND OF THE INVENTION
A. Background Regarding Lipase Inhibitors
[0003] During the past two decades, obesity has become a major health crisis in the western industrialized countries. For example, obesity among both adults and children has risen significantly in the U.S.: the latest data from the National Center for Health Statistics show that 30 percent of U.S. adults 20 years of age and older--over 60 million people--are obese. More disturbing is that over 9 million children (aged 6-19) in the U.S. are obese, this is more than triple the number of obese children noted in 1980. Obesity, which leads to other severe health conditions including diabetes, heart disease and cancer, is now the most common nutritional disorder in western industrialized nations.
[0004] The recent health crisis has spurred research in weight control, including studies in diet, exercise, surgery and pharmaceutical preparations to help reduce weight gain. Because obesity is caused by a build up of fat in the body due to, for example, the over-consumption of high fat foods, drug design strategies have included the blocking or stimulating of various biomolecules and enzymes involved in fat metabolism. Some strategies have encompassed serotonin and noradrenaline re-uptake inhibitors, .beta.3-adrenoreceptor agonists, leptin agonists, melanocortin-3 agonists, cannabinoid (CB1) receptor blockers, and lipase inhibitors.
[0005] Lipase inhibitors have been recognized as an effective way to manage obesity by inhibiting the absorption of some dietary fats. Lipase is an enzyme that hydrolyzes lipids, the ester bonds in triglycerides, to form fatty acids and glycerol. Inhibitors of lipase, therefore, prevent the breakdown and subsequent use of the fatty acids and glycerols in the body.
[0006] 1. Orlistat
[0007] Orlistat, also known as tetrahydroplipstatin (THL), is a reversible inhibitor of lipases. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine residue site of gastric and pancreatic lipases. The inactivated enzymes are thus unavailable to hydrolyze dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control. Systemic absorption of the drug is therefore not needed for activity. At the recommended therapeutic dose of 120 mg three times a day, orlistat inhibits dietary fat absorption by approximately 30%.
[0008] The chemical name of orlistat is (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]-dodecyl ester. Its empirical formula is C.sub.29H.sub.53NO.sub.5, and its molecular weight is 495.7. It is a single diastereomeric molecule that contains four chiral centers, with a negative optical rotation in ethanol at 529 nm having the following structure:
[0009] Orlistat is a white to off-white crystalline powder. Orlistat is practically insoluble in water, freely soluble in chloroform, and very soluble in methanol and ethanol. Orlistat has no pKa within the physiological pH range.
[0010] Orlistat is offered under the registered trademark XENICAL.RTM. by Hoffman-La Roche Inc. of Nutley, N.J. XENICAL.RTM. is available for oral administration in dark-blue, hard-gelatin capsules, with light-blue imprinting. Each capsule contains 120 mg of the active ingredient, orlistat. The capsules also contain the inactive ingredients microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate, povidone, and talc. Each capsule shell contains gelatin, titanium dioxide, and FD&C Blue No. 1, with printing of pharmaceutical glaze NF, titanium dioxide, and FD&C Blue No. 1 aluminum lake.
[0011] The recommended dose of XENICAL.RTM. is one 120-mg capsule 3 times a day, once with each main meal containing fat (during or up to 1 hour after the meal).
[0012] Lipase inhibitors are described in, for example, U.S. Pat. No. 4,598,089 for "Leucine Derivatives" and 6,004,996 for "Tetrahydrolipstatin Containing Compositions." U.S. Pat. No. 4,598,089 describes the orlistat compound, compositions for administration of an orlistat compound, and methods of treating obesity and hyperlipaemia in an afflicted mammal wherein orlistat is administered. U.S. Pat. No. 6,004,996 describes orlistat-containing particles having improved stability against moisture and heat during production and storage.
Building IP: Patent Application re Nanoparticulate Lipase Inhibitor Formulations
United States Patent Application 20060246141
Kind Code A1
Liversidge; Gary G. ; et al. November 2, 2006
Nanoparticulate lipase inhibitor formulations
Abstract
The invention relates to nanoparticulate lipase inhibitor compositions having improved pharmacokinetic profiles. The nanoparticulate lipase inhibitor compositions have an effective average particle size of less than about 2000 nm and are useful in the treatment of obesity and related diseases.
Inventors: Liversidge; Gary G.; (West Chester, PA) ; Jenkins; Scott; (Downingtown, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name and Adress: Elan Pharma International, Limited
FIELD OF THE INVENTION
[0002] The invention relates to nanoparticulate lipase inhibitor compositions, including nanoparticulate orlistat formulations, with an average effective particle size of less than about 2000 nm. The invention also relates to methods of preparing nanoparticulate lipase inhibitors as well as to methods of treating patients using the nanoparticulate lipase inhibitor compositions.
BACKGROUND OF THE INVENTION
A. Background Regarding Lipase Inhibitors
[0003] During the past two decades, obesity has become a major health crisis in the western industrialized countries. For example, obesity among both adults and children has risen significantly in the U.S.: the latest data from the National Center for Health Statistics show that 30 percent of U.S. adults 20 years of age and older--over 60 million people--are obese. More disturbing is that over 9 million children (aged 6-19) in the U.S. are obese, this is more than triple the number of obese children noted in 1980. Obesity, which leads to other severe health conditions including diabetes, heart disease and cancer, is now the most common nutritional disorder in western industrialized nations.
[0004] The recent health crisis has spurred research in weight control, including studies in diet, exercise, surgery and pharmaceutical preparations to help reduce weight gain. Because obesity is caused by a build up of fat in the body due to, for example, the over-consumption of high fat foods, drug design strategies have included the blocking or stimulating of various biomolecules and enzymes involved in fat metabolism. Some strategies have encompassed serotonin and noradrenaline re-uptake inhibitors, .beta.3-adrenoreceptor agonists, leptin agonists, melanocortin-3 agonists, cannabinoid (CB1) receptor blockers, and lipase inhibitors.
[0005] Lipase inhibitors have been recognized as an effective way to manage obesity by inhibiting the absorption of some dietary fats. Lipase is an enzyme that hydrolyzes lipids, the ester bonds in triglycerides, to form fatty acids and glycerol. Inhibitors of lipase, therefore, prevent the breakdown and subsequent use of the fatty acids and glycerols in the body.
[0006] 1. Orlistat
[0007] Orlistat, also known as tetrahydroplipstatin (THL), is a reversible inhibitor of lipases. It exerts its therapeutic activity in the lumen of the stomach and small intestine by forming a covalent bond with the active serine residue site of gastric and pancreatic lipases. The inactivated enzymes are thus unavailable to hydrolyze dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides. As undigested triglycerides are not absorbed, the resulting caloric deficit may have a positive effect on weight control. Systemic absorption of the drug is therefore not needed for activity. At the recommended therapeutic dose of 120 mg three times a day, orlistat inhibits dietary fat absorption by approximately 30%.
[0008] The chemical name of orlistat is (S)-2-formylamino-4-methyl-pentanoic acid (S)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl]methyl]-dodecyl ester. Its empirical formula is C.sub.29H.sub.53NO.sub.5, and its molecular weight is 495.7. It is a single diastereomeric molecule that contains four chiral centers, with a negative optical rotation in ethanol at 529 nm having the following structure:
[0009] Orlistat is a white to off-white crystalline powder. Orlistat is practically insoluble in water, freely soluble in chloroform, and very soluble in methanol and ethanol. Orlistat has no pKa within the physiological pH range.
[0010] Orlistat is offered under the registered trademark XENICAL.RTM. by Hoffman-La Roche Inc. of Nutley, N.J. XENICAL.RTM. is available for oral administration in dark-blue, hard-gelatin capsules, with light-blue imprinting. Each capsule contains 120 mg of the active ingredient, orlistat. The capsules also contain the inactive ingredients microcrystalline cellulose, sodium starch glycolate, sodium lauryl sulfate, povidone, and talc. Each capsule shell contains gelatin, titanium dioxide, and FD&C Blue No. 1, with printing of pharmaceutical glaze NF, titanium dioxide, and FD&C Blue No. 1 aluminum lake.
[0011] The recommended dose of XENICAL.RTM. is one 120-mg capsule 3 times a day, once with each main meal containing fat (during or up to 1 hour after the meal).
[0012] Lipase inhibitors are described in, for example, U.S. Pat. No. 4,598,089 for "Leucine Derivatives" and 6,004,996 for "Tetrahydrolipstatin Containing Compositions." U.S. Pat. No. 4,598,089 describes the orlistat compound, compositions for administration of an orlistat compound, and methods of treating obesity and hyperlipaemia in an afflicted mammal wherein orlistat is administered. U.S. Pat. No. 6,004,996 describes orlistat-containing particles having improved stability against moisture and heat during production and storage.
...und es hört nicht auf spannender zu werden, denn anbei die Patentschrift zu nanoformulierten Benzodiazepinen, mit welchen Roche gross geworden ist (bekanntestes Benzo von Roche ist Valium).
Building IP: Patent Application re Aerosol and Injectable Formulations of Nanoparticulate Benzodiazepine
United States Patent Application 20060198896
Kind Code A1
Liversidge; Gary ; et al. September 7, 2006
Aerosol and injectable formulations of nanoparticulate benzodiazepine
Abstract
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Inventors: Liversidge; Gary; (West Chester, PA) ; Jenkins; Scott; (Downingtown, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name and Adress: Elan Pharma International Limited
The present invention is directed to aerosol and injectable formulations of nanoparticulate benzodiazepine, and preferably, nanoparticulate lorazepam. The compositions of the invention are useful in treating status epilepticus, sleep induction, acute psychosis, irritable bowel syndrome, and for pre-anesthesia medication. Also encompassed by the invention are methods of making and using such compositions.
BACKGROUND OF THE INVENTION
I. Administration Routes for Drugs
[0002] The route of administration of a drug substance can be critical to its pharmacological effectiveness. Various routes of administration exist, and all have their own advantages and disadvantages. Oral drug delivery of tablets, capsules, liquids, and the like is the most convenient approach to drug delivery, but many drug compounds are not amenable to oral administration. For example, modern protein drugs which are unstable in the acidic gastric environment or which are rapidly degraded by proteolytic enzymes in the digestive tract are poor candidates for oral administration. Similarly, poorly water soluble compounds which do not dissolve rapidly enough to be orally absorbed are likely to be ineffective when given as oral dosage forms. Oral administration can also be undesirable because drugs which are administered orally are generally distributed to all tissues in the body, and not just to the intended site of pharmacological activity. Alternative types of systemic administration are subcutaneous or intravenous injection. This approach avoids the gastrointestinal tract and therefore can be an effective route for delivery of proteins and peptides. However, these routes of administration have a low rate of patient compliance, especially for drugs such as insulin which must be administered one or more times daily. Additional alternative methods of drug delivery have been developed including transdermal, rectal, vaginal, intranasal, and pulmonary delivery.
[0003] Nasal drug delivery relies on inhalation of an aerosol through the nose so that active drug substance can reach the nasal mucosa. Drugs intended for systemic activity can be absorbed into the bloodstream because the nasal mucosa is highly vascularized. Alternatively, if the drug is intended to act topically, it is delivered directly to the site of activity and does not have to distribute throughout the body; hence, relatively low doses may be used. Examples of such drugs are decongestants, antihistamines, and anti-inflammatory steroids for seasonal allergic rhinitis.
[0004] Pulmonary drug delivery relies on inhalation of an aerosol through the mouth and throat so that the drug substance can reach the lung. For systemically active drugs, it is desirable for the drug particles to reach the alveolar region of the lung, whereas drugs which act on the smooth muscle of the conducting airways should preferentially deposit in the bronchiole region. Such drugs can include beta-agonists, anti cholinergics, and corticosteroids.
[0005] A. Droplet/Particle Size Determines Deposition Site
[0006] In developing a therapeutic aerosol, the aerodynamic size distribution of the inhaled particles is the single most important variable in defining the site of droplet or particle deposition in the patient; in short, it will determine whether drug targeting succeeds or fails. See P. Byron, "Aerosol Formulation, Generation, and Delivery Using Nonmetered Systems," Respiratory Drug Delivery, 144-151, 144 (CRC Press, 1989). Thus, a prerequisite in developing a therapeutic aerosol is a preferential particle size. The deposition of inhaled aerosols involves different mechanisms for different size particles. D. Swift (1980); Parodi et al., "Airborne Particles and Their Pulmonary Deposition," in Scientific Foundations of Respiratory Medicine, Scaddings et al. (eds.), pp. 545-557 (W. B. Saunders, Philadelphia, 1981); J. Heyder, "Mechanism of Aerosol Particle Deposition," Chest, 80:820-823 (1981).
[0007] Generally, inhaled particles are subject to deposition by one of two mechanisms: impaction, which usually predominates for larger particles, and sedimentation, which is prevalent for smaller particles. Impaction occurs when the momentum of an inhaled particle is large enough that the particle does not follow the air stream and encounters a physiological surface. In contrast, sedimentation occurs primarily in the deep lung when very small particles which have traveled with the inhaled air stream encounter physiological surfaces as a result of random diffusion within the air stream. For intranasally administered drug compounds which are inhaled through the nose, it is desirable for the drug to impact directly on the nasal mucosa; thus, large (ca. 5 to 100 .mu.m) particles or droplets are generally preferred for targeting of nasal delivery.
[0008] Pulmonary drug delivery is accomplished by inhalation of an aerosol through the mouth and throat. Particles having aerodynamic diameters of greater than about 5 microns generally do not reach the lung; instead, they tend to impact the back of the throat and are swallowed and possibly orally absorbed. Particles having diameters of about 2 to about 5 microns are small enough to reach the upper- to mid-pulmonary region (conducting airways), but are too large to reach the alveoli. Even smaller particles, i.e., about 0.5 to about 2 microns, are capable of reaching the alveolar region. Particles having diameters smaller than about 0.5 microns can also be deposited in the alveolar region by sedimentation, although very small particles may be exhaled.
[0009] B. Devices Used For Nasal And Pulmonary Drug Delivery
[0010] Drugs intended for intranasal delivery (systemic and local) can be administered as aqueous solutions or suspensions, as solutions or suspensions in halogenated hydrocarbon propellants (pressurized metered-dose inhalers), or as dry powders. Metered-dose spray pumps for aqueous formulations, pMDIs, and DPIs for nasal delivery are available from, for example, Valois of America or Pfeiffer of America.
[0011] Drugs intended for pulmonary delivery can also be administered as aqueous formulations, as suspensions or solutions in halogenated hydrocarbon propellants, or as dry powders. Aqueous formulations must be aerosolized by liquid nebulizers employing either hydraulic or ultrasonic atomization, propellant-based systems require suitable pressurized metered-dose inhalers (pMDIs), and dry powders require dry powder inhaler devices (DPIs) which are capable of dispersing the drug substance effectively. For aqueous and other non-pressurized liquid systems, a variety of nebulizers (including small volume nebulizers) are available to aerosolize the formulations. Compressor-driven nebulizers incorporate jet technology and use compressed air to generate the liquid aerosol. Such devices are commercially available from, for example, Healthdyne Technologies, Inc.; Invacare, Inc.; Mountain Medical Equipment, Inc.; Pari Respiratory, Inc.; Mada Medical, Inc.; Puritan-Bennet; Schuco, Inc., DeVilbiss Health Care, Inc.; and Hospitak, Inc. Ultrasonic nebulizers rely on mechanical energy in the form of vibration of a piezoelectric crystal to generate inhalable liquid droplets and are commercially available from, for example, Omron Heathcare, Inc. and DeVilbiss Health Care, Inc.
[0012] A propellant driven inhaler (pMDI) releases a metered dose of medicine upon each actuation. The medicine is formulated as a suspension or solution of a drug substance in a suitable propellant such as a halogenated hydrocarbon. pMDIs are described in, for example, Newman, S. P., Aerosols and the Lung, Clarke et al., eds., pp. 197-224 (Butterworths, London, England, 1984).
[0013] Dry powder inhalers (DPIs), which involve deaggregation and aerosolization of dry powders, normally rely upon a burst of inspired air that is drawn through the unit to deliver a drug dosage. Such devices are described in, for example, U.S. Pat. No. 4,807,814 to Douche et al., which is directed to a pneumatic powder ejector having a suction stage and an injection stage; SU 628930 (Abstract), describing a hand-held powder disperser having an axial air flow tube; Fox et al., Powder and Bulk Engineering, pages 33-36 (March 1988), describing a venturi eductor having an axial air inlet tube upstream of a venturi restriction; EP 347 779, describing a hand-held powder disperser having a collapsible expansion chamber, and U.S. Pat. No. 5,785,049 to Smith et al., directed to dry powder delivery devices for drugs.
[0014] C. Problems with Conventional Aerosol and Injectable Compositions and Methods
[0015] Conventional techniques are extremely inefficient in delivering agents to the lung for a variety of reasons. Prior to the present invention, attempts to develop inhalable aqueous suspensions of poorly water soluble drugs have been largely unsuccessful. For example, it has been reported that ultrasonic nebulization of a suspension containing fluorescein and latex drug spheres, representing insoluble drug particles, resulted in only 1% aerosolization of the particles, while air-jet nebulization resulted in only a fraction of particles being aerosolized (Susan L. Tiano, "Functionality Testing Used to Rationally Assess Performance of a Model Respiratory Solution or Suspension in a Nebulizer," Dissertation Abstracts International, 56/12-B, pp. 6578 (1995)). Another problem encountered with nebulization of liquid formulations prior to the present invention was the long (4-20 min) period of time required for administration of a therapeutic dose. Long administration times are required because conventional liquid formulations for nebulization are very dilute solutions or suspensions of micronized drug substance. Prolonged administration times are undesirable because they lessen patient compliance and make it difficult to control the dose administered. Lastly, aerosol formulations of micronized drug are not feasible for deep lung delivery of insoluble compounds because the droplets needed to reach the alveolar region (0.5 to 2 microns) are too small to accommodate micronized drug crystals, which are typically 2-3 microns or more in diameter.
[0016] Conventional pMDIs are also inefficient in delivering drug substance to the lung. In most cases, pMDIs consist of suspensions of micronized drug substance in halogenated hydrocarbons such as chlorofluorocarbons (CFCs) or hydrofluoroalkanes (HFAs). Actuation of the pMDI results in delivery of a metered dose of drug and propellant, both of which exit the device at high velocities because of the propellant pressures. The high velocity and momentum of the drug particles results in a high degree of oropharyngeal impaction as well as loss to the device used to deliver the agent. These losses lead to variability in therapeutic agent levels and poor therapeutic control. In addition, oropharyngeal deposition of drugs intended for topical administration to the conducting airways (such as corticosteroids) can lead to systemic absorption with resultant undesirable side effects. Additionally, conventional micronization (air-jet milling) of pure drug substance can reduce the drug particle size to no less than about 2-3 microns. Thus, the micronized material typically used in pMDIs is inherently unsuitable for delivery to the alveolar region and is not expected to deposit below the central bronchiole region of the lung.
[0017] Prior to the present invention, delivery of dry powders to the lung typically used micronized drug substance. In the dry powder form, micronized substances tend to have substantial interparticle electrostatic attractive forces which prevent the powders from flowing smoothly and generally make them difficult to disperse. Thus, two key challenges to pulmonary delivery of dry powders are the ability of the device to accurately meter the intended dose and the ability of the device to fully disperse the micronized particles. For many devices and formulations, the extent of dispersion is dependent upon the patient's inspiration rate, which itself may be variable and can lead to a variability in the delivered dose.
[0018] Delivery of drugs to the nasal mucosa can also be accomplished with aqueous, propellant-based, or dry powder formulations. However, absorption of poorly soluble drugs can be problematic because of mucociliary clearance which transports deposited particles from the nasal mucosa to the throat where they are swallowed. Complete clearance generally occurs within about 15-20 minutes. Thus, poorly soluble drugs which do not dissolve within this time frame are unavailable for either local or systemic activity.
[0019] As described below in the Background of Nanoparticulate Active Agent Compositions, several published U.S. patents and patent applications describe aerosols of nanoparticulate drugs. However, none of these documents describe aerosols of a nanoparticulate benzodiazepine, such as lorazepam.
II. Background Regarding Lorazepam
[0020] Lorazepam is a benzodiazepine. It is also known as 7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-- one. Its molecular formula is C.sub.15H.sub.10Cl.sub.2N.sub.2O.sub.2, and it has a molecular weight of 321.16. Lorazepam has only slight solubility in water, i.e., 0.08 mg/mL. U.S. Pat. No. 6,699,849 to Loftsson et al., which is specifically incorporated by reference, refers to lorazepam and benzodiazepine. Lorazepam is a controlled substance. Merck Index, Thirteenth Ed., p. 999 (Merck & Co., Whitehouse Station, N.J. 2001). As pharmaceutically acceptable salts including organic salts or esters of lorazepam can be employed as a substitute for lorazepam, the references below to lorazepam are also intended to include lorazepam salts and esters and mixtures thereof.
[0021] Because of lorazepam's low water solubility, it is generally formulated for oral administration. However, oral administration of lorazepam has disadvantages. For example, lorazepam is susceptible to enzymatic degradation by glucuronyl transferase enzyme in the intestine or in the intestinal mucosa, as disclosed in U.S. Pat. No. 6,692,766 to Rubinstein et al., which is incorporated by reference. Sterile lorazepam typically includes a preservative such as benzyl alcohol and requires refrigeration. Lorazepam delivered orally may have a slow absorption and onset of action.
[0022] Injectable formulations of lorazepam are preferable over oral administration doses because intravenous (IV) or intramuscular (IM) administration of a drug results in a significantly shorter response time as compared to oral administration. Moreover, injectable formulations of pain medication are also preferable for post-operative health care, where oral administration may not be feasible. Injectable formulations of lorazepam are particularly preferred, as lorazepam is not addictive, in contrast to other injectable formulations of drugs, such as morphine and ketorolac (Toradol.RTM.).
[0023] However, injectable lorazepam formulations are difficult to formulate due to the low water-solubility of lorazepam. Moreover, current injectable formulations of lorazepam are undesirable because the formulations must include polyethylene glycol and propylene glycol as solubilizers, which can result in pain at the injection site.
There remains a need in the art for improved dosage forms of benzodiazepines, such as lorazepam. The present invention satisfies this need.
SUMMARY OF THE INVENTION
[0029] The present invention is directed to the surprising and unexpected discovery of new aerosol and injectable dosage forms of a nanoparticulate benzodiazepine, such as lorazepam. The formulations comprises a nanoparticulate benzodiazepine, such as nanoparticulate lorazepam, having an effective average particle size of less than about 2000 nm. The nanoparticulate benzodiazepine, such as lorazepam, preferably has at least one surface stabilizer either adsorbed onto or associated with the surface of the benzodizepine. In one embodiment of the invention, the surface stabilizer is a povidone polymer. Because lorazepam is practically insoluble in water, significant bioavailability can be problematic.
[0030] In one embodiment there is provided an aerosol that delivers an optimal dosage of a benzodiazepine, such as lorazepam. The aerosols of the invention do not require a preservative such as benzyl alcohol, which affects lorazepam stability.
[0031] In another embodiment, a safe and effective injectable formulation of a benzodiazepine, such as lorazepam, is provided. The injectable formulation eliminates the need for propylene glycol and polyethylene glycol, such as polyoxyl 60 hydrogenated castor oil (HCO-60), as solubilizers for injectable lorazepam compositions, and solves the problem of the insolubility of lorazepam in water. This is beneficial, as in convention non-nanoparticulate injectable benzodiazepine formulations comprising polyoxyl 60 hydrogenated castor oil as a solubilizer, the presence of this solubilizer can lead to anaphylactic shock (i.e., severe allergic reaction) and death. The injectable dosage forms of the invention surprisingly deliver the required therapeutic amount of the drug in vivo, and render the drug bioavailable in a rapid and constant manner, which is required for effective human therapy. Moreover, the invention provides for compositions comprising high concentrations of a benzodiazepine, such as lorazepam, in low injection volumes, with rapid drug dissolution upon administration.
[0032] The present invention is also directed to aqueous, propellant-based, and dry powder aerosols of a nanoparticulate benzodiazepine, such as lorazepam, for pulmonary and nasal delivery, in which essentially every inhaled particle contains at least one nanoparticulate benzodiazepine, such as lorazepam, nanoparticle. The nanoparticulate benzodiazepine, such as lorazepam, is highly water-insoluble. Preferably, the nanoparticulate benzodiazepine, such as lorazepam, has an effective average particle size of less than about 2 microns. Nanoparticulate aerosol formulations are described in U.S. Pat. No. 6,811,767 to Bosch et al., specifically incorporated by reference. Non-aerosol preparations of submicron sized water-insoluble drugs are described in U.S. Pat. No. 5,145,684 to Liversidge et al., specifically incorporated herein by reference.
[0033] The invention also includes the following embodiments directed to aerosol formulations of a benzodiazepine, such as lorazepam. One embodiment of the invention is directed to aqueous aerosols of nanoparticulate dispersion of a benzodiazepine, such as lorazepam. Another embodiment of the invention is directed to dry powder aerosol formulations comprising a benzodiazepine, such as lorazepam, for pulmonary and/or nasal administration. Yet another embodiment of the invention is directed to a process and composition for propellant-based systems comprising a nanoparticulate benzodiazepine, such as lorazepam.
[0034] The nanoparticulate benzodiazepine, such as lorazepam, formulations of the invention may optionally include one or more pharmaceutically acceptable excipients, such as non-toxic physiologically acceptable liquid carriers, pH adjusting agents, or preservatives.
[0035] In another aspect of the invention there is provided a method of preparing the nanoparticulate benzodiazepine, such as lorazepam, injectable and aerosol formulations of the invention. The nanoparticulate dispersions used in making aerosol and injectable nanoparticulate benzodiazepine compositions can be made by wet milling, homogenization, precipitation, or supercritical fluid methods known in the art. An exemplary method comprises: (1) dispersing a benzodiazepine, such as lorazepam, in a liquid dispersion media; and (2) mechanically reducing the particle size of the benzodiazepine to the desired effective average particle size, e.g., less than about 2000 nm. At least one surface stabilizer can be added to the dispersion media either before, during, or after particle size reduction of the benzodiazepine. In one embodiment for the injectable composition, the surface stabilizer is a povidone polymer with a molecular weight of less than about 40,000 daltons. Preferably, the liquid dispersion media is maintained at a physiologic pH, for example, within the range of from about 3 to about 8, during the size reduction process. The nanoparticulate benzodiazepine dispersion can be used as an injectable formulation.
[0036] Dry powders comprising a nanoparticulate benzodiazepine, such as lorazepam, can be made by spray drying or freeze-drying aqueous dispersions of the nanoparticles. The dispersions used in these systems may or may not comprise dissolved diluent material prior to drying. Additionally, both pressurized and non-pressurized milling operations can be employed to make nanoparticulate benzodiazepine, such as lorazepam, compositions in non-aqueous systems.
[0037] In yet another aspect of the invention, there is provided a method of treating a subject in need with the injectable and/or aerosol nanoparticulate benzodiazepine, such as lorazepam, compositions of the invention. In an exemplary method, therapeutically effective amount of an injectable or aerosol nanoparticulate benzodiazepine composition of the invention is administered to a subject in need. The methods of the invention encompass treating a subject for status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and pre-anesthesia medication. Diagnostic methods, comprising imaging of the administered dosage form, are also encompassed by the invention.
[0038] It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory and are intended to provide further explanation of the invention as claimed. Other objects, advantages, and novel features will be readily apparent to those skilled in the art from the following detailed description of the invention.
Building IP: Patent Application re Aerosol and Injectable Formulations of Nanoparticulate Benzodiazepine
United States Patent Application 20060198896
Kind Code A1
Liversidge; Gary ; et al. September 7, 2006
Aerosol and injectable formulations of nanoparticulate benzodiazepine
Abstract
Described are nanoparticulate formulations of a benzodiazepine, such as lorazepam, that does not require the presence of polyethylene glycol and propylene glycol as stabilizers, and methods of making and using such formulations. The formulations are particularly useful in aerosol and injectable dosage forms, and comprise nanoparticulate benzodiazepine, such as lorazepam, and at least one surface stabilizer. The formulations are useful in the treatment of status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and as a pre-anesthesia medication.
Inventors: Liversidge; Gary; (West Chester, PA) ; Jenkins; Scott; (Downingtown, PA)
Correspondence Name and Address: ELAN DRUG DELIVERY, INC.;C/O FOLEY & LARDNER LLP
3000 K STREET, N.W.
SUITE 500
WASHINGTON
DC
20007-5109
US
Assignee Name and Adress: Elan Pharma International Limited
The present invention is directed to aerosol and injectable formulations of nanoparticulate benzodiazepine, and preferably, nanoparticulate lorazepam. The compositions of the invention are useful in treating status epilepticus, sleep induction, acute psychosis, irritable bowel syndrome, and for pre-anesthesia medication. Also encompassed by the invention are methods of making and using such compositions.
BACKGROUND OF THE INVENTION
I. Administration Routes for Drugs
[0002] The route of administration of a drug substance can be critical to its pharmacological effectiveness. Various routes of administration exist, and all have their own advantages and disadvantages. Oral drug delivery of tablets, capsules, liquids, and the like is the most convenient approach to drug delivery, but many drug compounds are not amenable to oral administration. For example, modern protein drugs which are unstable in the acidic gastric environment or which are rapidly degraded by proteolytic enzymes in the digestive tract are poor candidates for oral administration. Similarly, poorly water soluble compounds which do not dissolve rapidly enough to be orally absorbed are likely to be ineffective when given as oral dosage forms. Oral administration can also be undesirable because drugs which are administered orally are generally distributed to all tissues in the body, and not just to the intended site of pharmacological activity. Alternative types of systemic administration are subcutaneous or intravenous injection. This approach avoids the gastrointestinal tract and therefore can be an effective route for delivery of proteins and peptides. However, these routes of administration have a low rate of patient compliance, especially for drugs such as insulin which must be administered one or more times daily. Additional alternative methods of drug delivery have been developed including transdermal, rectal, vaginal, intranasal, and pulmonary delivery.
[0003] Nasal drug delivery relies on inhalation of an aerosol through the nose so that active drug substance can reach the nasal mucosa. Drugs intended for systemic activity can be absorbed into the bloodstream because the nasal mucosa is highly vascularized. Alternatively, if the drug is intended to act topically, it is delivered directly to the site of activity and does not have to distribute throughout the body; hence, relatively low doses may be used. Examples of such drugs are decongestants, antihistamines, and anti-inflammatory steroids for seasonal allergic rhinitis.
[0004] Pulmonary drug delivery relies on inhalation of an aerosol through the mouth and throat so that the drug substance can reach the lung. For systemically active drugs, it is desirable for the drug particles to reach the alveolar region of the lung, whereas drugs which act on the smooth muscle of the conducting airways should preferentially deposit in the bronchiole region. Such drugs can include beta-agonists, anti cholinergics, and corticosteroids.
[0005] A. Droplet/Particle Size Determines Deposition Site
[0006] In developing a therapeutic aerosol, the aerodynamic size distribution of the inhaled particles is the single most important variable in defining the site of droplet or particle deposition in the patient; in short, it will determine whether drug targeting succeeds or fails. See P. Byron, "Aerosol Formulation, Generation, and Delivery Using Nonmetered Systems," Respiratory Drug Delivery, 144-151, 144 (CRC Press, 1989). Thus, a prerequisite in developing a therapeutic aerosol is a preferential particle size. The deposition of inhaled aerosols involves different mechanisms for different size particles. D. Swift (1980); Parodi et al., "Airborne Particles and Their Pulmonary Deposition," in Scientific Foundations of Respiratory Medicine, Scaddings et al. (eds.), pp. 545-557 (W. B. Saunders, Philadelphia, 1981); J. Heyder, "Mechanism of Aerosol Particle Deposition," Chest, 80:820-823 (1981).
[0007] Generally, inhaled particles are subject to deposition by one of two mechanisms: impaction, which usually predominates for larger particles, and sedimentation, which is prevalent for smaller particles. Impaction occurs when the momentum of an inhaled particle is large enough that the particle does not follow the air stream and encounters a physiological surface. In contrast, sedimentation occurs primarily in the deep lung when very small particles which have traveled with the inhaled air stream encounter physiological surfaces as a result of random diffusion within the air stream. For intranasally administered drug compounds which are inhaled through the nose, it is desirable for the drug to impact directly on the nasal mucosa; thus, large (ca. 5 to 100 .mu.m) particles or droplets are generally preferred for targeting of nasal delivery.
[0008] Pulmonary drug delivery is accomplished by inhalation of an aerosol through the mouth and throat. Particles having aerodynamic diameters of greater than about 5 microns generally do not reach the lung; instead, they tend to impact the back of the throat and are swallowed and possibly orally absorbed. Particles having diameters of about 2 to about 5 microns are small enough to reach the upper- to mid-pulmonary region (conducting airways), but are too large to reach the alveoli. Even smaller particles, i.e., about 0.5 to about 2 microns, are capable of reaching the alveolar region. Particles having diameters smaller than about 0.5 microns can also be deposited in the alveolar region by sedimentation, although very small particles may be exhaled.
[0009] B. Devices Used For Nasal And Pulmonary Drug Delivery
[0010] Drugs intended for intranasal delivery (systemic and local) can be administered as aqueous solutions or suspensions, as solutions or suspensions in halogenated hydrocarbon propellants (pressurized metered-dose inhalers), or as dry powders. Metered-dose spray pumps for aqueous formulations, pMDIs, and DPIs for nasal delivery are available from, for example, Valois of America or Pfeiffer of America.
[0011] Drugs intended for pulmonary delivery can also be administered as aqueous formulations, as suspensions or solutions in halogenated hydrocarbon propellants, or as dry powders. Aqueous formulations must be aerosolized by liquid nebulizers employing either hydraulic or ultrasonic atomization, propellant-based systems require suitable pressurized metered-dose inhalers (pMDIs), and dry powders require dry powder inhaler devices (DPIs) which are capable of dispersing the drug substance effectively. For aqueous and other non-pressurized liquid systems, a variety of nebulizers (including small volume nebulizers) are available to aerosolize the formulations. Compressor-driven nebulizers incorporate jet technology and use compressed air to generate the liquid aerosol. Such devices are commercially available from, for example, Healthdyne Technologies, Inc.; Invacare, Inc.; Mountain Medical Equipment, Inc.; Pari Respiratory, Inc.; Mada Medical, Inc.; Puritan-Bennet; Schuco, Inc., DeVilbiss Health Care, Inc.; and Hospitak, Inc. Ultrasonic nebulizers rely on mechanical energy in the form of vibration of a piezoelectric crystal to generate inhalable liquid droplets and are commercially available from, for example, Omron Heathcare, Inc. and DeVilbiss Health Care, Inc.
[0012] A propellant driven inhaler (pMDI) releases a metered dose of medicine upon each actuation. The medicine is formulated as a suspension or solution of a drug substance in a suitable propellant such as a halogenated hydrocarbon. pMDIs are described in, for example, Newman, S. P., Aerosols and the Lung, Clarke et al., eds., pp. 197-224 (Butterworths, London, England, 1984).
[0013] Dry powder inhalers (DPIs), which involve deaggregation and aerosolization of dry powders, normally rely upon a burst of inspired air that is drawn through the unit to deliver a drug dosage. Such devices are described in, for example, U.S. Pat. No. 4,807,814 to Douche et al., which is directed to a pneumatic powder ejector having a suction stage and an injection stage; SU 628930 (Abstract), describing a hand-held powder disperser having an axial air flow tube; Fox et al., Powder and Bulk Engineering, pages 33-36 (March 1988), describing a venturi eductor having an axial air inlet tube upstream of a venturi restriction; EP 347 779, describing a hand-held powder disperser having a collapsible expansion chamber, and U.S. Pat. No. 5,785,049 to Smith et al., directed to dry powder delivery devices for drugs.
[0014] C. Problems with Conventional Aerosol and Injectable Compositions and Methods
[0015] Conventional techniques are extremely inefficient in delivering agents to the lung for a variety of reasons. Prior to the present invention, attempts to develop inhalable aqueous suspensions of poorly water soluble drugs have been largely unsuccessful. For example, it has been reported that ultrasonic nebulization of a suspension containing fluorescein and latex drug spheres, representing insoluble drug particles, resulted in only 1% aerosolization of the particles, while air-jet nebulization resulted in only a fraction of particles being aerosolized (Susan L. Tiano, "Functionality Testing Used to Rationally Assess Performance of a Model Respiratory Solution or Suspension in a Nebulizer," Dissertation Abstracts International, 56/12-B, pp. 6578 (1995)). Another problem encountered with nebulization of liquid formulations prior to the present invention was the long (4-20 min) period of time required for administration of a therapeutic dose. Long administration times are required because conventional liquid formulations for nebulization are very dilute solutions or suspensions of micronized drug substance. Prolonged administration times are undesirable because they lessen patient compliance and make it difficult to control the dose administered. Lastly, aerosol formulations of micronized drug are not feasible for deep lung delivery of insoluble compounds because the droplets needed to reach the alveolar region (0.5 to 2 microns) are too small to accommodate micronized drug crystals, which are typically 2-3 microns or more in diameter.
[0016] Conventional pMDIs are also inefficient in delivering drug substance to the lung. In most cases, pMDIs consist of suspensions of micronized drug substance in halogenated hydrocarbons such as chlorofluorocarbons (CFCs) or hydrofluoroalkanes (HFAs). Actuation of the pMDI results in delivery of a metered dose of drug and propellant, both of which exit the device at high velocities because of the propellant pressures. The high velocity and momentum of the drug particles results in a high degree of oropharyngeal impaction as well as loss to the device used to deliver the agent. These losses lead to variability in therapeutic agent levels and poor therapeutic control. In addition, oropharyngeal deposition of drugs intended for topical administration to the conducting airways (such as corticosteroids) can lead to systemic absorption with resultant undesirable side effects. Additionally, conventional micronization (air-jet milling) of pure drug substance can reduce the drug particle size to no less than about 2-3 microns. Thus, the micronized material typically used in pMDIs is inherently unsuitable for delivery to the alveolar region and is not expected to deposit below the central bronchiole region of the lung.
[0017] Prior to the present invention, delivery of dry powders to the lung typically used micronized drug substance. In the dry powder form, micronized substances tend to have substantial interparticle electrostatic attractive forces which prevent the powders from flowing smoothly and generally make them difficult to disperse. Thus, two key challenges to pulmonary delivery of dry powders are the ability of the device to accurately meter the intended dose and the ability of the device to fully disperse the micronized particles. For many devices and formulations, the extent of dispersion is dependent upon the patient's inspiration rate, which itself may be variable and can lead to a variability in the delivered dose.
[0018] Delivery of drugs to the nasal mucosa can also be accomplished with aqueous, propellant-based, or dry powder formulations. However, absorption of poorly soluble drugs can be problematic because of mucociliary clearance which transports deposited particles from the nasal mucosa to the throat where they are swallowed. Complete clearance generally occurs within about 15-20 minutes. Thus, poorly soluble drugs which do not dissolve within this time frame are unavailable for either local or systemic activity.
[0019] As described below in the Background of Nanoparticulate Active Agent Compositions, several published U.S. patents and patent applications describe aerosols of nanoparticulate drugs. However, none of these documents describe aerosols of a nanoparticulate benzodiazepine, such as lorazepam.
II. Background Regarding Lorazepam
[0020] Lorazepam is a benzodiazepine. It is also known as 7-Chloro-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-- one. Its molecular formula is C.sub.15H.sub.10Cl.sub.2N.sub.2O.sub.2, and it has a molecular weight of 321.16. Lorazepam has only slight solubility in water, i.e., 0.08 mg/mL. U.S. Pat. No. 6,699,849 to Loftsson et al., which is specifically incorporated by reference, refers to lorazepam and benzodiazepine. Lorazepam is a controlled substance. Merck Index, Thirteenth Ed., p. 999 (Merck & Co., Whitehouse Station, N.J. 2001). As pharmaceutically acceptable salts including organic salts or esters of lorazepam can be employed as a substitute for lorazepam, the references below to lorazepam are also intended to include lorazepam salts and esters and mixtures thereof.
[0021] Because of lorazepam's low water solubility, it is generally formulated for oral administration. However, oral administration of lorazepam has disadvantages. For example, lorazepam is susceptible to enzymatic degradation by glucuronyl transferase enzyme in the intestine or in the intestinal mucosa, as disclosed in U.S. Pat. No. 6,692,766 to Rubinstein et al., which is incorporated by reference. Sterile lorazepam typically includes a preservative such as benzyl alcohol and requires refrigeration. Lorazepam delivered orally may have a slow absorption and onset of action.
[0022] Injectable formulations of lorazepam are preferable over oral administration doses because intravenous (IV) or intramuscular (IM) administration of a drug results in a significantly shorter response time as compared to oral administration. Moreover, injectable formulations of pain medication are also preferable for post-operative health care, where oral administration may not be feasible. Injectable formulations of lorazepam are particularly preferred, as lorazepam is not addictive, in contrast to other injectable formulations of drugs, such as morphine and ketorolac (Toradol.RTM.).
[0023] However, injectable lorazepam formulations are difficult to formulate due to the low water-solubility of lorazepam. Moreover, current injectable formulations of lorazepam are undesirable because the formulations must include polyethylene glycol and propylene glycol as solubilizers, which can result in pain at the injection site.
There remains a need in the art for improved dosage forms of benzodiazepines, such as lorazepam. The present invention satisfies this need.
SUMMARY OF THE INVENTION
[0029] The present invention is directed to the surprising and unexpected discovery of new aerosol and injectable dosage forms of a nanoparticulate benzodiazepine, such as lorazepam. The formulations comprises a nanoparticulate benzodiazepine, such as nanoparticulate lorazepam, having an effective average particle size of less than about 2000 nm. The nanoparticulate benzodiazepine, such as lorazepam, preferably has at least one surface stabilizer either adsorbed onto or associated with the surface of the benzodizepine. In one embodiment of the invention, the surface stabilizer is a povidone polymer. Because lorazepam is practically insoluble in water, significant bioavailability can be problematic.
[0030] In one embodiment there is provided an aerosol that delivers an optimal dosage of a benzodiazepine, such as lorazepam. The aerosols of the invention do not require a preservative such as benzyl alcohol, which affects lorazepam stability.
[0031] In another embodiment, a safe and effective injectable formulation of a benzodiazepine, such as lorazepam, is provided. The injectable formulation eliminates the need for propylene glycol and polyethylene glycol, such as polyoxyl 60 hydrogenated castor oil (HCO-60), as solubilizers for injectable lorazepam compositions, and solves the problem of the insolubility of lorazepam in water. This is beneficial, as in convention non-nanoparticulate injectable benzodiazepine formulations comprising polyoxyl 60 hydrogenated castor oil as a solubilizer, the presence of this solubilizer can lead to anaphylactic shock (i.e., severe allergic reaction) and death. The injectable dosage forms of the invention surprisingly deliver the required therapeutic amount of the drug in vivo, and render the drug bioavailable in a rapid and constant manner, which is required for effective human therapy. Moreover, the invention provides for compositions comprising high concentrations of a benzodiazepine, such as lorazepam, in low injection volumes, with rapid drug dissolution upon administration.
[0032] The present invention is also directed to aqueous, propellant-based, and dry powder aerosols of a nanoparticulate benzodiazepine, such as lorazepam, for pulmonary and nasal delivery, in which essentially every inhaled particle contains at least one nanoparticulate benzodiazepine, such as lorazepam, nanoparticle. The nanoparticulate benzodiazepine, such as lorazepam, is highly water-insoluble. Preferably, the nanoparticulate benzodiazepine, such as lorazepam, has an effective average particle size of less than about 2 microns. Nanoparticulate aerosol formulations are described in U.S. Pat. No. 6,811,767 to Bosch et al., specifically incorporated by reference. Non-aerosol preparations of submicron sized water-insoluble drugs are described in U.S. Pat. No. 5,145,684 to Liversidge et al., specifically incorporated herein by reference.
[0033] The invention also includes the following embodiments directed to aerosol formulations of a benzodiazepine, such as lorazepam. One embodiment of the invention is directed to aqueous aerosols of nanoparticulate dispersion of a benzodiazepine, such as lorazepam. Another embodiment of the invention is directed to dry powder aerosol formulations comprising a benzodiazepine, such as lorazepam, for pulmonary and/or nasal administration. Yet another embodiment of the invention is directed to a process and composition for propellant-based systems comprising a nanoparticulate benzodiazepine, such as lorazepam.
[0034] The nanoparticulate benzodiazepine, such as lorazepam, formulations of the invention may optionally include one or more pharmaceutically acceptable excipients, such as non-toxic physiologically acceptable liquid carriers, pH adjusting agents, or preservatives.
[0035] In another aspect of the invention there is provided a method of preparing the nanoparticulate benzodiazepine, such as lorazepam, injectable and aerosol formulations of the invention. The nanoparticulate dispersions used in making aerosol and injectable nanoparticulate benzodiazepine compositions can be made by wet milling, homogenization, precipitation, or supercritical fluid methods known in the art. An exemplary method comprises: (1) dispersing a benzodiazepine, such as lorazepam, in a liquid dispersion media; and (2) mechanically reducing the particle size of the benzodiazepine to the desired effective average particle size, e.g., less than about 2000 nm. At least one surface stabilizer can be added to the dispersion media either before, during, or after particle size reduction of the benzodiazepine. In one embodiment for the injectable composition, the surface stabilizer is a povidone polymer with a molecular weight of less than about 40,000 daltons. Preferably, the liquid dispersion media is maintained at a physiologic pH, for example, within the range of from about 3 to about 8, during the size reduction process. The nanoparticulate benzodiazepine dispersion can be used as an injectable formulation.
[0036] Dry powders comprising a nanoparticulate benzodiazepine, such as lorazepam, can be made by spray drying or freeze-drying aqueous dispersions of the nanoparticles. The dispersions used in these systems may or may not comprise dissolved diluent material prior to drying. Additionally, both pressurized and non-pressurized milling operations can be employed to make nanoparticulate benzodiazepine, such as lorazepam, compositions in non-aqueous systems.
[0037] In yet another aspect of the invention, there is provided a method of treating a subject in need with the injectable and/or aerosol nanoparticulate benzodiazepine, such as lorazepam, compositions of the invention. In an exemplary method, therapeutically effective amount of an injectable or aerosol nanoparticulate benzodiazepine composition of the invention is administered to a subject in need. The methods of the invention encompass treating a subject for status epilepticus, treatment of irritable bowel syndrome, sleep induction, acute psychosis, and pre-anesthesia medication. Diagnostic methods, comprising imaging of the administered dosage form, are also encompassed by the invention.
[0038] It is to be understood that both the foregoing general description and the following detailed description are exemplary and explanatory and are intended to provide further explanation of the invention as claimed. Other objects, advantages, and novel features will be readily apparent to those skilled in the art from the following detailed description of the invention.
Antwort auf Beitrag Nr.: 29.236.498 von Poppholz am 09.05.07 18:03:15Auf der Jahresversammlung der American Academy of Neurology präsentierte Daten liefern neue Informationen zur Anwendung und Verträglichkeit von TYSABR
Mittwoch 9. Mai 2007, 17:46 Uhr
...poppi, ich denke, dass viele Neurologen ihre Zurückhaltung mehr und mehr aufgeben werden.
Sofern keine weiteren PML-Fälle bei zunehmender Applikation bzw. Anwendungsdauer auftreten, desto progressiver wird das zukünftige Wachstum sein.
Mittwoch 9. Mai 2007, 17:46 Uhr
...poppi, ich denke, dass viele Neurologen ihre Zurückhaltung mehr und mehr aufgeben werden.
Sofern keine weiteren PML-Fälle bei zunehmender Applikation bzw. Anwendungsdauer auftreten, desto progressiver wird das zukünftige Wachstum sein.
Antwort auf Beitrag Nr.: 29.356.431 von Cyberhexe am 17.05.07 13:22:48hallo Cyberhexe,
habe ich das richtig verstanden, Du bist wieder mit Deinem ganzen Einsatz in Elan eingestiegen?
Also ich muss schon sagen Mut fehlt Dir nicht.
Ich wünsche Dir Erfolg.
Eine Frage zu meinem Selbstverständnis: Warum wird ELAN unter zwei WKN gehandelt (871331: z.Zt. 11,05 / 903801: z.Zt. 10,63 beides Fft)
(871331: z.Zt. 11,05 / 903801: z.Zt. 10,63 beides Fft)
Grüße
Levermann
habe ich das richtig verstanden, Du bist wieder mit Deinem ganzen Einsatz in Elan eingestiegen?
Also ich muss schon sagen Mut fehlt Dir nicht.
Ich wünsche Dir Erfolg.
Eine Frage zu meinem Selbstverständnis: Warum wird ELAN unter zwei WKN gehandelt
 (871331: z.Zt. 11,05 / 903801: z.Zt. 10,63 beides Fft)
(871331: z.Zt. 11,05 / 903801: z.Zt. 10,63 beides Fft)Grüße
Levermann
...die erste Produktionsstätte von BiogenIdec ausserhalb der USA wird 2009 in Hillerod/Dänemark fertig sein und zwar für die Produktion von Tysabri. Wenn ich mich richtig erinnere, dann werden dort Produktionskapazitäten zur Herstellung von 75.000 Jahresdosen des MAB Tysabri entstehen, welche mit dem ergiebigeren Verfahren, dem "high titer process", auf das Vierfache, also 300.000 Jahresdosen Tysabri, erweiterbar ist - eine gigantische Zahl.
http://biz.yahoo.com/prnews/070515/latu044.html?.v=95
PASADENA, Calif., May 15 /PRNewswire-FirstCall/ -- Jacobs Engineering Group Inc. (NYSE: JEC - News) announced today that they received a contract from Biogen Idec (Nasdaq: BIIB - News) to provide engineering, procurement, validation, and site support services for the first cell culture manufacturing facility at the greenfield biotechnology plant in Hillerod, Denmark. The manufacturing facility is expected to produce TYSABRI® (natalizumab), which is used in the treatment of multiple sclerosis.
http://biz.yahoo.com/prnews/070515/latu044.html?.v=95
PASADENA, Calif., May 15 /PRNewswire-FirstCall/ -- Jacobs Engineering Group Inc. (NYSE: JEC - News) announced today that they received a contract from Biogen Idec (Nasdaq: BIIB - News) to provide engineering, procurement, validation, and site support services for the first cell culture manufacturing facility at the greenfield biotechnology plant in Hillerod, Denmark. The manufacturing facility is expected to produce TYSABRI® (natalizumab), which is used in the treatment of multiple sclerosis.
...anbei eine interessante Studie über die Sicherheit von Tysabri - es hat den Anschein, dass BiogenIdec mit dem Aufbau der Produktionskapazitäten die bisher eher passive Strategie (aufgrund von kannibalisierenden Avonex-Umsätzen) gegenüber Tysabri aufgegeben hat:
http://clinicaltrials.gov/ct/show/NCT00297232?order=4
The purpose of this study it to evaluate the safety of natalizumab monotherapy following re-exposure to natalizumab. This includes assessing the risk of hypersensitivity and immunogenicity and confirming the safety of switching from interferon beta, glatiramer acetate, or other multiple sclerosis therapies to natalizumab.
http://clinicaltrials.gov/ct/show/NCT00297232?order=4
The purpose of this study it to evaluate the safety of natalizumab monotherapy following re-exposure to natalizumab. This includes assessing the risk of hypersensitivity and immunogenicity and confirming the safety of switching from interferon beta, glatiramer acetate, or other multiple sclerosis therapies to natalizumab.
...und noch eine weitere geplante Studie zu Tysabri:
http://clinicaltrials.gov/ct/show/NCT00464074?order=5
Purpose
This study aims to study the effects of TYSABRI® treatment on fatigue and cognition in patients with relapsing forms of MS.
http://clinicaltrials.gov/ct/show/NCT00464074?order=5
Purpose
This study aims to study the effects of TYSABRI® treatment on fatigue and cognition in patients with relapsing forms of MS.
...und auch auf den Verdacht hin es könnte langweilig werden, noch eine weitere Studie, die auf Schwangerschaftstauglichkeit fokussiert ist:
http://clinicaltrials.gov/ct/show/NCT00472992?order=6
Purpose
The purpose of this Registry is to monitor pregnant subjects and fetuses inadvertently exposed to TYSABRI® and to detect any potential increase in the risk of both major birth defects and spontaneous pregnancy loss.
http://clinicaltrials.gov/ct/show/NCT00472992?order=6
Purpose
The purpose of this Registry is to monitor pregnant subjects and fetuses inadvertently exposed to TYSABRI® and to detect any potential increase in the risk of both major birth defects and spontaneous pregnancy loss.
Antwort auf Beitrag Nr.: 29.356.845 von levermann_I am 17.05.07 13:59:31hallo levermann_i,
es gibt zwei WKN, da die eine für Europa und die andere für den US-Markt gedacht ist. Soweit ich weiß ist die 903801 nicht in den USA handelbar.
Generell sind beide Aktien gleich "wertvoll". Die 871331 steht allerdings meistens höher im Kurs. Bei Kursgleichheit sollte also die ADR gekauft werden.
Ansonsten haben wir einen Diskussions-Thread und einen Informations-Thread zu ELAN.
Dies ist der Informations-Thread.
Gib einfach ELAN als Suchbegriff ein, dann findest Du uns schon.
Gruß
Poppholz
es gibt zwei WKN, da die eine für Europa und die andere für den US-Markt gedacht ist. Soweit ich weiß ist die 903801 nicht in den USA handelbar.
Generell sind beide Aktien gleich "wertvoll". Die 871331 steht allerdings meistens höher im Kurs. Bei Kursgleichheit sollte also die ADR gekauft werden.
Ansonsten haben wir einen Diskussions-Thread und einen Informations-Thread zu ELAN.
Dies ist der Informations-Thread.

Gib einfach ELAN als Suchbegriff ein, dann findest Du uns schon.
Gruß
Poppholz
Gestern hat BiogenIdec die Öffentlichkeit über deren Forschungs- und Entwicklungsaktivitäten orientiert. Hierbei war Tysabri ein zentrales Thema. Bezüglich der PML-Gefahr ist man bemüht, entsprechende Indikatoren zu identifizieren, die auf eine bevorstehende Erkrankung hinweisen. Darüber hianus sind Aktivitäten im Gange, die eine erfolgreiche Behandlung der PML in der Klinik gewährleisten. Wenn PML einerseits aufgrund von entsprechenden Markern prognostizierbar ist und andererseits ein geeignete Therapie zur Verfügung steht, dann wird Tysabri den Markt zur Behandlung von MS beherrschen.
Determination of PML risk:
1. Assessment of potential risk factors for PML development in HIV+
population
– Assess whether also applies to MS patients receiving TYSABRI
2. Investigation of markers of cellular immune status as predictors of an increased risk
– Investigate effect of TYSABRI on immune system
Management of PML:
3. Discovery of early diagnostic markers of PML in HIV+ population
– Validation for MS TYSABRI patients
4. Rapid Removal of TYSABRI via plasmapheresis to allow immune
reconstitution and arrest of PML progression
5. Finding of an effective anti-JCV therapy
Da PML durch das JCVirus ausgelöst wird, zielen die Forschungsaktivitäten auf eine Blockade dieses Virus --> 5HT2a Blockers as Potential anti-JCV Therapy
Therapeutic Potential of JCV Specific siRNA
The siRNA would “save” uninfected oligodendrocytes
“Save” the patient
• Demonstrated JC virus suppression by JCV specific siRNA in vitro
• Demonstrated effective siRNA delivery to oligodendrocytes in vivo
• Options for delivery of RNAi therapeutic
• Direct intraparenchymal administration
• Systemic with hyperosmotic blood brain barrier disruption
Determination of PML risk:
1. Assessment of potential risk factors for PML development in HIV+
population
– Assess whether also applies to MS patients receiving TYSABRI
2. Investigation of markers of cellular immune status as predictors of an increased risk
– Investigate effect of TYSABRI on immune system
Management of PML:
3. Discovery of early diagnostic markers of PML in HIV+ population
– Validation for MS TYSABRI patients
4. Rapid Removal of TYSABRI via plasmapheresis to allow immune
reconstitution and arrest of PML progression
5. Finding of an effective anti-JCV therapy
Da PML durch das JCVirus ausgelöst wird, zielen die Forschungsaktivitäten auf eine Blockade dieses Virus --> 5HT2a Blockers as Potential anti-JCV Therapy
Therapeutic Potential of JCV Specific siRNA
The siRNA would “save” uninfected oligodendrocytes
“Save” the patient
• Demonstrated JC virus suppression by JCV specific siRNA in vitro
• Demonstrated effective siRNA delivery to oligodendrocytes in vivo
• Options for delivery of RNAi therapeutic
• Direct intraparenchymal administration
• Systemic with hyperosmotic blood brain barrier disruption
ebenfalls vom Biogen Idec Research & Development Day über Tysabri zur Behandlung von Morbus Crohn (MC):
Behandlung von MC mit Tysabri weitaus komplexer, da viele MC-Patienten eine immusupprimierte Vorgeschichte haben, die mitunter wie z.B. bei Azathioprin nachhaltig ist - der einzige PML-Fall in Ty-Mono ist bei einem MC-Patienten aufgetreten, der u.a. mit Azathioprin "vorbehandelt" wurde.
- Frühstadium bzw. milde MC:
Behandlung 1st Line mit 5-ASA
Behandlung 2nd Line mit oralen Steroiden
- mässige MC:
Behandlung 3rd Line mit immunsupprimierenden Medikamenten
Behandlung 4th Line mit REMICADE / HUMIRA
- schwerwiegende MC:
Behandlung 5th Line mit IV-Steroiden
im Anschluss daran gelten die Patienten als austherapiert und es besteht nur noch die Möglichkeit chirurgisch einzugreifen.
- 800.000 Betroffene in USA und EU
- vergleicht man die Daten nach einem Jahr, dann hat Tysabri (55%)gegenüber Remicade (29%) und HUMIRA (36%) eine wesentlich höhere Remissionsrate; auch hier gilt: kann die PML erkannt und bei Ausbruch behandelt werden, dann ist Tysabri mehr als eine Alternative.
- bisher 1754 Teilnehmer in MC-Studien mit Tysabri
Entscheidung über die Zulassung zur Behandlung von MC sowohl in den US (FDA) als auch in Europa (EMEA) noch in diesem Jahr.
Behandlung von MC mit Tysabri weitaus komplexer, da viele MC-Patienten eine immusupprimierte Vorgeschichte haben, die mitunter wie z.B. bei Azathioprin nachhaltig ist - der einzige PML-Fall in Ty-Mono ist bei einem MC-Patienten aufgetreten, der u.a. mit Azathioprin "vorbehandelt" wurde.
- Frühstadium bzw. milde MC:
Behandlung 1st Line mit 5-ASA
Behandlung 2nd Line mit oralen Steroiden
- mässige MC:
Behandlung 3rd Line mit immunsupprimierenden Medikamenten
Behandlung 4th Line mit REMICADE / HUMIRA
- schwerwiegende MC:
Behandlung 5th Line mit IV-Steroiden
im Anschluss daran gelten die Patienten als austherapiert und es besteht nur noch die Möglichkeit chirurgisch einzugreifen.
- 800.000 Betroffene in USA und EU
- vergleicht man die Daten nach einem Jahr, dann hat Tysabri (55%)gegenüber Remicade (29%) und HUMIRA (36%) eine wesentlich höhere Remissionsrate; auch hier gilt: kann die PML erkannt und bei Ausbruch behandelt werden, dann ist Tysabri mehr als eine Alternative.
- bisher 1754 Teilnehmer in MC-Studien mit Tysabri
Entscheidung über die Zulassung zur Behandlung von MC sowohl in den US (FDA) als auch in Europa (EMEA) noch in diesem Jahr.
...darüberhinaus wurden Forschungsaktivitäten bei der Behandlung von verschiedenen Krebsarten mit Natalizumab (also tysabri) erwähnt. Dies mag für den Laien (so auch für mich) auf den ersten Blick etwas verwirrend erscheinen, aber anscheinend soll ein möglicher Therapieansatz über eine Blockade von Rezeptoren (Integrine) an den Zelloberflächen von Krebszellen/Metastasen sehr viel versprechend sein. Und Natalizumab tritt eben mit einem derartigen Rezeptor (alpha4-Integrin) auf der Zelloberfläche in Verbindung.
...aber das ist natürlich Zukunftsmusik:
Natalizumab in Oncology
Integrin α4β1 (VLA-4) is best known as a leukocyte adhesion receptor mediating arrest and extravasation of lymphocytes via interaction with its receptor VCAM1 on activated endothelial cells.
Targeting VLA-4 in Oncology–
Potential Mechanisms of Action
• Direct antitumor activity against VLA-4 expressing
tumor cells (hematologic malignancies)
• Reversal of cell adhesion mediated drug resistance
(hematologic malignancies, solid tumors?)
• Inhibition of tumor angiogenesis &
lymphangiogenesis (solid tumors)
• Proof of concept trial in multiple myeloma planned to
start H1 ‘08
• Preclinical studies for further characterization of proof
of concept initiated
• Solid tumor clinical studies to follow
Humanized IgG4κ monoclonal antibody
• IV infusion
• Natalizumab binds to the alpha-4 subunit of integrin molecules
• Potential role in oncology:
• Direct antitumor activity against VLA-4+ tumor cells
• Reversal of cell adhesion mediated drug resistance
• Inhibition of tumor angiogenesis & lymphangiogenesis
• Opportunity for broad application across hematologic and solid
tumors
• Collaboration with Elan – August 2000
...aber das ist natürlich Zukunftsmusik:
Natalizumab in Oncology
Integrin α4β1 (VLA-4) is best known as a leukocyte adhesion receptor mediating arrest and extravasation of lymphocytes via interaction with its receptor VCAM1 on activated endothelial cells.
Targeting VLA-4 in Oncology–
Potential Mechanisms of Action
• Direct antitumor activity against VLA-4 expressing
tumor cells (hematologic malignancies)
• Reversal of cell adhesion mediated drug resistance
(hematologic malignancies, solid tumors?)
• Inhibition of tumor angiogenesis &
lymphangiogenesis (solid tumors)
• Proof of concept trial in multiple myeloma planned to
start H1 ‘08
• Preclinical studies for further characterization of proof
of concept initiated
• Solid tumor clinical studies to follow
Humanized IgG4κ monoclonal antibody
• IV infusion
• Natalizumab binds to the alpha-4 subunit of integrin molecules
• Potential role in oncology:
• Direct antitumor activity against VLA-4+ tumor cells
• Reversal of cell adhesion mediated drug resistance
• Inhibition of tumor angiogenesis & lymphangiogenesis
• Opportunity for broad application across hematologic and solid
tumors
• Collaboration with Elan – August 2000
Antwort auf Beitrag Nr.: 29.369.437 von Cyberhexe am 18.05.07 11:27:37...die mögliche Wirkungsweise von Natalizumab in der Onkologie besteht darin, dass eine Metastasierung verhindert werden soll. Wenn man bedenkt, dass viele Krebserkrankungen wie z.B. Prostatakrebs eigentlich nicht tödlich wären, wenn nicht eine Metastasierung stattfinden würde, dann ist dieser Ansatz bemerkenswert.
The long and short of the patent starts with the assumption that (1) tumor cancer cells from one organ will detach, (ii) float about in body, and (iii) attach to another organ's cells and grow. {Metatastize). This is the same way Velcro works. The cancer cell has a hook (the protein VLA-4) and the organ cell has a loop (the protein VCAM). Ty (natalizumab) gloms onto a cancer cells' hook (VLA-4) and blocks it from sticking.
Bei soliden Tumoren ist das Vorhandensein oder das Fehlen von Metastasen ein entscheidender Faktor für den Verlauf der Erkrankung. Würden Krebszellen nicht metastasieren, wäre ein Krebspatient nach der vollständigen örtlichen Tumorentfernung geheilt. Oft haben sich aber schon zum Zeitpunkt der Diagnosestellung Krebszellen abgelöst, wandern durch Blut- oder Lymphbahnen oder haben sich festgesetzt.
aus: http://www.krebsinformationsdienst.de/Fragen_und_Antworten/i…
The long and short of the patent starts with the assumption that (1) tumor cancer cells from one organ will detach, (ii) float about in body, and (iii) attach to another organ's cells and grow. {Metatastize). This is the same way Velcro works. The cancer cell has a hook (the protein VLA-4) and the organ cell has a loop (the protein VCAM). Ty (natalizumab) gloms onto a cancer cells' hook (VLA-4) and blocks it from sticking.
Bei soliden Tumoren ist das Vorhandensein oder das Fehlen von Metastasen ein entscheidender Faktor für den Verlauf der Erkrankung. Würden Krebszellen nicht metastasieren, wäre ein Krebspatient nach der vollständigen örtlichen Tumorentfernung geheilt. Oft haben sich aber schon zum Zeitpunkt der Diagnosestellung Krebszellen abgelöst, wandern durch Blut- oder Lymphbahnen oder haben sich festgesetzt.
aus: http://www.krebsinformationsdienst.de/Fragen_und_Antworten/i…
...erstaunlicherweise soll die positive Wirkung von Natalizumab über die Zeit zunehmen, so dass die Schubrate in Abhängigkeit von der Anwendungsdauer noch weiter abnimmt:
im 1.Jahr 0.27 Schübe/a, im 2.Jahr 0.20 Schübe/a und im 3.Jahr auf 0.15. Verbunden mit den vielerlei Aktivitätäten hinsichtlich PML-Monitoring und Therapie sollten immermehr Neurologen Tysabri ihren Patienten empfehlen - die Zuwachsraten dürften deswegen überproportional ansteigen.
http://www.mspatientsforchoice.org/clindata.htm
TYSABRI Efficacy Sustained through Three Years
Patients who participated in the Phase III TYSABRI program were eligible to enroll in an open-label extension study that evaluated the therapy's long-term effects. Included in this were patients from AFFIRM, a randomized, double-blind, placebo-controlled, two-year monotherapy study of TYSABRI that enrolled 942 patients (627 patients on TYSABRI, 315 on placebo). In AFFIRM, TYSABRI reduced the annualized relapse rate in patients with MS by 67% (p<0.001) and the risk of 12-week sustained disability progression by 42% (p<0.001) compared with placebo.
In the intent to treat analysis, the annualized relapse rate for patients treated with TYSABRI over the three-year period was 0.23, translating into an average of one relapse every 4.3 years. The relapse rate also continued to remain low over the three-year treatment period with TYSABRI: 0.27 during the first year; 0.20 during the second year; and 0.15 during the third year (based on 531 patients who entered the extension study, which includes approximately 250 patients with nearly three years of continuous therapy).
(1) The relapse reduction rates used were: TYSABRI was 67%, AVONEX (Interferon beta-1a IM) 32%, Betaseron(R) (Interferon beta-1b) 34%, Copaxone(R) (glatiramer acetate) 29%, and Rebif(R) (Interferon beta-1a SC) 32%.
im 1.Jahr 0.27 Schübe/a, im 2.Jahr 0.20 Schübe/a und im 3.Jahr auf 0.15. Verbunden mit den vielerlei Aktivitätäten hinsichtlich PML-Monitoring und Therapie sollten immermehr Neurologen Tysabri ihren Patienten empfehlen - die Zuwachsraten dürften deswegen überproportional ansteigen.
http://www.mspatientsforchoice.org/clindata.htm
TYSABRI Efficacy Sustained through Three Years
Patients who participated in the Phase III TYSABRI program were eligible to enroll in an open-label extension study that evaluated the therapy's long-term effects. Included in this were patients from AFFIRM, a randomized, double-blind, placebo-controlled, two-year monotherapy study of TYSABRI that enrolled 942 patients (627 patients on TYSABRI, 315 on placebo). In AFFIRM, TYSABRI reduced the annualized relapse rate in patients with MS by 67% (p<0.001) and the risk of 12-week sustained disability progression by 42% (p<0.001) compared with placebo.
In the intent to treat analysis, the annualized relapse rate for patients treated with TYSABRI over the three-year period was 0.23, translating into an average of one relapse every 4.3 years. The relapse rate also continued to remain low over the three-year treatment period with TYSABRI: 0.27 during the first year; 0.20 during the second year; and 0.15 during the third year (based on 531 patients who entered the extension study, which includes approximately 250 patients with nearly three years of continuous therapy).
(1) The relapse reduction rates used were: TYSABRI was 67%, AVONEX (Interferon beta-1a IM) 32%, Betaseron(R) (Interferon beta-1b) 34%, Copaxone(R) (glatiramer acetate) 29%, and Rebif(R) (Interferon beta-1a SC) 32%.
http://www.nature.com/search/executeSearch?sp-q=bapineuzumab…
1. Therapies for Alzheimer's disease
Irena Melnikova
Elan/Wyeth could initially bring bapineuzumab to the market as a symptom-management drug and then secure disease-modifying claims in post-approval studies. In an 8-week Phase I study, bapineuzumab showed statistically significant improvement in cognitive function. If interim data from the ongoing Phase II trial, expected in mid 2007, confirm this result, Elan/Wyeth could initiate a short Phase III trial (3–6 months as opposed to an 18–month study required for a DMD) and file for a cognitive improvement/symptom-management label.
Nature Reviews Drug Discovery 6, 341 - 342 (01 May 2007) News and Analysis
...dieser Zielhorizont scheint mir jedoch viel zu optimistisch!
1. Therapies for Alzheimer's disease
Irena Melnikova
Elan/Wyeth could initially bring bapineuzumab to the market as a symptom-management drug and then secure disease-modifying claims in post-approval studies. In an 8-week Phase I study, bapineuzumab showed statistically significant improvement in cognitive function. If interim data from the ongoing Phase II trial, expected in mid 2007, confirm this result, Elan/Wyeth could initiate a short Phase III trial (3–6 months as opposed to an 18–month study required for a DMD) and file for a cognitive improvement/symptom-management label.
Nature Reviews Drug Discovery 6, 341 - 342 (01 May 2007) News and Analysis
...dieser Zielhorizont scheint mir jedoch viel zu optimistisch!
















Elan and Wyeth to Initiate Phase 3 Clinical Trial of Bapineuzumab (AAB-001) in Alzheimer's Disease
Monday May 21, 2:00 am ET
DUBLIN, Ireland & Madison, N.J.--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Wyeth Pharmaceuticals, a division of Wyeth (NYSE: WYE - News), today announced the decision to initiate a Phase 3 clinical program of their lead immunotherapeutic candidate, Bapineuzumab (AAB-001), for the treatment of patients with mild to moderate Alzheimer's Disease. This decision was based on the seriousness of the disease and the totality of what the companies have learned from their immunotherapy programs, including a scheduled Interim look at data from an ongoing Phase 2 study, which remains blinded. No conclusion about the Phase 2 study can be drawn until the study is completed and the final data are analyzed and released in 2008. Phase 3 clinical trial design will be finalized with regulatory agencies, and subject to regulatory approval, it is intended for the trial to begin in the second half of 2007.
ADVERTISEMENT
It is important to remember that Alzheimer's disease is a complex and formidable challenge, and our immunotherapeutic programs still contain inherent risks.
About Bapineuzumab
Bapineuzumab (AAB-001) is a humanized monoclonal antibody that received Fast Track designation from the United States Food and Drug Administration (FDA) for treatment of mild to moderate Alzheimer's disease. Fast Track designation facilitates development and may expedite regulatory review of drugs that the FDA recognizes as potentially addressing an unmet medical need for serious or life threatening conditions.
There are two ongoing Phase 2 studies with Bapineuzumab. The first Phase 2 trial is a randomized, double-blind, placebo controlled, multiple ascending dose study of 4 cohorts of the approximately 240 total patients with mild to moderate Alzheimer's disease. The primary objective of the trial is to assess the safety of bapineuzumab. Assessments of cognitive and functional status are also being made in the trail, and each patient's participation lasts approximately 18 months. The key end-points include: ADAS-Cog (assesses cognition), Neuropsychological Test Battery (NTB) and DAD score (measures quality of life). The second Phase 2 trial is an Alzheimer's beta-amyloid imaging study in 30 patients and is being conducted in Europe. The companies do not expect that any Phase 2 data will be released into the public domain until the completion of the Phase 2 trials in 2008.
About Alzheimer's Disease
Alzheimer's disease is a progressive brain disorder that gradually destroys a person's memory and ability to learn, reason, make judgments, communicate and carry out daily activities. As Alzheimer's progresses, individuals may also experience changes in personality and behavior, such as anxiety, suspiciousness or agitation, as well as delusions or hallucinations. It is currently estimated that more than 5 million Americans and more than 24 million people worldwide have Alzheimer's disease (Source: Alzheimer's Association and Alzheimer's Disease International)
About the Elan and Wyeth Collaboration
The Elan and Wyeth Alzheimer's Immunotherapy Program (AIP) is a 50:50 collaboration to research, develop and commercialize an immunotherapeutic approach that may be used for the treatment of mild to moderate AD and possibly to prevent the onset of the disease. Current AIP programs include bapineuzumab (AAB-001), AAB-001 SubQ, ACC-001 and AAB-002. Wyeth and Elan equally share all costs and potential revenues from this collaboration.
About Elan
Elan Corporation (NYSE: ELN - News), plc is a neuroscience-based biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. Elan shares trade on the New York, London and Dublin Stock Exchanges. For additional information about the company, please visit http://www.elan.com
About Wyeth
Wyeth Pharmaceuticals, a division of Wyeth, has leading products in the areas of women's health care, infectious disease, gastrointestinal health, central nervous system, inflammation, transplantation, hemophilia, oncology, vaccines and nutritional products.
Wyeth is one of the world's largest research-driven pharmaceutical and health care products companies. It is a leader in the discovery, development, manufacturing and marketing of pharmaceuticals, vaccines, biotechnology products and non-prescription medicines that improve the quality of life for people worldwide. The Company's major divisions include Wyeth Pharmaceuticals, Wyeth Consumer Healthcare and Fort Dodge Animal Health. For additional information about the company, please visit https://www.wyeth.com.
Safe Harbor / Forward-Looking Statements
The statements in this press release that are not historical facts are forward-looking statements that involve risks and uncertainties and include, without limitation, the risks associated with the inherent uncertainty of the clinical development of AAB-001 for Alzheimer's disease and whether AAB-001 will ever be approved for commercialization. Factors which could cause actual results to differ materially from the companies' current expectations include the risks that problems or delays may arise during preparations for the proposed Phase 3 trial or, if the proposed Phase 3 trial is initiated, during the course of the Phase 3 trial, that the Phase 2 trials may not be successfully completed, and even if the Phase 2 trials are successfully completed, that results in the proposed Phase 3 trial may not show that AAB-001 is safe and effective, as well as the other risks and uncertainties described from time to time in the companies' periodic and other reports filed with the Securities and Exchange Commission.
http://biz.yahoo.com/bw/070521/20070520005042.html?.v=1
NCB
Elan and Wyeth are to initiate a Phase III study with AAB-001 in patients with mild to moderate Alzheimer’s Disease. This decision to initiate Phase III studies was based on the “seriousness of the disease and the totality of what the companies have learned
from their immunotherapy programs” included the interim data from the on-going Phase II study. The Phase II data is not expected to be released until the Phase II studies complete in 2008.
• The Phase II interim analysis data, the Phase I AAB-001 data and the follow-on study with the AN-1792 treated patients likely supported Elan/Wyeth’s decision to progress to a Phase III study. The Phase I study with AAB-001 showed that patients (6 treated patients) treated with 1.5mg/kg of AAB-001 showed an statistically significant improvement in the MMSE (mini-mental state examination (assess short term memory)) compared to the placebo treated patients at week 16. This memory improvement trend continued through 12 months. A 4.5 yr follow-up study of patients (c.160) that participated in the AN-1792 study showed that the high antibody responder patients in this study were 40% less dependent on caregivers than patients treated with placebo.
• Our AAB-001 forecasts assume a launch of the drug in 2010, pricing of $15k (at the low end of the biologics range), peak market share of c.10% in the US and 1.5% in EU/RoW and 50% profit split with Wyeth. Our NPV assessment was based on a 25% probability of success which we will be increasing to reflect the progress of AAB-001 to Phase III. At this point, however, it is difficult to assess the real market potential of this
news in the absence of detail on the actual Phase II data and the label the data supports (disease modifying versus symptomatic). What we can assume is that Elan has seen clinically meaningful trends (no interpretation on statistical significance of the
Phase II data can be made) in the Phase II study that has lead to the decision on initiating a Phase III study.
The Phase III study is expected to begin in H2 2007 and is likely to be an 18-month study. Data dependent, the earliest that Elan could file for US approval could be H2 2008/H1 2009.
Our SOTPs is under review.
• Click Here to access the Elan page on NCB’s online Irish Equity Stock Guide
Orla Hartford +353 1 611 5844 orla.hartford@ncb.
http://www.investorvillage.com/smbd.asp?mb=160&mn=109205&pt=…
Elan and Wyeth are to initiate a Phase III study with AAB-001 in patients with mild to moderate Alzheimer’s Disease. This decision to initiate Phase III studies was based on the “seriousness of the disease and the totality of what the companies have learned
from their immunotherapy programs” included the interim data from the on-going Phase II study. The Phase II data is not expected to be released until the Phase II studies complete in 2008.
• The Phase II interim analysis data, the Phase I AAB-001 data and the follow-on study with the AN-1792 treated patients likely supported Elan/Wyeth’s decision to progress to a Phase III study. The Phase I study with AAB-001 showed that patients (6 treated patients) treated with 1.5mg/kg of AAB-001 showed an statistically significant improvement in the MMSE (mini-mental state examination (assess short term memory)) compared to the placebo treated patients at week 16. This memory improvement trend continued through 12 months. A 4.5 yr follow-up study of patients (c.160) that participated in the AN-1792 study showed that the high antibody responder patients in this study were 40% less dependent on caregivers than patients treated with placebo.
• Our AAB-001 forecasts assume a launch of the drug in 2010, pricing of $15k (at the low end of the biologics range), peak market share of c.10% in the US and 1.5% in EU/RoW and 50% profit split with Wyeth. Our NPV assessment was based on a 25% probability of success which we will be increasing to reflect the progress of AAB-001 to Phase III. At this point, however, it is difficult to assess the real market potential of this
news in the absence of detail on the actual Phase II data and the label the data supports (disease modifying versus symptomatic). What we can assume is that Elan has seen clinically meaningful trends (no interpretation on statistical significance of the
Phase II data can be made) in the Phase II study that has lead to the decision on initiating a Phase III study.
The Phase III study is expected to begin in H2 2007 and is likely to be an 18-month study. Data dependent, the earliest that Elan could file for US approval could be H2 2008/H1 2009.
Our SOTPs is under review.
• Click Here to access the Elan page on NCB’s online Irish Equity Stock Guide
Orla Hartford +353 1 611 5844 orla.hartford@ncb.
http://www.investorvillage.com/smbd.asp?mb=160&mn=109205&pt=…
...no comment!
Analysten-Bewertung - 26.04.07
Elan neues Kursziel
Rating-Update: Frankfurt (aktiencheck.de AG) - Die Analysten von Deutsche Securities stufen die Aktie von Elan (<-->/ ) unverändert mit "hold" ein. Das Kursziel werde von 14 auf 13 USD gesenkt. (26.04.2007/ac/a/u)
Quelle: Deutsche Securities
Analysten-Bewertung - 26.04.07
Elan neues Kursziel
Rating-Update: Frankfurt (aktiencheck.de AG) - Die Analysten von Deutsche Securities stufen die Aktie von Elan (<-->/ ) unverändert mit "hold" ein. Das Kursziel werde von 14 auf 13 USD gesenkt. (26.04.2007/ac/a/u)
Quelle: Deutsche Securities
Antwort auf Beitrag Nr.: 29.401.385 von Cyberhexe am 21.05.07 11:52:56....das kennen wir ie Analysten stufen herunter und die Brokerhäuser,bei denen sie arbeiten,kaufen ohne Unterlass....
ie Analysten stufen herunter und die Brokerhäuser,bei denen sie arbeiten,kaufen ohne Unterlass.... und versuchen solange,den Kurs unter der Decke zu halten...
und versuchen solange,den Kurs unter der Decke zu halten...
 ie Analysten stufen herunter und die Brokerhäuser,bei denen sie arbeiten,kaufen ohne Unterlass....
ie Analysten stufen herunter und die Brokerhäuser,bei denen sie arbeiten,kaufen ohne Unterlass.... und versuchen solange,den Kurs unter der Decke zu halten...
und versuchen solange,den Kurs unter der Decke zu halten...
...das wäre ein potenter Kursbeschleuniger: Zulassungsantrag für Bapineuzumab aufgrund der Phase-2-Daten - die Phantasie wächst!
Bin gespannt, ob Elan auf dem AGM den Bau einer neuen Produktionsstätte in Irland bekanntgibt.
http://ftp.ncb.ie/equities/Elan201006Final.pdf
Potential Filing of AAB-001 with Phase II Data
AAB-001 (bapineuzumab) is in Phase II studies with interim analysis of Phase II data expected by the end of 2006. AAB-001 has been designated fast track status and there may be the option (data dependent) to file for approval in the US following completion of Phase II studies. We believe that the market is not aware of the potential for AAB-001 to progress directly from the Phase II study to filing for marketing approval. In our view, the
Phase II trial of AAB-001 has been designed to generate sufficient data to allow an accelerated filing for marketing approval should the data be strong. There are several precedents where products have been filed for approval on Phase II data including the
biologic, Remicade, which was approved by the FDA, under subpart E, with Phase II data (involving a 108 Crohn’s patient study).
Bin gespannt, ob Elan auf dem AGM den Bau einer neuen Produktionsstätte in Irland bekanntgibt.
http://ftp.ncb.ie/equities/Elan201006Final.pdf
Potential Filing of AAB-001 with Phase II Data
AAB-001 (bapineuzumab) is in Phase II studies with interim analysis of Phase II data expected by the end of 2006. AAB-001 has been designated fast track status and there may be the option (data dependent) to file for approval in the US following completion of Phase II studies. We believe that the market is not aware of the potential for AAB-001 to progress directly from the Phase II study to filing for marketing approval. In our view, the
Phase II trial of AAB-001 has been designed to generate sufficient data to allow an accelerated filing for marketing approval should the data be strong. There are several precedents where products have been filed for approval on Phase II data including the
biologic, Remicade, which was approved by the FDA, under subpart E, with Phase II data (involving a 108 Crohn’s patient study).
aus einer Pressekonferenz vom 14.März 2007:
Jive to Kelly Martin...."What you have mentioned, the Phase 3 issue for AAB-001, any chance we can file for a BLA at that time?"
Kelly Martin's "We will move to Phase 3 when we have enough confirmatory data to do that and make reasonable judgements about those 2 things [timing and dosage], and we need the Phase 3 because we need more safety data. So Phase 2, it may stand on its own with the data, the data will drive it, and if it does we will file, but we will also need a Phase 3 going, because you'll need more patients from a safety point of view for the FDA."
Jive to Kelly Martin...."What you have mentioned, the Phase 3 issue for AAB-001, any chance we can file for a BLA at that time?"
Kelly Martin's "We will move to Phase 3 when we have enough confirmatory data to do that and make reasonable judgements about those 2 things [timing and dosage], and we need the Phase 3 because we need more safety data. So Phase 2, it may stand on its own with the data, the data will drive it, and if it does we will file, but we will also need a Phase 3 going, because you'll need more patients from a safety point of view for the FDA."
Ken Kam hält eine gemeinsame Übernahme von Elan durch Wyeth und Biogen für möglich:
http://articles.moneycentral.msn.com/Investing/StrategyLab/R…
http://articles.moneycentral.msn.com/Investing/StrategyLab/R…
erstes Generika angekündigt für Copaxone:
http://www.thehindubusinessline.com/2007/05/17/stories/20070…
der Patentschutz für Avonex dauert auch nicht mehr all zu lange, so dass Biogen demnächst voll und ganz auf Tysabri setzen könnte - gut für Elan!
http://www.thehindubusinessline.com/2007/05/17/stories/20070…
der Patentschutz für Avonex dauert auch nicht mehr all zu lange, so dass Biogen demnächst voll und ganz auf Tysabri setzen könnte - gut für Elan!
http://www.davy.ie/other/pubarticles/eqbrief20070523.pdf" target="_blank" rel="nofollow ugc noopener">http://www.davy.ie/other/pubarticles/eqbrief20070523.pdf
Further discussions with Elan’s senior management have provided additional insights on the prospects for its emerging AD franchise. Management is very upbeat on AD prospects. The move to Phase III is only the first of many steps for AAB-001, and in itself AAB-001 is the first of many products in the Wyeth
collaboration and across the overall AD pipeline. As such our $21 SOTP valuation has further AD upside from (a) the disclosure of
further AAB-001 data and (b) progress in the other AD products through Phase I and II. The design of Phase III for AAB-001 remains under active discussion, and a pre-Phase III meeting is being scheduled with the FDA. European and US arms are
expected in what should be a programme with more than 1,000 patients – a decision whether to explore single or multiple doses has not been disclosed to date. The strong sense is that management wishes to develop its own manufacturing capability for the overall AD franchise – to help provide dual source
capacity but also from a strategic desire to have expertise in all elements of the operational process.
We felt that management was a little definitive than previously on the filing strategy for AAB-001, preferring not to speculate on whether Phase II would be used as part of a filing submission. This may be no more than prudence on Elan’s part while in the midst of FDA discussions and while the blinded Phase II study is
ongoing.
Other aspects of Elan’s AD programme are also progressing well. A Phase I trial on AAB-001 is being conducted in Japan, while its subcutaneous formulation is in Phase I and if successful should enter Phase II sometime next year. Dosing in the EU arm of the ACC-001 Phase II trial should begin in the coming weeks.
Further discussions with Elan’s senior management have provided additional insights on the prospects for its emerging AD franchise. Management is very upbeat on AD prospects. The move to Phase III is only the first of many steps for AAB-001, and in itself AAB-001 is the first of many products in the Wyeth
collaboration and across the overall AD pipeline. As such our $21 SOTP valuation has further AD upside from (a) the disclosure of
further AAB-001 data and (b) progress in the other AD products through Phase I and II. The design of Phase III for AAB-001 remains under active discussion, and a pre-Phase III meeting is being scheduled with the FDA. European and US arms are
expected in what should be a programme with more than 1,000 patients – a decision whether to explore single or multiple doses has not been disclosed to date. The strong sense is that management wishes to develop its own manufacturing capability for the overall AD franchise – to help provide dual source
capacity but also from a strategic desire to have expertise in all elements of the operational process.
We felt that management was a little definitive than previously on the filing strategy for AAB-001, preferring not to speculate on whether Phase II would be used as part of a filing submission. This may be no more than prudence on Elan’s part while in the midst of FDA discussions and while the blinded Phase II study is
ongoing.
Other aspects of Elan’s AD programme are also progressing well. A Phase I trial on AAB-001 is being conducted in Japan, while its subcutaneous formulation is in Phase I and if successful should enter Phase II sometime next year. Dosing in the EU arm of the ACC-001 Phase II trial should begin in the coming weeks.

International Conference on Prevention of Dementia
09.06.07 -12.06.07
Tuesday, June 12, 2007I
INTERVENTION AND TREATMENTS
..u.a.
- Immunotherapy
Dale Schenk , Élan Pharmaceuticals, South San Francisco, Calif., United States
http://www.alz.org/preventionconference/pc2007/session_detai…
Edward Owens Buys OSI Pharmaceuticals Inc., Elan Corp. Plc, Amylin Pharmaceuticals Inc., Sells Biomet Inc., Bausch & Lomb Inc., CVS Corp.
Ticker Date* Price* buy/sell Picked By
SIAL 2007-03-31 $39.8 Add David Dreman
BOL 2007-03-31 $52.3 Add David Dreman
AMLN 2007-03-31 $38.8 Buy Edward Owens
BAY 2007-03-31 $56.7 Reduce Edward Owens
BOL 2007-03-31 $52.3 Sell Edward Owens
*The price and date might not be the actual time and price at which the transactions were made. In the case of institutional owners, the date is stated as the last day of their fiscal quarter. The prices are estimates if no accurate information available.
Want to buy healthcare stocks, see what Edward Owens is buying. He is the manager of Vanguard Healthcare Fund since 1984, averaging more than 19% a year. These are his buys and sells during the first quarter. Edward Owens owns 81 stocks with a total value of $24.5 billion.
Edward Owens buys Osi Pharmaceuticals Inc., Elan Corp. Plc, Amylin Pharmaceuticals Inc., sells Biomet Inc., Bausch & Lomb Inc., Cvs Corp. during the 3-months ended 03/31/2007, according to the most recent filings of his investment company, Vanguard Health Care Fund. Edward Owens owns 81 stocks with a total value of $24.5 billion. These are the details of the buys and sells.
New Purchases: AMLN, ELN, OSIP,
Added Positions: SIAL, WAG,
Reduced Positions: BAY, GILD, HGSI, VMSI,
Sold Out: BMET, BOL, CVS,
.......
....
New Purchase: Elan Corp. Plc (ELN)
Edward Owens initiated holdings in Pharmaceuticals company Elan Corp. Plc. His purchase prices were between $12.35 and $14.81, with an estimated average price of $13.3. The impact to his portfolio due to this purchase was 0.1%. His holdings were 1,820,000 shares as of 03/31/2007. Shares of Elan Corp. Plc were traded at around $19.04.
http://www.gurufocus.com/news.php?id=6015
..ich würde sagen, das war eine weise Entscheidung Edward..
Ticker Date* Price* buy/sell Picked By
SIAL 2007-03-31 $39.8 Add David Dreman
BOL 2007-03-31 $52.3 Add David Dreman
AMLN 2007-03-31 $38.8 Buy Edward Owens
BAY 2007-03-31 $56.7 Reduce Edward Owens
BOL 2007-03-31 $52.3 Sell Edward Owens
*The price and date might not be the actual time and price at which the transactions were made. In the case of institutional owners, the date is stated as the last day of their fiscal quarter. The prices are estimates if no accurate information available.
Want to buy healthcare stocks, see what Edward Owens is buying. He is the manager of Vanguard Healthcare Fund since 1984, averaging more than 19% a year. These are his buys and sells during the first quarter. Edward Owens owns 81 stocks with a total value of $24.5 billion.
Edward Owens buys Osi Pharmaceuticals Inc., Elan Corp. Plc, Amylin Pharmaceuticals Inc., sells Biomet Inc., Bausch & Lomb Inc., Cvs Corp. during the 3-months ended 03/31/2007, according to the most recent filings of his investment company, Vanguard Health Care Fund. Edward Owens owns 81 stocks with a total value of $24.5 billion. These are the details of the buys and sells.
New Purchases: AMLN, ELN, OSIP,
Added Positions: SIAL, WAG,
Reduced Positions: BAY, GILD, HGSI, VMSI,
Sold Out: BMET, BOL, CVS,
.......
....
New Purchase: Elan Corp. Plc (ELN)
Edward Owens initiated holdings in Pharmaceuticals company Elan Corp. Plc. His purchase prices were between $12.35 and $14.81, with an estimated average price of $13.3. The impact to his portfolio due to this purchase was 0.1%. His holdings were 1,820,000 shares as of 03/31/2007. Shares of Elan Corp. Plc were traded at around $19.04.
http://www.gurufocus.com/news.php?id=6015
..ich würde sagen, das war eine weise Entscheidung Edward..

DJ Elan:Co Alzheimer's Treatment Will Change Disease's Pathology
By Quentin Fottrell
Of DOW JONES NEWSWIRES
DUBLIN (Dow Jones)--Elan Corp. PLC's (ELN) head of research and
development, Lars Ekman, said Thursday he thinks the company's
Alzheimer's disease treatment, AAB-001, will change the underlying
pathology of the disease. Speaking to Dow Jones Newswires ahead of the company's annual general meeting, Ekman also said that Elan hasn't ruled out initially releasing AAB-001 on a symptomatic label, but that the company's objective is to ultimately release a label for the treatment which
tackles the whole spectrum of the disease.
Crucially, Ekman said he couldn't comment on the ongoing Phase II
AAB-001 trial as that would destroy the trial. But he said Elan's
joint-venture partner Wyeth (WYE) has the capacity to supply AAB-001 for a couple of years.
--------------------------------------------------
DJ Elan: Alzheimer's Treatment Will Change Disease's Pathology-2
"As the market grows, Elan has the right to produce AAB-001," Ekman told Dow Jones Newswires. "Elan doesn't have, at this point, a biological manufacturing plan, but that is a strategic consideration that Elan has to make."
Earlier this week, Elan's share surged after the Irish drug company and its U.S. partner Wyeth said they are to start Phase III clinical trials of AAB-001, their treatment for mild to moderate Alzheimer's disease, earlier than planned.
With Phase III trials starting in mid-2007, rather than mid-2008 as expected, Elan said AAB-001 could potentially receive approval from the U.S. Food & Drug Administration and reach the market in 2009 or 2010.
(MORE TO FOLLOW) Dow Jones Newswires
05-24-07 0611ET
Copyright (c) 2007 Dow Jones & Company, Inc.
- - 06 11 AM EDT 05-24-07
--------------------------------------------------
DJ Elan: Alzheimer's Treatment Will Change Disease's Pathology-3
"Almost all the big pharmas have bought programs that mimic ours, but they are a number of years behind," Ekman said. "That's the best compliment you can get. We have a very strong intellectual property position."
Ekman stopped short of saying the treatment will "cure" Alzheimer's, but said this was only because he didn't want to give patients false hope.
Referring to Pfizer Inc.'s (PFE) Aricept, the leading drug of its kind, Ekman said: "It changes the symptoms, but doesn't change the course of the disease. That is what makes AAB-001 dramatically different."
"AAB-001 changes the underlying pathology of the disease, which gives the possibility to influence the disease at the heart of the
problem," he said. "It takes away the toxic substances that drive the disease."
(MORE TO FOLLOW) Dow Jones Newswires
05-24-07 0616ET
Copyright (c) 2007 Dow Jones & Company, Inc.
- - 06 16 AM EDT 05-24-07
--------------------------------------------------
DJ Elan: Alzheimer's Treatment Will Change Disease's Pathology-4
Ekman said there are about 10 million Alzheimer's patients
collectively in the U.S. and Europe, and about 14 million in the rest of the world, supporting the new drug's potential as a blockbuster. Ian Hunter, analyst with Dublin-based Goodbody Stockbrokers, estimates that "conservative" peak annual sales for AAB-001 could be $2.2 billion by 2014 should the treatment get final approval from the regulatory authorities.
Company Web site: http://www.elan.com
-By Quentin Fottrell, Dow Jones Newswires; +353-1-6762189;
quentin.fottrell@dowjones.com
(END) Dow Jones Newswires
05-24-07 0620ET
Copyright (c) 2007 Dow Jones & Company, Inc.
- - 06 20 AM EDT 05-24-07
http://www.investorvillage.com/smbd.asp?mb=160&mn=111828&pt=…
By Quentin Fottrell
Of DOW JONES NEWSWIRES
DUBLIN (Dow Jones)--Elan Corp. PLC's (ELN) head of research and
development, Lars Ekman, said Thursday he thinks the company's
Alzheimer's disease treatment, AAB-001, will change the underlying
pathology of the disease. Speaking to Dow Jones Newswires ahead of the company's annual general meeting, Ekman also said that Elan hasn't ruled out initially releasing AAB-001 on a symptomatic label, but that the company's objective is to ultimately release a label for the treatment which
tackles the whole spectrum of the disease.
Crucially, Ekman said he couldn't comment on the ongoing Phase II
AAB-001 trial as that would destroy the trial. But he said Elan's
joint-venture partner Wyeth (WYE) has the capacity to supply AAB-001 for a couple of years.
--------------------------------------------------
DJ Elan: Alzheimer's Treatment Will Change Disease's Pathology-2
"As the market grows, Elan has the right to produce AAB-001," Ekman told Dow Jones Newswires. "Elan doesn't have, at this point, a biological manufacturing plan, but that is a strategic consideration that Elan has to make."
Earlier this week, Elan's share surged after the Irish drug company and its U.S. partner Wyeth said they are to start Phase III clinical trials of AAB-001, their treatment for mild to moderate Alzheimer's disease, earlier than planned.
With Phase III trials starting in mid-2007, rather than mid-2008 as expected, Elan said AAB-001 could potentially receive approval from the U.S. Food & Drug Administration and reach the market in 2009 or 2010.
(MORE TO FOLLOW) Dow Jones Newswires
05-24-07 0611ET
Copyright (c) 2007 Dow Jones & Company, Inc.
- - 06 11 AM EDT 05-24-07
--------------------------------------------------
DJ Elan: Alzheimer's Treatment Will Change Disease's Pathology-3
"Almost all the big pharmas have bought programs that mimic ours, but they are a number of years behind," Ekman said. "That's the best compliment you can get. We have a very strong intellectual property position."
Ekman stopped short of saying the treatment will "cure" Alzheimer's, but said this was only because he didn't want to give patients false hope.
Referring to Pfizer Inc.'s (PFE) Aricept, the leading drug of its kind, Ekman said: "It changes the symptoms, but doesn't change the course of the disease. That is what makes AAB-001 dramatically different."
"AAB-001 changes the underlying pathology of the disease, which gives the possibility to influence the disease at the heart of the
problem," he said. "It takes away the toxic substances that drive the disease."
(MORE TO FOLLOW) Dow Jones Newswires
05-24-07 0616ET
Copyright (c) 2007 Dow Jones & Company, Inc.
- - 06 16 AM EDT 05-24-07
--------------------------------------------------
DJ Elan: Alzheimer's Treatment Will Change Disease's Pathology-4
Ekman said there are about 10 million Alzheimer's patients
collectively in the U.S. and Europe, and about 14 million in the rest of the world, supporting the new drug's potential as a blockbuster. Ian Hunter, analyst with Dublin-based Goodbody Stockbrokers, estimates that "conservative" peak annual sales for AAB-001 could be $2.2 billion by 2014 should the treatment get final approval from the regulatory authorities.
Company Web site: http://www.elan.com
-By Quentin Fottrell, Dow Jones Newswires; +353-1-6762189;
quentin.fottrell@dowjones.com
(END) Dow Jones Newswires
05-24-07 0620ET
Copyright (c) 2007 Dow Jones & Company, Inc.
- - 06 20 AM EDT 05-24-07
http://www.investorvillage.com/smbd.asp?mb=160&mn=111828&pt=…
Elan to Present at the Goldman Sachs 28th Annual Global Healthcare Conference

 -
- 
 -
- 

Elan Corporation, plc announces that it will present at the Goldman Sachs 28th Annual Global Healthcare Conference in Laguna Niguel, California on Wednesday, June 13th, 2007 at 10.40 a.m. Pacific Time, 1.40 p.m. Eastern Time and 6.40 p.m. British Summer Time.
Interested parties may access a live audio webcast of the presentation by visiting Elan’s website at www.elan.com and clicking on the Investor Relations section, then on the event icon.

 -
- 
 -
- 

Elan Corporation, plc announces that it will present at the Goldman Sachs 28th Annual Global Healthcare Conference in Laguna Niguel, California on Wednesday, June 13th, 2007 at 10.40 a.m. Pacific Time, 1.40 p.m. Eastern Time and 6.40 p.m. British Summer Time.
Interested parties may access a live audio webcast of the presentation by visiting Elan’s website at www.elan.com and clicking on the Investor Relations section, then on the event icon.
G O L D M A N S A C H S
Elan Corporation (ADR) (ELN) Buy:
Tysabri, tax losses and Bapineuzumab; initiating as
a Buy
Company Name: Elan Corporation (ADR)
Ticker: ELNPrice: 19.1 52-Week Price Range (US$): 19-12
Year Net EPS . . Gross
Source of opportunity
We initiate coverage of Elan as Buy with a 12-month price target of US$25/ADR. In our view, the value of Tysabri, the leveraging potential of the Alzheimer?s Disease franchise and the value of the group?s c.US$3 bn of utilizable tax losses are inadequately reflected in the shares. Despite near-term risks for Tysabri (new PML cases), in our view, the group?s improved risk profile is under-appreciated by investors. As an alternative to buying the stock, our Credit Research team recommends buying long-dated (2013) bonds which are more sensitive to Tysabri than the R&D pipeline, for yield. Our Options team also discusses derivatives strategies.
Catalyst
Continuing growth of Tysabri sales (in MS) should provide solid support for the shares, and if growth stays at current levels, could push the stock higher. US and/or EU approvals
for Tysabri in Crohn?s Disease are potential catalysts before year-end. Phase II Bapineuzumab data (30-patient PET scan study) could be a significant catalyst if positive, as could a sub part E filing for Bapineuzumab in the US.
Valuation
Using our risk-adjusted DCF valuation methodology, we calculate a 12-month price target of US$25/ADR; this implies a technology value of US$27.40/ADR. Should Tysabri be approved in Crohn?s Disease, it could add up to US$4/ADR to our price target.
Key risks
The main near-term risk to our price target and view is an increase in cases of PML reported with Tysabri. In this scenario, we would view the risk of another product withdrawal as low, unless there were a significant number of new cases. However, we would expect acute share price weakness if any cases are reported. Elan also has product development risks, in common with other biotech companies.
The Goldman Sachs Group Inc. does and seeks to do business with companies covered in its research reports. As a result, investors should be aware that the firm may have a conflict of interest that could affect the objectivity of this report.
Investors should consider this report as only a single factor in making their investment decision.
http://www.investorvillage.com/smbd.asp?mb=160&mn=112536&pt=…
Elan Corporation (ADR) (ELN) Buy:
Tysabri, tax losses and Bapineuzumab; initiating as
a Buy
Company Name: Elan Corporation (ADR)
Ticker: ELNPrice: 19.1 52-Week Price Range (US$): 19-12
Year Net EPS . . Gross
Source of opportunity
We initiate coverage of Elan as Buy with a 12-month price target of US$25/ADR. In our view, the value of Tysabri, the leveraging potential of the Alzheimer?s Disease franchise and the value of the group?s c.US$3 bn of utilizable tax losses are inadequately reflected in the shares. Despite near-term risks for Tysabri (new PML cases), in our view, the group?s improved risk profile is under-appreciated by investors. As an alternative to buying the stock, our Credit Research team recommends buying long-dated (2013) bonds which are more sensitive to Tysabri than the R&D pipeline, for yield. Our Options team also discusses derivatives strategies.
Catalyst
Continuing growth of Tysabri sales (in MS) should provide solid support for the shares, and if growth stays at current levels, could push the stock higher. US and/or EU approvals
for Tysabri in Crohn?s Disease are potential catalysts before year-end. Phase II Bapineuzumab data (30-patient PET scan study) could be a significant catalyst if positive, as could a sub part E filing for Bapineuzumab in the US.
Valuation
Using our risk-adjusted DCF valuation methodology, we calculate a 12-month price target of US$25/ADR; this implies a technology value of US$27.40/ADR. Should Tysabri be approved in Crohn?s Disease, it could add up to US$4/ADR to our price target.
Key risks
The main near-term risk to our price target and view is an increase in cases of PML reported with Tysabri. In this scenario, we would view the risk of another product withdrawal as low, unless there were a significant number of new cases. However, we would expect acute share price weakness if any cases are reported. Elan also has product development risks, in common with other biotech companies.
The Goldman Sachs Group Inc. does and seeks to do business with companies covered in its research reports. As a result, investors should be aware that the firm may have a conflict of interest that could affect the objectivity of this report.
Investors should consider this report as only a single factor in making their investment decision.
http://www.investorvillage.com/smbd.asp?mb=160&mn=112536&pt=…
...zum Thema Alzheimer - von AMA News April 9,
http://www.ama-assn.org/amednews/2007/04/09/hlsb0409.htm
........New drugs are in the pipeline and could arrive at the Food and Drug Administration by fall for possible approval next year, Sam Gandy, MD, PhD, director of Thomas Jefferson University's Farber Institute for Neurosciences, in Philadelphia, told the Senate panel. Dr. Gandy's laboratory is participating in clinical trials for two of the new drugs.
So far, the FDA has approved several drugs that temporarily slow symptoms in some people, but the medicines being tested are intended to attack the disease directly. Among them are two that target the amyloid plaques that are a molecular hallmark of Alzheimer's, Dr. Gandy said. Reports on the new entities are very encouraging, he added. "These drugs are safe. Patients tolerate them well. And they appear to show significant positive impact, slowing progression of the disease."
The drugs could transform Alzheimer's from a death sentence to a manageable chronic illness, he told the Senate panel.
http://www.ama-assn.org/amednews/2007/04/09/hlsb0409.htm
........New drugs are in the pipeline and could arrive at the Food and Drug Administration by fall for possible approval next year, Sam Gandy, MD, PhD, director of Thomas Jefferson University's Farber Institute for Neurosciences, in Philadelphia, told the Senate panel. Dr. Gandy's laboratory is participating in clinical trials for two of the new drugs.
So far, the FDA has approved several drugs that temporarily slow symptoms in some people, but the medicines being tested are intended to attack the disease directly. Among them are two that target the amyloid plaques that are a molecular hallmark of Alzheimer's, Dr. Gandy said. Reports on the new entities are very encouraging, he added. "These drugs are safe. Patients tolerate them well. And they appear to show significant positive impact, slowing progression of the disease."
The drugs could transform Alzheimer's from a death sentence to a manageable chronic illness, he told the Senate panel.
Antwort auf Beitrag Nr.: 29.464.907 von bernie55 am 25.05.07 12:31:27Is it me, or does it seem as if there is almost an effort to suppress the GS news?
Any other company that received an upgrade that raised their price target by these percentages would have the news being screamed from the roof tops.
If it were not for the incredible investigative shareholders on this board, I still would not know about this upgrade.
Why is it that this has still not hit the Yahoo news postings?
http://www.investorvillage.com/smbd.asp?mb=160&mn=112549&pt=…
...mmmmhhh....wahr mit dem Upgrade von GS wohl nur ein Verar---ung......grrrrrr.. ......
......
....sorry...
Any other company that received an upgrade that raised their price target by these percentages would have the news being screamed from the roof tops.
If it were not for the incredible investigative shareholders on this board, I still would not know about this upgrade.
Why is it that this has still not hit the Yahoo news postings?
http://www.investorvillage.com/smbd.asp?mb=160&mn=112549&pt=…
...mmmmhhh....wahr mit dem Upgrade von GS wohl nur ein Verar---ung......grrrrrr..
 ......
..........sorry...

Goldman Sachs starts coverage of Elan at buy
By Sarah Turner
Last Update: 7:50 AM ET May 25, 2007
LONDON (MarketWatch) -- Goldman Sachs initiated coverage on Irish pharmaceutical Elan (UK:ELA : elan corp ord eur0.05
12:40pm 05/25/2007
UK:ELA13.98, +0.47, +3.5%) (ELN : Elan Corporation, plc
Last: 19.01-0.09-0.47%
6:50am 05/25/2007
ELN19.01, -0.09, -0.5%) with a buy rating on Friday. "In our view, the value of Tysabri, the leveraging potential of the Alzheimer's disease franchise and the value of the group's $3 billion of utilizable tax losses are inadequately reflected in the shares," said the broker. Elan shares rose 3.9% in London.
http://www.marketwatch.com/News/Story/Story.aspx?guid=%7bDF6…
...also doch oder wie oder was ?????
By Sarah Turner
Last Update: 7:50 AM ET May 25, 2007
LONDON (MarketWatch) -- Goldman Sachs initiated coverage on Irish pharmaceutical Elan (UK:ELA : elan corp ord eur0.05
12:40pm 05/25/2007
UK:ELA13.98, +0.47, +3.5%) (ELN : Elan Corporation, plc
Last: 19.01-0.09-0.47%
6:50am 05/25/2007
ELN19.01, -0.09, -0.5%) with a buy rating on Friday. "In our view, the value of Tysabri, the leveraging potential of the Alzheimer's disease franchise and the value of the group's $3 billion of utilizable tax losses are inadequately reflected in the shares," said the broker. Elan shares rose 3.9% in London.
http://www.marketwatch.com/News/Story/Story.aspx?guid=%7bDF6…
...also doch oder wie oder was ?????

Antwort auf Beitrag Nr.: 29.466.352 von bernie55 am 25.05.07 14:01:20UPGRADE - BUY von GS
YEPP !!!!!
YEPP !!!!!
...und schon wieder ein Advisory Committee:
http://www.fda.gov/OHRMS/DOCKETS/98fr/07n-0204-nm00001.pdf
http://www.fda.gov/OHRMS/DOCKETS/98fr/07n-0204-nm00001.pdf
Antwort auf Beitrag Nr.: 29.472.308 von Cyberhexe am 25.05.07 20:04:38was bedeutet das füs uns? 

zu den Nebenwirkungen von Natalizumab:
http://www.fda.gov/OHRMS/DOCKETS/ac/06/slides/2006-4208S1-01…
man beachte speziell slide 14 von 39, in welcher der Unterschied bei Infektionen gegenüber einem Scheinpräparat thematisiert wird - die Kurven sind erstaunlicherweise fast deckungsgleich!
http://www.fda.gov/OHRMS/DOCKETS/ac/06/slides/2006-4208S1-01…
man beachte speziell slide 14 von 39, in welcher der Unterschied bei Infektionen gegenüber einem Scheinpräparat thematisiert wird - die Kurven sind erstaunlicherweise fast deckungsgleich!
Antwort auf Beitrag Nr.: 29.472.771 von surga am 25.05.07 20:44:32...die Experten des AC werden sich sehr wahrscheinlich für eine Zulassung von Tysabri zur Behandlung von MC aussprechen - ob die FDA diesem Votum entsprechend handeln wird, bin ich seit der Provenge-Entscheidung nicht mehr ganz so sicher (aber ziemlich sicher  )!
)!
 )!
)!
http://news.moneycentral.msn.com/provider/providerarticle.as…
...mmmmhhh...wenn man sich jetzt bereits öffentlich zur Preisgestaltung eines Alzheimer-Medikamentes äussert, dann dürfte m.E. die Zulassung nicht erst 2010 zur Diskussion stehen - die PII-Ergebnisse scheinen tatsächlich spektakulär zu sein, dass eine Zulassung mit diesen PII-Daten für möglich erscheint.
...mmmmhhh...wenn man sich jetzt bereits öffentlich zur Preisgestaltung eines Alzheimer-Medikamentes äussert, dann dürfte m.E. die Zulassung nicht erst 2010 zur Diskussion stehen - die PII-Ergebnisse scheinen tatsächlich spektakulär zu sein, dass eine Zulassung mit diesen PII-Daten für möglich erscheint.
Antwort auf Beitrag Nr.: 29.526.713 von Cyberhexe am 29.05.07 16:54:39FDA to weigh Tysabri for Crohn's disease
By SHAWN POGATCHNIK
DUBLIN, Ireland
Elan Corp. PLC and Biogen Idec Inc., the makers of Tysabri, announced Tuesday that U.S. regulators would soon review the drug for its possible use by sufferers of the gastrointestinal ailment Crohn's disease.
Both companies said two review committees of the U.S. Food and Drug Administration would jointly consider on July 31 whether to permit sale of Tysabri to treat Crohn's, which causes chronic but nonfatal inflammation of the intestines and afflicts 1 million people worldwide.
Clinical trials of Tysabri -- which last year was approved for use in the United States and European Union to combat the most advanced cases of multiple sclerosis -- have indicated that the drug is effective in preventing inflammatory immune cells from penetrating the wall of the intestine, limiting the damage they can cause.
Both MS and Crohn's are diseases that cause the immune system to attack the body's soft tissues, including those that line the intestines and nerves.
Tysabri's use for MS patients has been heavily restricted because of its link to a rare, usually fatal disease of the central nervous system called progressive multifocal leukoencephalopathy, or PML. Both companies temporarily withdrew the drug from sale in February 2005 after three patients in clinical trials contracted PML; two, including a Crohn's sufferer, died.
Elan of Dublin, Ireland, and Biogen Idec of Cambridge, Mass., applied in December for FDA approval of Tysabri for Crohn's sufferers.
The disease most commonly develops in people in their teens and 20s and has no cure. It can cause diarrhea, abdominal cramps, fever and bowel obstructions, leading to lost appetite and decreased weight.
The leading current treatment for Crohn's is Remicade, manufactured by Johnson & Johnson. Analysts say, if given FDA approval, Tysabri probably would be prescribed only to those Crohn's sufferers who were not responding to treatment from longer-established, lower-risk drugs.
Elan shares fell 0.03 euros ($0.04) to 14.15 euros ($19.09) on the Irish Stock Exchange.
By SHAWN POGATCHNIK
DUBLIN, Ireland
Elan Corp. PLC and Biogen Idec Inc., the makers of Tysabri, announced Tuesday that U.S. regulators would soon review the drug for its possible use by sufferers of the gastrointestinal ailment Crohn's disease.
Both companies said two review committees of the U.S. Food and Drug Administration would jointly consider on July 31 whether to permit sale of Tysabri to treat Crohn's, which causes chronic but nonfatal inflammation of the intestines and afflicts 1 million people worldwide.
Clinical trials of Tysabri -- which last year was approved for use in the United States and European Union to combat the most advanced cases of multiple sclerosis -- have indicated that the drug is effective in preventing inflammatory immune cells from penetrating the wall of the intestine, limiting the damage they can cause.
Both MS and Crohn's are diseases that cause the immune system to attack the body's soft tissues, including those that line the intestines and nerves.
Tysabri's use for MS patients has been heavily restricted because of its link to a rare, usually fatal disease of the central nervous system called progressive multifocal leukoencephalopathy, or PML. Both companies temporarily withdrew the drug from sale in February 2005 after three patients in clinical trials contracted PML; two, including a Crohn's sufferer, died.
Elan of Dublin, Ireland, and Biogen Idec of Cambridge, Mass., applied in December for FDA approval of Tysabri for Crohn's sufferers.
The disease most commonly develops in people in their teens and 20s and has no cure. It can cause diarrhea, abdominal cramps, fever and bowel obstructions, leading to lost appetite and decreased weight.
The leading current treatment for Crohn's is Remicade, manufactured by Johnson & Johnson. Analysts say, if given FDA approval, Tysabri probably would be prescribed only to those Crohn's sufferers who were not responding to treatment from longer-established, lower-risk drugs.
Elan shares fell 0.03 euros ($0.04) to 14.15 euros ($19.09) on the Irish Stock Exchange.
Antwort auf Beitrag Nr.: 29.527.354 von bernie55 am 29.05.07 17:32:40http://www.businessweek.com/ap/financialnews/D8PE2GNO0.htm
Antwort auf Beitrag Nr.: 29.526.713 von Cyberhexe am 29.05.07 16:54:39CH, Danke! 
Im leben lernen wir nie aus

Im leben lernen wir nie aus

Antwort auf Beitrag Nr.: 29.527.152 von Cyberhexe am 29.05.07 17:21:36 ...mmmmhhh...wenn man sich jetzt bereits öffentlich zur Preisgestaltung eines Alzheimer-Medikamentes äussert, dann dürfte m.E. die Zulassung nicht erst 2010 zur Diskussion stehen - die PII-Ergebnisse scheinen tatsächlich spektakulär zu sein, dass eine Zulassung mit diesen PII-Daten für möglich erscheint.
..in diesem Zusammenhang sei Goodbody's Analyst Ian Hunter zu erwähnen....
"The implication is that the AAB-001 data might be strong enough to trigger a request for approval on Phase II data,".
"If this were the case, the drug could be on the market in late 2008, early 2009."
..in diesem Zusammenhang sei Goodbody's Analyst Ian Hunter zu erwähnen....
"The implication is that the AAB-001 data might be strong enough to trigger a request for approval on Phase II data,".
"If this were the case, the drug could be on the market in late 2008, early 2009."
Antwort auf Beitrag Nr.: 29.533.390 von bernie55 am 30.05.07 00:00:41wow...das Vakzin ist auch schon in der Spur:
http://clinicaltrials.gov/ct/show/NCT00479557?order=1
http://clinicaltrials.gov/ct/show/NCT00479557?order=1
Antwort auf Beitrag Nr.: 29.536.142 von Cyberhexe am 30.05.07 10:43:52interessant ist zudem, dass für die beginnende Phase-2-Studie mit dem Vakzin ausschliesslich europäische Studienzentren in F, D und E ausgewählt wurden; ich denke, dass dies mit einer möglichen (oder sogar wahrscheinlichen) Zulassung von AAB-001 in den Vereinigten Staaten zu tun hat. Denn falls AAB-001 von der FDA zugelassen ist, wäre es fast unmöglich für das Vakzin entsprechende Studienteilnehmer zu rekrutieren.
anbei eine sehr bedeutungsvolle Studie zu Tysabri:
http://clinicaltrials.gov/ct/show/NCT00424788?order=2
...welche darauf abzielt, die Natalizumab-Antikörper schnellstmöglich aus dem Organismus zu eliminieren. Dies dürfte ein möglicher Therapieschritt im PML-Verdachtsfall sein - denn falls es gelingen sollte, durch ein gezieltes Monitoring und einer entsprechenden Therapie die PML-Gefahr sehr gering zu halten, dann würden bei Tysabri alle Dämme brechen!
http://clinicaltrials.gov/ct/show/NCT00424788?order=2
...welche darauf abzielt, die Natalizumab-Antikörper schnellstmöglich aus dem Organismus zu eliminieren. Dies dürfte ein möglicher Therapieschritt im PML-Verdachtsfall sein - denn falls es gelingen sollte, durch ein gezieltes Monitoring und einer entsprechenden Therapie die PML-Gefahr sehr gering zu halten, dann würden bei Tysabri alle Dämme brechen!
...und wieder gibts neue Patente!
Im Mai 2007 wurden folgende Urheberrechte bestätigt:
29.5.2007
Benzamide 2-hydroxy-3-diaminoalkanes
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Beta-amino acid derivatives-inhibitors of leukocyte adhesion mediated by VLA-4
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
22.5.2007
Derivatised carbohydrates and their use in solid delivery systems
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
8.5.2007
Compounds to treat Alzheimer's disease
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
...Elan dürfte mittelfristig zu den ganz grossen Biotechschmieden aufsteigen!
Im Mai 2007 wurden folgende Urheberrechte bestätigt:
29.5.2007
Benzamide 2-hydroxy-3-diaminoalkanes
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Beta-amino acid derivatives-inhibitors of leukocyte adhesion mediated by VLA-4
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
22.5.2007
Derivatised carbohydrates and their use in solid delivery systems
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
8.5.2007
Compounds to treat Alzheimer's disease
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
...Elan dürfte mittelfristig zu den ganz grossen Biotechschmieden aufsteigen!
zukünftig von grösster Bedeutung ist möglicherweise folgendes Urheberrecht...
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
..., mit welchem für alle wasserunlöslichen Wirkstoffe eine neue Formulierung mit entsprechend höherer Bioverfügbarkeit möglich ist. Gerade bei auslaufendem Patentschutz von wasserunlöslichen Aktivsubstanzen könnte dies eine wirkungsvolle Möglichkeit sein, sich von generischen Anbietern abzugrenzen. Das Potential ist riesig! Ich frage mich nur, wann die Pharmaschwergewichte sich um dieses Sahnehäubchen streiten.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
..., mit welchem für alle wasserunlöslichen Wirkstoffe eine neue Formulierung mit entsprechend höherer Bioverfügbarkeit möglich ist. Gerade bei auslaufendem Patentschutz von wasserunlöslichen Aktivsubstanzen könnte dies eine wirkungsvolle Möglichkeit sein, sich von generischen Anbietern abzugrenzen. Das Potential ist riesig! Ich frage mich nur, wann die Pharmaschwergewichte sich um dieses Sahnehäubchen streiten.
es gibt aktuell 2 Phase-II-Studien mit Bapineuzumab (AAB-001):
http://clinicaltrials.gov/ct/show/NCT00112073?order=17
Total Enrollment: 240
Study start: April 2005; Study completion: April 2008
Purpose
The purpose of this study is to assess the safety and tolerability of multiple doses of AAB-001 passive immunization in patients with mild to moderate Alzheimer's disease (AD).
http://clinicaltrials.gov/ct/show/NCT00174525?order=3
Study start: April 2005; Study completion: April 2008
Purpose
This research study will assess whether AAB-001 is safe, well tolerated and effective for use in patients with Alzheimer's Disease. AAB-001 is a new drug that is not available outside this study. AAB-001 is an antibody (a type of protein usually produced by white blood cells to destroy other substances in the body). In Alzheimer's disease a protein called amyloid gathers in the brain and is thought to cause symptoms like memory loss and confusion. It is hoped that AAB-001 will attach to the amyloid protein in your brain and help your body to remove it.
Sollten die Ergebnisse wirklich überwältigend sein, ist eine Zulassung auf Basis dieser relativ mächtigen Phase-II-Studien durchaus vorstellbar!!
http://clinicaltrials.gov/ct/show/NCT00112073?order=17
Total Enrollment: 240
Study start: April 2005; Study completion: April 2008
Purpose
The purpose of this study is to assess the safety and tolerability of multiple doses of AAB-001 passive immunization in patients with mild to moderate Alzheimer's disease (AD).
http://clinicaltrials.gov/ct/show/NCT00174525?order=3
Study start: April 2005; Study completion: April 2008
Purpose
This research study will assess whether AAB-001 is safe, well tolerated and effective for use in patients with Alzheimer's Disease. AAB-001 is a new drug that is not available outside this study. AAB-001 is an antibody (a type of protein usually produced by white blood cells to destroy other substances in the body). In Alzheimer's disease a protein called amyloid gathers in the brain and is thought to cause symptoms like memory loss and confusion. It is hoped that AAB-001 will attach to the amyloid protein in your brain and help your body to remove it.
Sollten die Ergebnisse wirklich überwältigend sein, ist eine Zulassung auf Basis dieser relativ mächtigen Phase-II-Studien durchaus vorstellbar!!
interessanter Artikel:
http://www.ama-assn.org/amednews/2007/04/09/hlsb0409.htm
Push for Alzheimer's treatment grows along with numbers
New drugs in the pipeline have yielded positive early reports, says a researcher involved in the studies.
New drugs are in the pipeline and could arrive at the Food and Drug Administration by fall for possible approval next year, Sam Gandy, MD, PhD, director of Thomas Jefferson University's Farber Institute for Neurosciences, in Philadelphia, told the Senate panel. Dr. Gandy's laboratory is participating in clinical trials for two of the new drugs.
So far, the FDA has approved several drugs that temporarily slow symptoms in some people, but the medicines being tested are intended to attack the disease directly. Among them are two that target the amyloid plaques that are a molecular hallmark of Alzheimer's, Dr. Gandy said. Reports on the new entities are very encouraging, he added. "These drugs are safe. Patients tolerate them well. And they appear to show significant positive impact, slowing progression of the disease."
http://www.ama-assn.org/amednews/2007/04/09/hlsb0409.htm
Push for Alzheimer's treatment grows along with numbers
New drugs in the pipeline have yielded positive early reports, says a researcher involved in the studies.
New drugs are in the pipeline and could arrive at the Food and Drug Administration by fall for possible approval next year, Sam Gandy, MD, PhD, director of Thomas Jefferson University's Farber Institute for Neurosciences, in Philadelphia, told the Senate panel. Dr. Gandy's laboratory is participating in clinical trials for two of the new drugs.
So far, the FDA has approved several drugs that temporarily slow symptoms in some people, but the medicines being tested are intended to attack the disease directly. Among them are two that target the amyloid plaques that are a molecular hallmark of Alzheimer's, Dr. Gandy said. Reports on the new entities are very encouraging, he added. "These drugs are safe. Patients tolerate them well. And they appear to show significant positive impact, slowing progression of the disease."
...ich bin sehr zuversichtlich, dass das AC am 31.7.07 eine Empfehlung auf Zulassung von Tysabri zur Behandlung von MC aussprechen wird (allerdings unter Anwendung eines Risikoplans analog MS) und die FDA in diesem Fall auch dieser Empfehlung folgen wird!

http://www.pharmaceutical-business-review.com/article_news.a…

http://www.pharmaceutical-business-review.com/article_news.a…
Elans Anzahl an Patenten ist schon beeindruckend:
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Dale Schenk, Elans Chief Scientific Officer, ist für mich ein zukünftiger Nobelpreisträger, in dessen Forschungsaktivitäten die potente Alzheimer-Pipeline begründet ist.
http://www.jbc.org/cgi/content/abstract/281/40/29739?etoc
http://www.jbc.org/cgi/content/abstract/281/40/29739?etoc
bei den beiden Wirkstoffen dürfte es sich um das Immunokonjugat ACC-001 und die abgebrochene Studie mit AN-1792 handeln:
Neuer Impfstoff gegen Alzheimer-
Krankheit
Forscher aus Boston und Zürich haben
einen neuen Impfstoff gegen Alzheimer
erfolgreich bei Mäusen getestet.
Der Impfstoff reduziert das krankmachende
Eiweiss Beta-Amyloid, den
wichtigsten Bestandteil der krankhaften
Ablagerungen im Alzheimer-Gehirn.
Die Studie wird im Mai 2006 im
«Journal of Neuroscience» publiziert.
Die Forscher zeigen in ihrer Studie,
dass der neue Impfstoff, bestehend
aus zwei aneinander gereihten Kopien
des verkürzten Beta-Amyloid-Eiweisses,
die so genannten Amyloid-Plaques
wirksam reduziert. Die Amyloid-Plaques
sind eine der wichtigsten krankhaften
Ablagerungen im Gehirn von
Alzheimer-Patienten. Ebenso verbesserten
sich die Lern- und Gedächtnisfähigkeiten
in Verhaltenstests mit den
Alzheimer-Mäusen. Diese Verbesserungen
wurden erreicht, indem die
Impfstoffe wöchentlich auf der Nasenschleimhaut
appliziert wurden.
Der neue Impfstoff bildet Antikörper
gegen Beta-Amyloid, ohne eine Beta-
Amyloid-spezifische zelluläre Immunreaktion
auszulösen. Diese Immunreaktion
gegen das körpereigene
Beta-Amyloid-Eiweiss war höchstwahrscheinlich
die Ursache der Hirnhautentzündungen,
die bei einigen
Patienten in der abgebrochenen klinischen
Studie des ersten Alzheimer-
Impfstoffes aufgetreten sind.
Quelle: Universität Zürich/
www.journalmed.de
Neuer Impfstoff gegen Alzheimer-
Krankheit
Forscher aus Boston und Zürich haben
einen neuen Impfstoff gegen Alzheimer
erfolgreich bei Mäusen getestet.
Der Impfstoff reduziert das krankmachende
Eiweiss Beta-Amyloid, den
wichtigsten Bestandteil der krankhaften
Ablagerungen im Alzheimer-Gehirn.
Die Studie wird im Mai 2006 im
«Journal of Neuroscience» publiziert.
Die Forscher zeigen in ihrer Studie,
dass der neue Impfstoff, bestehend
aus zwei aneinander gereihten Kopien
des verkürzten Beta-Amyloid-Eiweisses,
die so genannten Amyloid-Plaques
wirksam reduziert. Die Amyloid-Plaques
sind eine der wichtigsten krankhaften
Ablagerungen im Gehirn von
Alzheimer-Patienten. Ebenso verbesserten
sich die Lern- und Gedächtnisfähigkeiten
in Verhaltenstests mit den
Alzheimer-Mäusen. Diese Verbesserungen
wurden erreicht, indem die
Impfstoffe wöchentlich auf der Nasenschleimhaut
appliziert wurden.
Der neue Impfstoff bildet Antikörper
gegen Beta-Amyloid, ohne eine Beta-
Amyloid-spezifische zelluläre Immunreaktion
auszulösen. Diese Immunreaktion
gegen das körpereigene
Beta-Amyloid-Eiweiss war höchstwahrscheinlich
die Ursache der Hirnhautentzündungen,
die bei einigen
Patienten in der abgebrochenen klinischen
Studie des ersten Alzheimer-
Impfstoffes aufgetreten sind.
Quelle: Universität Zürich/
www.journalmed.de
Newly Discovered Antibody May Be Body's Natural Defense Against Alzheimer's, Other Neurological Diseases
Main Category:
Alzheimer's / Dementia News
Article Date: 12 Jun 2007 - 0:00 PDT
In an important advance in the battle against Alzheimer's disease, physician-scientists at NewYork-Presbyterian Hospital/Weill Cornell Medical Center have identified naturally occurring antibodies in human blood that may help to defend against this form of dementia as well as other neurodegenerative diseases.
The newly found antibodies selectively target aggregates of beta amyloid proteins called "oligomers" that are toxic to brain cells, while ignoring the benign single-molecule forms of the same proteins. The existence of such antibodies was predicted by animal studies, but they were never previously demonstrated to be present in substantial quantities in blood from normal humans.
Lead researcher Dr. Norman Relkin, a behavioral neurologist and neuroscientist at NewYork-Presbyterian Hospital/Weill Cornell Medical Center, will present the findings on Monday, June 11, at 3:30 pm, at the "2nd Alzheimer's Association International Conference on Prevention of Dementia," in Washington, DC.
Dr. Relkin is director of the Memory Disorders Program at NewYork-Presbyterian/Weill Cornell and associate professor of clinical neurology at Weill Cornell Medical College.
Dr. Relkin's team has been testing an antibody-based immunotherapy called Intravenous Immunoglobulin (IVIG) as a potential new treatment for Alzheimer's. IVIG is made from the blood of healthy donors and was previously reported to contain small quantities of antibodies against beta amyloid.
"The effects of IVIG in lowering beta amyloid levels in Alzheimer's patients in our Phase I clinical trial were much more profound than we expected," Dr. Relkin explains. "We couldn't readily explain this based on the low levels of anti-amyloid antibodies known to be present in IVIG. We suspected there might be another, unseen player."
His laboratory studies demonstrated that IVIG initially bound very little single-molecule ("monomer") beta amyloid in a test tube. However, it gathered up much more of the protein when the amyloid was "aged" in a way that allowed soluble aggregates to form.
These beta amyloid "oligomers" can grow into insoluble "fibrils" that cluster around brain cells and are a hallmark of Alzheimer's disease. While monomers are produced from birth and appear to be relatively benign, oligomers have been implicated as potent toxins responsible for Alzheimer's-linked memory loss and brain cell death. This has led many scientists to speculate that oligomers may be the main culprit in Alzheimer's and therefore a prime target for a new generation of disease-modifying treatments.
To further confirm that these natural antibodies bind with oligomers, Dr. Relkin and his colleagues collaborated with Drs. Charles Glabe and Rakez Kayed from the University of California at Irvine, who had previously created anti-oligomer antibodies in rabbits. Using techniques pioneered by Dr. Glabe's group, the NewYork-Presbyterian/Weill Cornell scientists were able to measure and extract the human version of anti-oligomer antibodies from IVIG and then demonstrate that these antibodies are present in the blood of normal individuals.
The basis for the selective recognition of oligomers by these antibodies appears to be their capacity to recognize the oligomer's misfolded shape.
"That was a surprise, because most antibodies work by recognizing some aspect of the chemical structure of their target -- not their shape," explains Weill Cornell Medical College molecular biologist and study co-author, Dr. Paul Szabo. "That means that even though beta amyloid monomers and oligomers have the same fundamental chemical makeup, human anti-oligomer antibodies can distinguish between them. The antibodies recognize a particular shape that proteins assume only when they become these toxic aggregates."
The ability of the antibodies to recognize toxic proteins based on their shape may have important implications for immune therapy of other neurologic disorders, the researchers explain.
"We were able to confirm that the antibodies we found not only recognize oligomers of beta amyloid but also unhealthy forms of other proteins that accumulate in a wide variety of diseases, such as Parkinson's, Lewy body dementia and Prion disease (the human form of 'Mad Cow' disease), to name a few," says Dr. Relkin.
Since beta amyloid oligomers are much less abundant in the body than the single-molecule variety, the relatively high amount of oligomer-specific antibody found in human blood suggests that the immune system recognizes these aggregates to be a particularly noxious threat.
"This could be part of an innate defense mechanism against Alzheimer's and other age-related neurodegenerative disorders," comments Dr. Marc Weksler, The Irving Wright Sherwood Professor of Geriatrics and professor of medicine at Weill Cornell Medical College, and senior investigator in the Phase I IVIG study that led to this discovery.
However, the clear demonstration of the relationship of these scientific findings to clinical benefit in patients requires much more study, the experts say.
NewYork-Presbyterian/Weill Cornell is currently leading a six-month Phase II study of IVIG in 24 patients with mild and moderate Alzheimer's disease, which is planned to be complete later this year. While this study may provide a signal of the effect of IVIG therapy to clinical outcomes, further investigation in larger controlled and longer-term trials will be needed to definitively demonstrate whether IVIG is useful in treating Alzheimer's.
Still, this discovery substantially boosts our understanding of Alzheimer's and other neurodegenerative illnesses, the experts say.
This work was funded by grants from Baxter Healthcare, which developed and produces GAMMAGARD IVIG; the Citigroup Foundation; and private philanthropy.
Other collaborators on the "Alzheimer's Association Prevention Conference" presentation include Drs. Marc Weksler, Diana Mujalli, Sushila Shenoy and Basia Adamiak -- all of NewYork-Presbyterian Hospital/Weill Cornell Medical Center.
http://www.medicalnewstoday.com/medicalnews.php?newsid=73889
Main Category:
Alzheimer's / Dementia News
Article Date: 12 Jun 2007 - 0:00 PDT
In an important advance in the battle against Alzheimer's disease, physician-scientists at NewYork-Presbyterian Hospital/Weill Cornell Medical Center have identified naturally occurring antibodies in human blood that may help to defend against this form of dementia as well as other neurodegenerative diseases.
The newly found antibodies selectively target aggregates of beta amyloid proteins called "oligomers" that are toxic to brain cells, while ignoring the benign single-molecule forms of the same proteins. The existence of such antibodies was predicted by animal studies, but they were never previously demonstrated to be present in substantial quantities in blood from normal humans.
Lead researcher Dr. Norman Relkin, a behavioral neurologist and neuroscientist at NewYork-Presbyterian Hospital/Weill Cornell Medical Center, will present the findings on Monday, June 11, at 3:30 pm, at the "2nd Alzheimer's Association International Conference on Prevention of Dementia," in Washington, DC.
Dr. Relkin is director of the Memory Disorders Program at NewYork-Presbyterian/Weill Cornell and associate professor of clinical neurology at Weill Cornell Medical College.
Dr. Relkin's team has been testing an antibody-based immunotherapy called Intravenous Immunoglobulin (IVIG) as a potential new treatment for Alzheimer's. IVIG is made from the blood of healthy donors and was previously reported to contain small quantities of antibodies against beta amyloid.
"The effects of IVIG in lowering beta amyloid levels in Alzheimer's patients in our Phase I clinical trial were much more profound than we expected," Dr. Relkin explains. "We couldn't readily explain this based on the low levels of anti-amyloid antibodies known to be present in IVIG. We suspected there might be another, unseen player."
His laboratory studies demonstrated that IVIG initially bound very little single-molecule ("monomer") beta amyloid in a test tube. However, it gathered up much more of the protein when the amyloid was "aged" in a way that allowed soluble aggregates to form.
These beta amyloid "oligomers" can grow into insoluble "fibrils" that cluster around brain cells and are a hallmark of Alzheimer's disease. While monomers are produced from birth and appear to be relatively benign, oligomers have been implicated as potent toxins responsible for Alzheimer's-linked memory loss and brain cell death. This has led many scientists to speculate that oligomers may be the main culprit in Alzheimer's and therefore a prime target for a new generation of disease-modifying treatments.
To further confirm that these natural antibodies bind with oligomers, Dr. Relkin and his colleagues collaborated with Drs. Charles Glabe and Rakez Kayed from the University of California at Irvine, who had previously created anti-oligomer antibodies in rabbits. Using techniques pioneered by Dr. Glabe's group, the NewYork-Presbyterian/Weill Cornell scientists were able to measure and extract the human version of anti-oligomer antibodies from IVIG and then demonstrate that these antibodies are present in the blood of normal individuals.
The basis for the selective recognition of oligomers by these antibodies appears to be their capacity to recognize the oligomer's misfolded shape.
"That was a surprise, because most antibodies work by recognizing some aspect of the chemical structure of their target -- not their shape," explains Weill Cornell Medical College molecular biologist and study co-author, Dr. Paul Szabo. "That means that even though beta amyloid monomers and oligomers have the same fundamental chemical makeup, human anti-oligomer antibodies can distinguish between them. The antibodies recognize a particular shape that proteins assume only when they become these toxic aggregates."
The ability of the antibodies to recognize toxic proteins based on their shape may have important implications for immune therapy of other neurologic disorders, the researchers explain.
"We were able to confirm that the antibodies we found not only recognize oligomers of beta amyloid but also unhealthy forms of other proteins that accumulate in a wide variety of diseases, such as Parkinson's, Lewy body dementia and Prion disease (the human form of 'Mad Cow' disease), to name a few," says Dr. Relkin.
Since beta amyloid oligomers are much less abundant in the body than the single-molecule variety, the relatively high amount of oligomer-specific antibody found in human blood suggests that the immune system recognizes these aggregates to be a particularly noxious threat.
"This could be part of an innate defense mechanism against Alzheimer's and other age-related neurodegenerative disorders," comments Dr. Marc Weksler, The Irving Wright Sherwood Professor of Geriatrics and professor of medicine at Weill Cornell Medical College, and senior investigator in the Phase I IVIG study that led to this discovery.
However, the clear demonstration of the relationship of these scientific findings to clinical benefit in patients requires much more study, the experts say.
NewYork-Presbyterian/Weill Cornell is currently leading a six-month Phase II study of IVIG in 24 patients with mild and moderate Alzheimer's disease, which is planned to be complete later this year. While this study may provide a signal of the effect of IVIG therapy to clinical outcomes, further investigation in larger controlled and longer-term trials will be needed to definitively demonstrate whether IVIG is useful in treating Alzheimer's.
Still, this discovery substantially boosts our understanding of Alzheimer's and other neurodegenerative illnesses, the experts say.
This work was funded by grants from Baxter Healthcare, which developed and produces GAMMAGARD IVIG; the Citigroup Foundation; and private philanthropy.
Other collaborators on the "Alzheimer's Association Prevention Conference" presentation include Drs. Marc Weksler, Diana Mujalli, Sushila Shenoy and Basia Adamiak -- all of NewYork-Presbyterian Hospital/Weill Cornell Medical Center.
http://www.medicalnewstoday.com/medicalnews.php?newsid=73889
Elans Partner bei der Entwicklung des Alzheimer-Wirkstoffes AZD103/ELND005 TransitionTherapeutics plant ein Listing an der NASDAQ:
http://www.transitiontherapeutics.com/news/article.php
Bei den von Kelly Martin angesprochenen möglichen Übernahmen, die das wissenschaftliche "Know how" erweitern bzw. Synergien bieten, dürfte TT zumindest ein potentieller Übernahmekandidat darstellen. Time will tell!
http://www.transitiontherapeutics.com/news/article.php
Bei den von Kelly Martin angesprochenen möglichen Übernahmen, die das wissenschaftliche "Know how" erweitern bzw. Synergien bieten, dürfte TT zumindest ein potentieller Übernahmekandidat darstellen. Time will tell!
...die "Alzheimer-Geschichte" gewinnt langsam an Fahrt:
http://www.upi.com/Health_Business/Analysis/2007/06/11/analy…
Elan/Wyeth's bapineuzumab, the first biologic agent expected to win approval for the treatment of Alzheimer's disease, will triple the market for medications for the brain illness to $8.8 billion by 2016, according to an analyst's report issued Monday.
http://www.upi.com/Health_Business/Analysis/2007/06/11/analy…
Elan/Wyeth's bapineuzumab, the first biologic agent expected to win approval for the treatment of Alzheimer's disease, will triple the market for medications for the brain illness to $8.8 billion by 2016, according to an analyst's report issued Monday.
neuste Schätzungen (2006) gehen aktuell von über 25 Mio Alzheimer-Patienten aus, wobei sich diese Zahl bis 2050 vervierfachen wird:
http://www.alz.org/preventionconference/pc2007/releases/6100…
The latest worldwide estimate of Alzheimer’s disease prevalence shows that 26.6 million people were living with the disease in 2006, according to research reported today at the 2nd Alzheimer’s Association International Conference on Prevention of Dementia in Washington, D.C.
The researchers predict that global prevalence of Alzheimer’s will quadruple by 2050 to more than 100 million, at which time 1 in 85 persons worldwide will be living with the disease. More than 40 percent of those cases will be in late stage Alzheimer’s requiring a high level of attention equivalent to nursing home care.
http://www.alz.org/preventionconference/pc2007/releases/6100…
The latest worldwide estimate of Alzheimer’s disease prevalence shows that 26.6 million people were living with the disease in 2006, according to research reported today at the 2nd Alzheimer’s Association International Conference on Prevention of Dementia in Washington, D.C.
The researchers predict that global prevalence of Alzheimer’s will quadruple by 2050 to more than 100 million, at which time 1 in 85 persons worldwide will be living with the disease. More than 40 percent of those cases will be in late stage Alzheimer’s requiring a high level of attention equivalent to nursing home care.

Elan Corporation, plc at Goldman Sachs 28th Annual Global Healthcare Conference 13-Jun-07 1:40 PM
..etws nach unten scrollen, dann habt ihr den ELAN Audio-Link von der GS Conference...
http://www.fulldisclosure.com/company.asp?client=cb&ticker=E…





























 (sorry-musste sein!)
(sorry-musste sein!)
aus der Alzheimer-Pipeline:
1.) zu AAB-001 bzw. "Bapineuzumab" (generic name) --> No Time to Waste? Elan-Wyeth Vaccine to Enter Phase 3 Trial
http://www.alzforum.org/new/detail.asp?id=1591
2.) zu Eli Lillys LY450139, zu welchem Elan die Möglichkeit hat, 50% der Rechte zu erwerben --> Washington: γ-secretase Inhibitor Survived Phase 2, Moving to 3
http://www.alzforum.org/new/detail.asp?id=1607
...das interessante daran ist, dass Elan bei diesen beiden Wirkstoffen entweder über 50% der Rechte verfügt (Bapineuzumab) oder aber die Möglichkeit hat, 50% der Rechte zu erwerben (LY450139). Ich denke Elan wird diese Option für LY450139 jedoch nur dann ausüben, wenn die in der eigenen Pipeline entwickelten gamma-Secretase-Hemmer hinter den Erwartungen zurückbleiben. Auf alle Fälle spielt Elan kräftig mit im Konzert bzw. Rennen der Grossen um eine Führungsrolle im grössten aller Märkte in der Pharmazie überhaupt, der das bisher Dagewesene komplett in den Schatten stellen könnte!
1.) zu AAB-001 bzw. "Bapineuzumab" (generic name) --> No Time to Waste? Elan-Wyeth Vaccine to Enter Phase 3 Trial
http://www.alzforum.org/new/detail.asp?id=1591
2.) zu Eli Lillys LY450139, zu welchem Elan die Möglichkeit hat, 50% der Rechte zu erwerben --> Washington: γ-secretase Inhibitor Survived Phase 2, Moving to 3
http://www.alzforum.org/new/detail.asp?id=1607
...das interessante daran ist, dass Elan bei diesen beiden Wirkstoffen entweder über 50% der Rechte verfügt (Bapineuzumab) oder aber die Möglichkeit hat, 50% der Rechte zu erwerben (LY450139). Ich denke Elan wird diese Option für LY450139 jedoch nur dann ausüben, wenn die in der eigenen Pipeline entwickelten gamma-Secretase-Hemmer hinter den Erwartungen zurückbleiben. Auf alle Fälle spielt Elan kräftig mit im Konzert bzw. Rennen der Grossen um eine Führungsrolle im grössten aller Märkte in der Pharmazie überhaupt, der das bisher Dagewesene komplett in den Schatten stellen könnte!
June 18, 2007, 10:22 am
Tysabri Update: No New Infections for MS DrugPosted by Jacob Goldstein
Tysabri, the multiple sclerosis drug that was withdrawn temporarily from the market after reports of serious brain infections, hasn’t been connected with any such infections since shipments resumed last July, the latest data show.
Biogen Idec, which co-markets the drug with Elan, made the update in a securities filing today. Since the companies resumed selling the drug, there have been “no confirmed cases of PML or other serious opportunistic infections,” according to a single PowerPoint slide Biogen Idec filed with the SEC. The filing occurred in advance of a presentation today at a neurology meeting in Greece.
Tysabri was approved in late 2004 then withdrawn in early 2005, after it emerged that three people in clinical trials of the drug developed PML, or progressive multifocal leukoencephalopathy, a very rare, very serious infection of the brain. As of May 23rd of this year, about 12,000 patients worldwide were on the drug, including about 7,600 in this country.
The agreement the company worked out with the FDA — described here — suggests the agency will continue to scrutinize the drug. The agreement requires the company monitor all patients on the drug, and to report to the FDA every quarter for the first year, every six months for the next two years, and annually after that. If any cases of PML or other serious opportunistic infections emerge, they must be reported within 15 days.
http://blogs.wsj.com/health/2007/06/18/tysabri-update-no-new…
Tysabri Update: No New Infections for MS DrugPosted by Jacob Goldstein
Tysabri, the multiple sclerosis drug that was withdrawn temporarily from the market after reports of serious brain infections, hasn’t been connected with any such infections since shipments resumed last July, the latest data show.
Biogen Idec, which co-markets the drug with Elan, made the update in a securities filing today. Since the companies resumed selling the drug, there have been “no confirmed cases of PML or other serious opportunistic infections,” according to a single PowerPoint slide Biogen Idec filed with the SEC. The filing occurred in advance of a presentation today at a neurology meeting in Greece.
Tysabri was approved in late 2004 then withdrawn in early 2005, after it emerged that three people in clinical trials of the drug developed PML, or progressive multifocal leukoencephalopathy, a very rare, very serious infection of the brain. As of May 23rd of this year, about 12,000 patients worldwide were on the drug, including about 7,600 in this country.
The agreement the company worked out with the FDA — described here — suggests the agency will continue to scrutinize the drug. The agreement requires the company monitor all patients on the drug, and to report to the FDA every quarter for the first year, every six months for the next two years, and annually after that. If any cases of PML or other serious opportunistic infections emerge, they must be reported within 15 days.
http://blogs.wsj.com/health/2007/06/18/tysabri-update-no-new…
...Eli Lilly müsste bald einmal erste Ergebnisse der Phase2-Studie des gamma-Secretase-Hemmers LY450139 bekannt geben, an welchem Elan die Option auf eine 50%ige Beteiligung besitzt:
http://www.clinicaltrials.gov/ct/show/NCT00244322?order=1
http://www.clinicaltrials.gov/ct/show/NCT00244322?order=1
aus einer Präsentation von BiogenIdec bei der Hauptversammlung Ende Mai 2007:
2007
U.S. commercial patients on therapy
Anfang Februar: 5,000
Mitte April: 6,600
Ende Mai: 7,600
International commercial patients on therapy (rest of world)
Anfang Februar: 1,600 (Total: 6,600)
Mitte April: 2,500 (Total: 9,100)
Ende Mai: 3,200 (Total: 10,800)
Total commercial & clinical trial patients on therapy
Anfang Februar: 7,500
Mitte April: 10,000
Ende Mai: 12,000
Welche Schlüsse kann man daraus ziehen?
1.) Die Anzahl der Studienteilnehmer ist um 300 gestiegen! Dies ist in den vielen Studien begründet, die im Laufe der letzten Wochen gestartet wurden:
http://clinicaltrials.gov/ct/search;jsessionid=0112C78ED4A6E…
2.) Die "kommerzielle" Zuwachsrate bei Tysabri ist leicht, wenn auch unter den Ertwartungen, gestiegen:
Anfang Feb (6,600) bis Mitte April (9,100) = 10 Wochen
2500 => durchschnittlicher Zuwachs: 250 Ty-Empfänger/Woche
Mitte April (9,100) bis Ende Mai (10,800) = 5.5 Wochen
1700 => durchschnittlicher Zuwachs: 309 Ty-Empf./Woche
Aber die wichtigste Aussage war die Folgende:
As of April 23, 2007: No new reports of confirmed cases of PML or other serious opportunistic infections!
Die zukünfzige Wachstumsrate dürfte weiter ansteigen,...
1.)...da Ty in weiteren Ländern eingeführt wurde/wird: z.B. Italy, Canada, Slovakia, Greece, Luxembourg, Switzerland, France etc.
2.)...wenn keine weiteren PML-Fälle vermeldet werden.
3.)...falls Ty für MorbusCrohn in der EU und den US zugelassen wird.
2007
U.S. commercial patients on therapy
Anfang Februar: 5,000
Mitte April: 6,600
Ende Mai: 7,600
International commercial patients on therapy (rest of world)
Anfang Februar: 1,600 (Total: 6,600)
Mitte April: 2,500 (Total: 9,100)
Ende Mai: 3,200 (Total: 10,800)
Total commercial & clinical trial patients on therapy
Anfang Februar: 7,500
Mitte April: 10,000
Ende Mai: 12,000
Welche Schlüsse kann man daraus ziehen?
1.) Die Anzahl der Studienteilnehmer ist um 300 gestiegen! Dies ist in den vielen Studien begründet, die im Laufe der letzten Wochen gestartet wurden:
http://clinicaltrials.gov/ct/search;jsessionid=0112C78ED4A6E…
2.) Die "kommerzielle" Zuwachsrate bei Tysabri ist leicht, wenn auch unter den Ertwartungen, gestiegen:
Anfang Feb (6,600) bis Mitte April (9,100) = 10 Wochen
2500 => durchschnittlicher Zuwachs: 250 Ty-Empfänger/Woche
Mitte April (9,100) bis Ende Mai (10,800) = 5.5 Wochen
1700 => durchschnittlicher Zuwachs: 309 Ty-Empf./Woche
Aber die wichtigste Aussage war die Folgende:
As of April 23, 2007: No new reports of confirmed cases of PML or other serious opportunistic infections!
Die zukünfzige Wachstumsrate dürfte weiter ansteigen,...
1.)...da Ty in weiteren Ländern eingeführt wurde/wird: z.B. Italy, Canada, Slovakia, Greece, Luxembourg, Switzerland, France etc.
2.)...wenn keine weiteren PML-Fälle vermeldet werden.
3.)...falls Ty für MorbusCrohn in der EU und den US zugelassen wird.
...in den amerikanischen Foren erwarten die Optimisten eine EMEA-Empfehlung zur Therapie von MorbusCrohn mit Tysabri bereits von der heutigen Sitzung der entsprechenden Expertenrunde - dies würde dann Morgen auf folgender Seite bekannt gegeben:
http://www.emea.europa.eu/whatsnewp.htm
...ich kann mir jedoch nicht vorstellen, dass die EMEA der Expertenrunde (Advisory Committee) der FDA vorgreift, welche am 31.7. zusammentrifft, um über eine Zulassungsempfehlung bei der behandlung von Morbus Crohn zu beraten. Die EMEA spielt wohl immernoch nur die 2.Geige, obwohl die Zulassungsunterlagen bei der EMEA vor bereits knapp 3 Jahre eingereicht wurden.
http://www.emea.europa.eu/whatsnewp.htm
...ich kann mir jedoch nicht vorstellen, dass die EMEA der Expertenrunde (Advisory Committee) der FDA vorgreift, welche am 31.7. zusammentrifft, um über eine Zulassungsempfehlung bei der behandlung von Morbus Crohn zu beraten. Die EMEA spielt wohl immernoch nur die 2.Geige, obwohl die Zulassungsunterlagen bei der EMEA vor bereits knapp 3 Jahre eingereicht wurden.
Artikel zur Alzheimer Vaccine Therapie
Science Daily — Scientists have provided new details about how proteins used to destroy bacteria and viruses may help treat Alzheimer's disease. Gunnar K. Gouras, associate professor of neurology and neuroscience at Weill Medical College of Cornell University, New York, and colleagues provide new insights into how these proteins, called antibodies, reduce the main hallmarks of Alzheimer's disease and raise hopes for a vaccine against the disease.
"Antibodies are probably the most promising experimental approach to fight Alzheimer's disease at this time," Gouras says. "The discoveries made using antibodies are so encouraging that results of ongoing vaccine trials against the disease are much anticipated."
Alzheimer's disease, the most common form of dementia, gradually destroys a person's memory and ability to learn, communicate, and carry out daily activities. According to the American Health Assistance Foundation, more than 4.5 million people in the United States live with the disease and more than 26 million people are affected worldwide. By 2050, the number of people who will suffer from the disease is estimated to nearly triple in the United States and to be four times as high worldwide.
Although no cure for the disease is available yet, scientists are actively looking for new treatments. One of the main goals of such treatments is to destroy clumps of a protein called beta amyloid, which are found in the brains of people with the disease, either inside the nerve cells or around them. Antibodies have been shown to be effective at removing these clumps but how they do it is not completely understood.
In their new study, appearing in the June 29 issue of the Journal of Biological Chemistry, Gouras and his colleagues provide new details about how the antibodies attack these clumps inside the nerve cells. The study was selected as a "Paper of the Week" by the journal's editor, meaning that it belongs to the top one percent of papers reviewed in significance and overall importance.
Using cultured cells from mice, the scientists showed that the antibodies first bind to the surface of the cells and connect to a protein called amyloid precursor protein (APP), which is already present on the cell surface. Then both proteins are internalized inside the cell.
Once inside the cell, APP is broken down into pieces, some of which are the amyloid beta proteins. If the antibodies are not present, the proteins start clustering and ultimately kill the cell. The scientists showed that the antibodies prevent this from happening by reducing accumulation of the amyloid beta proteins in vesicles inside the cell called endosomes.
"A lot of research has been done on protein clusters outside nerve cells," Gouras says. "In this study, we investigated for the first time what happens inside the cells and how antibodies can help prevent clusters from forming."
The researchers also found that the antibodies helped restore communication between nerve cells. In Alzheimer's patients, the protein clusters alter parts of the cell surfaces -- the synapses -- that help nerve cells talk to one another. As a result, thoughts are not transmitted, memory is lost, and new learning is hindered. But Gouras and his team showed that the antibodies cleared the protein clusters and helped cells talk to one another again.
Over the past seven years, research results on the use of antibodies against Alzheimer's disease have been so promising that two pharmaceutical companies, Ireland's Elan Corp. and U.S. partner Wyeth, have been conducting clinical trials of a potential vaccine. Although the first trials were stopped when 6 percent of the patients developed encephalitis -- an inflammation of brain tissue -- other clinical tests on the treated patients have been encouraging. In the second half of this year, the two companies will test a potential drug, called Bapineuzumab, on patients with mild to moderate Alzheimer's symptoms.
If successful, these trials could result in a new type of vaccine containing antibodies that would directly attack the amyloid beta protein clusters. Unlike common vaccines, which, in this case, would contain pieces of amyloid beta proteins and would stimulate the immune system to produce antibodies, the new vaccine would directly provide the antibodies to patients.
"These new developments are encouraging, but possible side effects may arise," Gouras says. He adds that although clinical trials need to be conducted as soon as possible to help alleviate the suffering of the increasing number of Alzheimer's patients, more research is still needed both to understand how amyloid beta proteins wreak havoc in the brain and to improve potential drugs.
Gouras and his colleagues are now trying to figure out how the protein clusters inside and outside the cells work together to destroy the cells. They already noticed that the clusters outside the cells affect those inside cells by making them grow. Another challenge will be to better understand what the clusters do inside the cells that leads to their death. The scientists are also using imaging and biochemical techniques to see, in cultured cells, how the antibodies affect the clusters.
"We have many indications that antibodies work," Gouras says. "Now we need to understand how they do it."
Article: "Internalized antibodies to the A-beta domain of APP reduce neuronal A-beta and protect against synaptic alterations," by Davide Tampellini, Jordi Magrane', Reisuke H. Takahashi, Feng Li, Michael T. Lin, Claudia G. Almeida, and Gunnar K. Gouras
Note: This story has been adapted from a news release issued by American Society for Biochemistry and Molecular Biology.
http://www.dentalplans.com/articles/20262/
Science Daily — Scientists have provided new details about how proteins used to destroy bacteria and viruses may help treat Alzheimer's disease. Gunnar K. Gouras, associate professor of neurology and neuroscience at Weill Medical College of Cornell University, New York, and colleagues provide new insights into how these proteins, called antibodies, reduce the main hallmarks of Alzheimer's disease and raise hopes for a vaccine against the disease.
"Antibodies are probably the most promising experimental approach to fight Alzheimer's disease at this time," Gouras says. "The discoveries made using antibodies are so encouraging that results of ongoing vaccine trials against the disease are much anticipated."
Alzheimer's disease, the most common form of dementia, gradually destroys a person's memory and ability to learn, communicate, and carry out daily activities. According to the American Health Assistance Foundation, more than 4.5 million people in the United States live with the disease and more than 26 million people are affected worldwide. By 2050, the number of people who will suffer from the disease is estimated to nearly triple in the United States and to be four times as high worldwide.
Although no cure for the disease is available yet, scientists are actively looking for new treatments. One of the main goals of such treatments is to destroy clumps of a protein called beta amyloid, which are found in the brains of people with the disease, either inside the nerve cells or around them. Antibodies have been shown to be effective at removing these clumps but how they do it is not completely understood.
In their new study, appearing in the June 29 issue of the Journal of Biological Chemistry, Gouras and his colleagues provide new details about how the antibodies attack these clumps inside the nerve cells. The study was selected as a "Paper of the Week" by the journal's editor, meaning that it belongs to the top one percent of papers reviewed in significance and overall importance.
Using cultured cells from mice, the scientists showed that the antibodies first bind to the surface of the cells and connect to a protein called amyloid precursor protein (APP), which is already present on the cell surface. Then both proteins are internalized inside the cell.
Once inside the cell, APP is broken down into pieces, some of which are the amyloid beta proteins. If the antibodies are not present, the proteins start clustering and ultimately kill the cell. The scientists showed that the antibodies prevent this from happening by reducing accumulation of the amyloid beta proteins in vesicles inside the cell called endosomes.
"A lot of research has been done on protein clusters outside nerve cells," Gouras says. "In this study, we investigated for the first time what happens inside the cells and how antibodies can help prevent clusters from forming."
The researchers also found that the antibodies helped restore communication between nerve cells. In Alzheimer's patients, the protein clusters alter parts of the cell surfaces -- the synapses -- that help nerve cells talk to one another. As a result, thoughts are not transmitted, memory is lost, and new learning is hindered. But Gouras and his team showed that the antibodies cleared the protein clusters and helped cells talk to one another again.
Over the past seven years, research results on the use of antibodies against Alzheimer's disease have been so promising that two pharmaceutical companies, Ireland's Elan Corp. and U.S. partner Wyeth, have been conducting clinical trials of a potential vaccine. Although the first trials were stopped when 6 percent of the patients developed encephalitis -- an inflammation of brain tissue -- other clinical tests on the treated patients have been encouraging. In the second half of this year, the two companies will test a potential drug, called Bapineuzumab, on patients with mild to moderate Alzheimer's symptoms.
If successful, these trials could result in a new type of vaccine containing antibodies that would directly attack the amyloid beta protein clusters. Unlike common vaccines, which, in this case, would contain pieces of amyloid beta proteins and would stimulate the immune system to produce antibodies, the new vaccine would directly provide the antibodies to patients.
"These new developments are encouraging, but possible side effects may arise," Gouras says. He adds that although clinical trials need to be conducted as soon as possible to help alleviate the suffering of the increasing number of Alzheimer's patients, more research is still needed both to understand how amyloid beta proteins wreak havoc in the brain and to improve potential drugs.
Gouras and his colleagues are now trying to figure out how the protein clusters inside and outside the cells work together to destroy the cells. They already noticed that the clusters outside the cells affect those inside cells by making them grow. Another challenge will be to better understand what the clusters do inside the cells that leads to their death. The scientists are also using imaging and biochemical techniques to see, in cultured cells, how the antibodies affect the clusters.
"We have many indications that antibodies work," Gouras says. "Now we need to understand how they do it."
Article: "Internalized antibodies to the A-beta domain of APP reduce neuronal A-beta and protect against synaptic alterations," by Davide Tampellini, Jordi Magrane', Reisuke H. Takahashi, Feng Li, Michael T. Lin, Claudia G. Almeida, and Gunnar K. Gouras
Note: This story has been adapted from a news release issued by American Society for Biochemistry and Molecular Biology.
http://www.dentalplans.com/articles/20262/
ALLGEMEINE INFOS zur Zulassung neuer Arzneien
22.06.2007 - 09:28 Uhr
FTD: Das kann ja heiter werden
Die Zulassung neuer Arzneien ist für die Pharmabranche längst keine Kleinigkeit mehr. Regelmäßig lassen die Behörden Hoffnungsträger durchfallen und Börsenträume platzen. Künftig kommt es noch dicker.
Sanofi-Aventis ist fassungslos über die Ablehnung seiner Diätpille Acomplia bei der US-Behörde FDA. Begründung: psychische Nebenwirkungen.
GlaxoSmithKline fürchtet das Aus für das umsatzstarke Diabetesmittel Avandia. Risiko: Herzinfarkt.
Das neue Krebsmittel Vectibix vom weltgrößten Biotechkonzern Amgen findet nicht den Zuspruch der Kontrolleure bei der EU-Behörde Emea. Erklärung: Wirksamkeit zweifelhaft.
Drei Beispiele aus der jüngsten Vergangenheit - eine gemeinsame Wirkung: Aufruhr an der Börse. Die Aktienkurse der betreffenden Konzerne sackten nacheinander auf ein Zweijahrestief. Allen Beteiligten, den Managern, Analysten und Kontrolleuren steckt noch der Skandal um das Schmerzmittel Vioxx in den Knochen. Die Folge für den Hersteller Merck & Co. sowie die Branche waren Tausende Schadensersatzklagen, Imagekrisen und Milliardenverluste. Das mahnte zur Vorsicht. Im Jahr drei nach Vioxx zieht die Politik auf beiden Seiten des Atlantiks die Zügel nun noch fester an.
So feilten am Donnerstag in Washington US-Senatoren an letzten Details für ein Gesetz, das der FDA mehr Einfluss und Geld für zusätzliche Sicherheitskontrollen von Medikamenten gibt. Dazu zahlt die Industrie an die FDA Gebühren von rund 400 Mio. $ jährlich, weitere 225 Mio. $ bringt sie in den kommenden fünf Jahren für die FDA-Observierung von Neueinführungen auf. "Die Nation hat aus den Sicherheitsproblemen mit dem Diabetesmedikament Avandia gelernt", sagte der Vorsitzende des Kongressausschusses, John Dingell, vor wenigen Tagen. Zudem können Verstöße gegen Marketing- und Sicherheitsauflagen mit bis zu 100 Mio. $
Strafe geahndet werden.
Und auch die Emea kann nun härter durchgreifen. Die EU-Kommission hat am 15. Juni eine Verordnung in Kraft gesetzt die Verstöße gegen Emea-Regeln mit hohen Geldbußen ahndet - etwa, wenn Firmen Vorgaben ihrer Arzneimittelzulassungen nicht einhalten, Informationen zur Risikobewertung ihrer Produkte zurückhalten oder deren Nebenwirkungen gar nicht oder erst sehr spät melden.
"Sehr harte Sanktionen"
Die Höchstgrenze der Geldbußen liegt bei fünf Prozent des Jahresumsatzes des betroffenen Zulassungsinhabers. Zulassungsinhaber kann auch eine Konzerntochter sein. "Das dürfte zu ungerechten Bestrafungen führen", sagte Unternehmensanwalt Uwe Fröhlich vom Pharmakonzern Baxter. "Zufälligerweise oder sogar absichtlich kann ein besonders umsatzstarker oder umsatzschwacher Teil eines Konzerns Inhaber der Zulassung sein. Ein Schlupfloch könnten auch Vermarktungspartnerschaften unabhängiger Unternehmen bieten, von denen nur eines die Zulassung hält." Gerechter sei es, die Buße am EU-weiten Umsatz der Arznei festzumachen. "Das sind alles in allem sehr harte Sanktionen", sagt Anwalt Jörg Schickert von der Kanzlei Lovells in München. Er findet manches an der Verordnung unausgereift. "Es gibt noch Schwachstellen, darunter die Frage, wie die Abgrenzung zwischen einzelstaatlichen Strafmaßnahmen und EU-weiten Sanktionen geregelt werden soll."
Das für Zulassungen zuständige Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) habe in der jüngeren Vergangenheit einige Straf- und Bußgeldverfahren eingeleitet, mit unterschiedlichem Ausgang entsprechend der Beweislage, heißt es auf Anfrage. "Ob es in Zukunft wichtiger sein wird, derartige Instrumentarien zur Verfügung zu haben, lässt sich nur schwer einschätzen", so das BfArM. Die im Arzneimittelgesetz für Ordnungswidrigkeiten vorgesehenen Bußgelder von maximal 25.000 Euro seien vergleichsweise gering.
Die Verordnung (EG) 658/2007 zur Auferlegung von Geldbußen durch die Kommission ist ein neuartiges Sanktionsmittel, das es für von der Emea zugelassene Arzneimittel bislang noch nicht gab. "Die Industrie sollte schnellstmöglich alle Schwachstellen abklopfen und Verfahren aufsetzen, die zukünftig Verstöße vermeiden. Dies sollte auch dokumentiert werden", sagte der Anwalt Schickert.
Emea-Chef Thomas Lönngren zumindest will nie mehr einen Tag wie den 30. September 2004 erleben: Weder der Vioxx-Hersteller Merck & Co. noch die FDA-Kollegen hatten ihn frühzeitig über den geplanten Rückruf der Schmerzpille informiert. Die Börse wusste früher Bescheid als er. "Wir wollen von Konzernen so schnell wie möglich informiert werden", sagte er.
Autor/Autoren: Peter Kuchenbuch
http://www.finanztreff.de/ftreff/news.htm?sektion=topthemen&…
22.06.2007 - 09:28 Uhr
FTD: Das kann ja heiter werden
Die Zulassung neuer Arzneien ist für die Pharmabranche längst keine Kleinigkeit mehr. Regelmäßig lassen die Behörden Hoffnungsträger durchfallen und Börsenträume platzen. Künftig kommt es noch dicker.
Sanofi-Aventis ist fassungslos über die Ablehnung seiner Diätpille Acomplia bei der US-Behörde FDA. Begründung: psychische Nebenwirkungen.
GlaxoSmithKline fürchtet das Aus für das umsatzstarke Diabetesmittel Avandia. Risiko: Herzinfarkt.
Das neue Krebsmittel Vectibix vom weltgrößten Biotechkonzern Amgen findet nicht den Zuspruch der Kontrolleure bei der EU-Behörde Emea. Erklärung: Wirksamkeit zweifelhaft.
Drei Beispiele aus der jüngsten Vergangenheit - eine gemeinsame Wirkung: Aufruhr an der Börse. Die Aktienkurse der betreffenden Konzerne sackten nacheinander auf ein Zweijahrestief. Allen Beteiligten, den Managern, Analysten und Kontrolleuren steckt noch der Skandal um das Schmerzmittel Vioxx in den Knochen. Die Folge für den Hersteller Merck & Co. sowie die Branche waren Tausende Schadensersatzklagen, Imagekrisen und Milliardenverluste. Das mahnte zur Vorsicht. Im Jahr drei nach Vioxx zieht die Politik auf beiden Seiten des Atlantiks die Zügel nun noch fester an.
So feilten am Donnerstag in Washington US-Senatoren an letzten Details für ein Gesetz, das der FDA mehr Einfluss und Geld für zusätzliche Sicherheitskontrollen von Medikamenten gibt. Dazu zahlt die Industrie an die FDA Gebühren von rund 400 Mio. $ jährlich, weitere 225 Mio. $ bringt sie in den kommenden fünf Jahren für die FDA-Observierung von Neueinführungen auf. "Die Nation hat aus den Sicherheitsproblemen mit dem Diabetesmedikament Avandia gelernt", sagte der Vorsitzende des Kongressausschusses, John Dingell, vor wenigen Tagen. Zudem können Verstöße gegen Marketing- und Sicherheitsauflagen mit bis zu 100 Mio. $
Strafe geahndet werden.
Und auch die Emea kann nun härter durchgreifen. Die EU-Kommission hat am 15. Juni eine Verordnung in Kraft gesetzt die Verstöße gegen Emea-Regeln mit hohen Geldbußen ahndet - etwa, wenn Firmen Vorgaben ihrer Arzneimittelzulassungen nicht einhalten, Informationen zur Risikobewertung ihrer Produkte zurückhalten oder deren Nebenwirkungen gar nicht oder erst sehr spät melden.
"Sehr harte Sanktionen"
Die Höchstgrenze der Geldbußen liegt bei fünf Prozent des Jahresumsatzes des betroffenen Zulassungsinhabers. Zulassungsinhaber kann auch eine Konzerntochter sein. "Das dürfte zu ungerechten Bestrafungen führen", sagte Unternehmensanwalt Uwe Fröhlich vom Pharmakonzern Baxter. "Zufälligerweise oder sogar absichtlich kann ein besonders umsatzstarker oder umsatzschwacher Teil eines Konzerns Inhaber der Zulassung sein. Ein Schlupfloch könnten auch Vermarktungspartnerschaften unabhängiger Unternehmen bieten, von denen nur eines die Zulassung hält." Gerechter sei es, die Buße am EU-weiten Umsatz der Arznei festzumachen. "Das sind alles in allem sehr harte Sanktionen", sagt Anwalt Jörg Schickert von der Kanzlei Lovells in München. Er findet manches an der Verordnung unausgereift. "Es gibt noch Schwachstellen, darunter die Frage, wie die Abgrenzung zwischen einzelstaatlichen Strafmaßnahmen und EU-weiten Sanktionen geregelt werden soll."
Das für Zulassungen zuständige Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) habe in der jüngeren Vergangenheit einige Straf- und Bußgeldverfahren eingeleitet, mit unterschiedlichem Ausgang entsprechend der Beweislage, heißt es auf Anfrage. "Ob es in Zukunft wichtiger sein wird, derartige Instrumentarien zur Verfügung zu haben, lässt sich nur schwer einschätzen", so das BfArM. Die im Arzneimittelgesetz für Ordnungswidrigkeiten vorgesehenen Bußgelder von maximal 25.000 Euro seien vergleichsweise gering.
Die Verordnung (EG) 658/2007 zur Auferlegung von Geldbußen durch die Kommission ist ein neuartiges Sanktionsmittel, das es für von der Emea zugelassene Arzneimittel bislang noch nicht gab. "Die Industrie sollte schnellstmöglich alle Schwachstellen abklopfen und Verfahren aufsetzen, die zukünftig Verstöße vermeiden. Dies sollte auch dokumentiert werden", sagte der Anwalt Schickert.
Emea-Chef Thomas Lönngren zumindest will nie mehr einen Tag wie den 30. September 2004 erleben: Weder der Vioxx-Hersteller Merck & Co. noch die FDA-Kollegen hatten ihn frühzeitig über den geplanten Rückruf der Schmerzpille informiert. Die Börse wusste früher Bescheid als er. "Wir wollen von Konzernen so schnell wie möglich informiert werden", sagte er.
Autor/Autoren: Peter Kuchenbuch
http://www.finanztreff.de/ftreff/news.htm?sektion=topthemen&…
Antwort auf Beitrag Nr.: 30.144.959 von bernie55 am 22.06.07 11:29:39erwartungsgemäss hat sich das CHMP noch nicht zu Tysabri und MC geäussert:
http://www.emea.europa.eu/whatsnewp.htm
http://www.emea.europa.eu/whatsnewp.htm
Präsentation von Dr. Sperling vor der NYAS:
- bei Punkt 32 geht es um AAB-001....
http://www.nyas.org/ebriefreps/ebrief/000508/presentations/s…
- bei Punkt 32 geht es um AAB-001....
http://www.nyas.org/ebriefreps/ebrief/000508/presentations/s…
The next great growth stock
We'll be watching this universe as we continue our search for great growth stocks, and so should you. Take a look at Elan (NYSE: ELN), a biotechnology company with a promising pipeline producing drugs to treat Alzheimer's, multiple sclerosis, and more.
http://www.fool.com/investing/high-growth/2007/06/27/a-unive…
We'll be watching this universe as we continue our search for great growth stocks, and so should you. Take a look at Elan (NYSE: ELN), a biotechnology company with a promising pipeline producing drugs to treat Alzheimer's, multiple sclerosis, and more.
http://www.fool.com/investing/high-growth/2007/06/27/a-unive…
Eine Superzusammenfassung zu AAB-001
240 patients, 4 cohorts of 60 each.
For AAB-001 Phase II trial setup see also:
http://www.alzforum.org/drg/drc/detail.asp?id=101
http://www.clinicaltrials.gov/ct/show/NCT00112073
Peeks are done by 'an independant 3rd party'.
My assumption is that it is done by the trials DSMB.
http://www1.investorvillage.com/smbd.asp?mb=160&mn=76777&pt=…
We know the Phase I results for AAB-001.
This trial looked more like a Phase II in it's setup than a typical Phase I.
They showed positive efficacy (not stat sig, too few people) in the 0.5 dose and statistical significant efficacy (despite not that many patients in trial) in the 1.5 dose.
"At week 16, the prespecified primary timepoint for analysis, the treatment difference relative to placebo favored the treated group at the 0.5mg/kg dose (treatment vs. placebo difference of 2.0, p=.152) and reached statistical significance at the 1.5mg/kg dose (treatment vs. placebo difference of 2.5, p=.047)."
Ronald S. Black et.al.
Background: Bapineuzumab (AAB-001) is a humanized monoclonal antibody to A-beta being developed by Wyeth and Elan for the treatment of AD.
http://library.corporate-ir.net/library/88/883/88326/items/1…
We had an extensive discussion about the peeks & the schedule, but be warned that most information is only fragmentary and much of what is reported is at best only likely (that includes information as to what dosage group was first, when each group started, when it is likely to end etc).
See for a part of one of those discussions last January where we talk about the interim peek schedule:
http://www1.investorvillage.com/smbd.asp?mb=160&mn=67245&pt…
We seem to be following this schedule (as explained in above post):
"May-07: peek at Cohort 3, Jun-07: Study results, July-07: Move to P3 (or not) ..."
Then we had a good PR in May:
"On 21 May 2007, Elan and Wyeth announced their plans to initiate phase 3 clinical trials of Bapineuzumab. The decision to launch phase 3 studies prior to the conclusion of the ongoing phase 2 was based on the totality of the accumulated clinical data from phase 1, phase 2 and a 4.5- year follow-up study of those patients involved in the original AN-1792 trial."
Now the fact that May-07 was spot on is likely just a coincidence, so the move to P3 need not be already in this month (as in officially start recruiting patients, not already the start of dosing; dosing would start no earlier than a few months later, say September 2007 (all IMHO as usual) ).
Apart from the Business & Finance article, which gave us some clues, we also have this post by living_with_elan which describes what KM said about it:
http://www1.investorvillage.com/smbd.asp?mb=160&mn=89426&pt=…
The important part of the B&F article is (posted by hunstmann_ie, msg 2139 at elanians):
B&F interview with KM. Extract #2.
"The decision to move from Phase 2 to Phase 3 is a large one because we have responsibilities to the patients. If you publicly annouce that you are at the last stage of a disease modifying therapy for alzheimer's, first you better be really sure that you are. And second, you better be ready to handle hundreds of thousands of calls and enquiries from people wanting to get their loved ones into the trial and onto the drug. that is a big social responsibility. We have had 15 to 20 years of work on this. The data will tell us whether we can move from Phase 2 to Phase 3 in February, July or next November. It might feel like a big deal in the short term but we have come very far and the responsibility is awful big. For the sake of a few weeks or months, let's not screw it up"
Zamboni_kid updated us in April 2007 that one of the cohorts ended in June 2007 (went OLE, would happen after 18 months according to some, after 15 or 16 months according to most).
Likely this was the cohort #3 which was peeked at in May 2007 (assuming the above mentioned schedule is somewhat correct).
http://www1.investorvillage.com/smbd.asp?mb=160&mn=100013&p…
240 patients, 4 cohorts of 60 each.
For AAB-001 Phase II trial setup see also:
http://www.alzforum.org/drg/drc/detail.asp?id=101
http://www.clinicaltrials.gov/ct/show/NCT00112073
Peeks are done by 'an independant 3rd party'.
My assumption is that it is done by the trials DSMB.
http://www1.investorvillage.com/smbd.asp?mb=160&mn=76777&pt=…
We know the Phase I results for AAB-001.
This trial looked more like a Phase II in it's setup than a typical Phase I.
They showed positive efficacy (not stat sig, too few people) in the 0.5 dose and statistical significant efficacy (despite not that many patients in trial) in the 1.5 dose.
"At week 16, the prespecified primary timepoint for analysis, the treatment difference relative to placebo favored the treated group at the 0.5mg/kg dose (treatment vs. placebo difference of 2.0, p=.152) and reached statistical significance at the 1.5mg/kg dose (treatment vs. placebo difference of 2.5, p=.047)."
Ronald S. Black et.al.
Background: Bapineuzumab (AAB-001) is a humanized monoclonal antibody to A-beta being developed by Wyeth and Elan for the treatment of AD.
http://library.corporate-ir.net/library/88/883/88326/items/1…
We had an extensive discussion about the peeks & the schedule, but be warned that most information is only fragmentary and much of what is reported is at best only likely (that includes information as to what dosage group was first, when each group started, when it is likely to end etc).
See for a part of one of those discussions last January where we talk about the interim peek schedule:
http://www1.investorvillage.com/smbd.asp?mb=160&mn=67245&pt…
We seem to be following this schedule (as explained in above post):
"May-07: peek at Cohort 3, Jun-07: Study results, July-07: Move to P3 (or not) ..."
Then we had a good PR in May:
"On 21 May 2007, Elan and Wyeth announced their plans to initiate phase 3 clinical trials of Bapineuzumab. The decision to launch phase 3 studies prior to the conclusion of the ongoing phase 2 was based on the totality of the accumulated clinical data from phase 1, phase 2 and a 4.5- year follow-up study of those patients involved in the original AN-1792 trial."
Now the fact that May-07 was spot on is likely just a coincidence, so the move to P3 need not be already in this month (as in officially start recruiting patients, not already the start of dosing; dosing would start no earlier than a few months later, say September 2007 (all IMHO as usual) ).
Apart from the Business & Finance article, which gave us some clues, we also have this post by living_with_elan which describes what KM said about it:
http://www1.investorvillage.com/smbd.asp?mb=160&mn=89426&pt=…
The important part of the B&F article is (posted by hunstmann_ie, msg 2139 at elanians):
B&F interview with KM. Extract #2.
"The decision to move from Phase 2 to Phase 3 is a large one because we have responsibilities to the patients. If you publicly annouce that you are at the last stage of a disease modifying therapy for alzheimer's, first you better be really sure that you are. And second, you better be ready to handle hundreds of thousands of calls and enquiries from people wanting to get their loved ones into the trial and onto the drug. that is a big social responsibility. We have had 15 to 20 years of work on this. The data will tell us whether we can move from Phase 2 to Phase 3 in February, July or next November. It might feel like a big deal in the short term but we have come very far and the responsibility is awful big. For the sake of a few weeks or months, let's not screw it up"
Zamboni_kid updated us in April 2007 that one of the cohorts ended in June 2007 (went OLE, would happen after 18 months according to some, after 15 or 16 months according to most).
Likely this was the cohort #3 which was peeked at in May 2007 (assuming the above mentioned schedule is somewhat correct).
http://www1.investorvillage.com/smbd.asp?mb=160&mn=100013&p…
Die Zulassung von Ty zur Behandlung von Morbus Crohn scheint eine sicher Angelegenheit zu sein --> siehe Kapitel 4.4
http://guidance.nice.org.uk/page.aspx?o=436077
4.4 Relevant comparators
Given that (anticipated) licences for all four drugs are for use only when conventional treatment has failed, it is unlikely that the drugs would be compared to conventional treatment within the same trial population. It should be noted that conventional treatment may include infliximab. Therefore the most likely comparator will be no treatment or placebo where all patients are receiving conventional therapy. Another relevant comparator may be a different dosing regimen of the same drug.
Die Zulassung wird erwartet!!
http://guidance.nice.org.uk/page.aspx?o=436077
4.4 Relevant comparators
Given that (anticipated) licences for all four drugs are for use only when conventional treatment has failed, it is unlikely that the drugs would be compared to conventional treatment within the same trial population. It should be noted that conventional treatment may include infliximab. Therefore the most likely comparator will be no treatment or placebo where all patients are receiving conventional therapy. Another relevant comparator may be a different dosing regimen of the same drug.
Die Zulassung wird erwartet!!
hallo hexe 
WAS hat das für ELAN zu bedeuten ?
hat ELAN keinen anteil an dieser oralen form von tysabri ?
Oral Tysabri steps up to the plate
By Anna Lewcock
Get the latest Market Reports on
Biogen Idec
UCB
multiple sclerosis
oral formulation
Related News
New strategies to treat neurological disorders
Active Biotech drug could have the edge in oral MS race
Extended storage for Teva MS drug
FDA panel set to decide on expanded use for Tysabri
Exubera has opened door for pulmonary delivery growth
New therapy attacks multiple sclerosis on two fronts
Novartis and Bayer bury the Betaseron hatchet
News Archives
All news for July 2007
All news for June 2007
27/06/2007 - With a number of companies competing in the race to develop the first oral treatment option for multiple sclerosis, UCB and Biogen Idec's oral version of the infamous Tysabri has entered Phase II trials.
The two companies' candidate, currently going by the name CDP323, is an oral VLA-4 antagonist (as is Tysabri) intended for the treatment of relapsing-remitting multiple sclerosis (MS). The Phase II trials are expected to yield results by the end of 2008.
Biogen Idec hopped on board to partner with UCB in developing and commercialising the small molecule compound back in October last year, following encouraging Phase I trials. The two companies are initially investigating the compound for the treatment of MS, but will also be considering its use in treating other autoimmune disease indications.
UCB and Biogen are co-developing and co-commercialising the compound, with all commercialisation costs and profits to be shared equally. Over $200m (€149m) will be added to UCB's coffers courtesy of the Biogen deal.
CDP323 is one of the few oral alpha-4 integrin antagonists according to UCB, and it's hoped that by tapping Biogen's expertise in multiple sclerosis the two companies can come up with a competitive product.
Biogen is already responsible for the market-leading MS drug worldwide, Avonex (interferon beta-1a), which brought the company revenues of around $1.7bn over 2006. However, the company ahs taken a few punches over the last few years regarding an existing injectable MS treatment, Tysabri (natalizumab).
Tysabri, also a VLA-4 antagonist, was approved by the US Food and Drug Administration (FDA) for relapsing-remitting forms of MS back in 2004, however was hastily withdrawn three months later after several cases of patients developing a life-threatening viral infection of the brain.
The occurrence of progressive multifocal leukoencephalopathy (PML) in certain patients held Tysabri of the market for a significant period of time, only returning with stringent guidelines in June last year.
Damage to Biogen and Tysabri partners Elan's potential revenues was significant, with original analyst estimates for the treatment predicting annual revenues of up to $3bn, but following its withdrawal and late relaunch the drug only managed to generate $74m over 2006 - split between Elan and Biogen.
Although the companies have initiated risk management plans in the US and Europe to facilitate the correct use of Tysabri, the firms clearly recognise that the damage have may have already been done, with Biogen foreseeing "many rumours and speculation regarding Tysabri and suspected cases of PML" in the future.
Despite the unfavourable aura surrounding the existing version of Tysabri, a spokesperson for UCB told in-PharmaTechnologist.com that the two companies "strongly believe an oral solution is worthwhile exploring," and that the product could potentially become one of the firm's bigger products.
A 24-hour oral formulation of the drug could have significant advantages over the current monthly version of Tysabri in that it could reduce the likelihood of PML taking hold in a patient, as well as the more obvious non-invasive aspect of drug administration.
It's perhaps unsurprising that Biogenis hoping to catch the next big wave in MS treatment by successfully producing an oral formulation whilst simultaneously reaffirming its position in the MS market and hopefully demonstrating the safety of a Tysabri-like treatment option.
In addition to this, the MS market is growing fairly rapidly, and the first non-invasive oral treatment to become available would earn itself a particularly favourable position and at least a temporary monopoly while competitors catch up.
Several firms are fighting to be the first on the market with a non-invasive treatment option for MS sufferers. Only earlier this month Teva and partners Active Biotech announced their oral candidate was entering Phase II trials, and Novartis' hotly tipped oral MS treatment (FTY720, fingolimod) is on track for submissions in 2009.

WAS hat das für ELAN zu bedeuten ?
hat ELAN keinen anteil an dieser oralen form von tysabri ?

Oral Tysabri steps up to the plate
By Anna Lewcock
Get the latest Market Reports on
Biogen Idec
UCB
multiple sclerosis
oral formulation
Related News
New strategies to treat neurological disorders
Active Biotech drug could have the edge in oral MS race
Extended storage for Teva MS drug
FDA panel set to decide on expanded use for Tysabri
Exubera has opened door for pulmonary delivery growth
New therapy attacks multiple sclerosis on two fronts
Novartis and Bayer bury the Betaseron hatchet
News Archives
All news for July 2007
All news for June 2007
27/06/2007 - With a number of companies competing in the race to develop the first oral treatment option for multiple sclerosis, UCB and Biogen Idec's oral version of the infamous Tysabri has entered Phase II trials.
The two companies' candidate, currently going by the name CDP323, is an oral VLA-4 antagonist (as is Tysabri) intended for the treatment of relapsing-remitting multiple sclerosis (MS). The Phase II trials are expected to yield results by the end of 2008.
Biogen Idec hopped on board to partner with UCB in developing and commercialising the small molecule compound back in October last year, following encouraging Phase I trials. The two companies are initially investigating the compound for the treatment of MS, but will also be considering its use in treating other autoimmune disease indications.
UCB and Biogen are co-developing and co-commercialising the compound, with all commercialisation costs and profits to be shared equally. Over $200m (€149m) will be added to UCB's coffers courtesy of the Biogen deal.
CDP323 is one of the few oral alpha-4 integrin antagonists according to UCB, and it's hoped that by tapping Biogen's expertise in multiple sclerosis the two companies can come up with a competitive product.
Biogen is already responsible for the market-leading MS drug worldwide, Avonex (interferon beta-1a), which brought the company revenues of around $1.7bn over 2006. However, the company ahs taken a few punches over the last few years regarding an existing injectable MS treatment, Tysabri (natalizumab).
Tysabri, also a VLA-4 antagonist, was approved by the US Food and Drug Administration (FDA) for relapsing-remitting forms of MS back in 2004, however was hastily withdrawn three months later after several cases of patients developing a life-threatening viral infection of the brain.
The occurrence of progressive multifocal leukoencephalopathy (PML) in certain patients held Tysabri of the market for a significant period of time, only returning with stringent guidelines in June last year.
Damage to Biogen and Tysabri partners Elan's potential revenues was significant, with original analyst estimates for the treatment predicting annual revenues of up to $3bn, but following its withdrawal and late relaunch the drug only managed to generate $74m over 2006 - split between Elan and Biogen.
Although the companies have initiated risk management plans in the US and Europe to facilitate the correct use of Tysabri, the firms clearly recognise that the damage have may have already been done, with Biogen foreseeing "many rumours and speculation regarding Tysabri and suspected cases of PML" in the future.
Despite the unfavourable aura surrounding the existing version of Tysabri, a spokesperson for UCB told in-PharmaTechnologist.com that the two companies "strongly believe an oral solution is worthwhile exploring," and that the product could potentially become one of the firm's bigger products.
A 24-hour oral formulation of the drug could have significant advantages over the current monthly version of Tysabri in that it could reduce the likelihood of PML taking hold in a patient, as well as the more obvious non-invasive aspect of drug administration.
It's perhaps unsurprising that Biogenis hoping to catch the next big wave in MS treatment by successfully producing an oral formulation whilst simultaneously reaffirming its position in the MS market and hopefully demonstrating the safety of a Tysabri-like treatment option.
In addition to this, the MS market is growing fairly rapidly, and the first non-invasive oral treatment to become available would earn itself a particularly favourable position and at least a temporary monopoly while competitors catch up.
Several firms are fighting to be the first on the market with a non-invasive treatment option for MS sufferers. Only earlier this month Teva and partners Active Biotech announced their oral candidate was entering Phase II trials, and Novartis' hotly tipped oral MS treatment (FTY720, fingolimod) is on track for submissions in 2009.
Antwort auf Beitrag Nr.: 30.451.279 von zenman am 02.07.07 16:53:19die Wirkungsweise des neuen Wiorkstoffes ist derjenigen von Natalizumab sehr ähnlich: die verabreichte Aktivsubstanz bindet an einen Rezeptor auf der Oberfläche von T-Lymphozyten und verhindert somit die Passage der T-Lymphozyten durch die Blut-Hirnschranke (--> MS) oder in das Gewebe des Verdauungstraktes (--> chronisch-entzündlichen Darmerkrankungen = MorbusCrohn)
Natalizumab kann sowohl an den T-Lymphozyten andocken oder aber die Andockrezeptoren der Blut-Hirnschranke belegen. Da Natalizumab als humanisierter monoklonaler Antikörper (MAK) im Magen denaturiert (also Zerstörung der komplexen Eiweiss-Struktur) , besteht die Notwendigkeit, den MAK unter Umgehung des Verdauungstraktes direkt in die Blutbahn zu verabreichen.
Bei dem von Biogen und UCB entwickelten Wirkstoff CDP323 handelt es sich um ein im Verhältnis kleines Molekül, welches ebenfalls an den Rezeptor VLA-4 der T-Lymphozyten bindet und dadurch die Passage durch die Blut-Hirnschranke verhindern soll. Da es sich hierbei nicht um eine Eiweiss-Struktur handelt, kann der Wirkstoff oral verabreicht werden --> dies ist gegenüber einer intravenösen Applikation ein riesiger Vorteil!
Allerdings muss dieser Wirkstoff zuerst in den klinischen Phasen seine Wirkung unter Beweis stellen, und zudem verfügt Elan mit ELND-001 (in Phase I) und ELND-002 (Präklinik) ebenfalls 2 derartige Kandidaten, welche einen ähnlichen Wirkungsansatz verfolgen.
Deswegen bleibt Folgendes festzuhalten:
1.) Wirksamkeit von CDP323 ist noch nicht erwiesen
2.) das Konkurrenzprodukt wird nicht vor 2011 eine Marktzulassung erhalten
3.) Elan verfügt in den neurodegenerativen Indikationen über eine anerkannt führende Expertise, so dass Elan-Aktionäre durchaus darauf spekulieren können, dass Elans "kleine Moleküle" im Minimum genau so wirksam sind wie CDP323
Natalizumab kann sowohl an den T-Lymphozyten andocken oder aber die Andockrezeptoren der Blut-Hirnschranke belegen. Da Natalizumab als humanisierter monoklonaler Antikörper (MAK) im Magen denaturiert (also Zerstörung der komplexen Eiweiss-Struktur) , besteht die Notwendigkeit, den MAK unter Umgehung des Verdauungstraktes direkt in die Blutbahn zu verabreichen.
Bei dem von Biogen und UCB entwickelten Wirkstoff CDP323 handelt es sich um ein im Verhältnis kleines Molekül, welches ebenfalls an den Rezeptor VLA-4 der T-Lymphozyten bindet und dadurch die Passage durch die Blut-Hirnschranke verhindern soll. Da es sich hierbei nicht um eine Eiweiss-Struktur handelt, kann der Wirkstoff oral verabreicht werden --> dies ist gegenüber einer intravenösen Applikation ein riesiger Vorteil!
Allerdings muss dieser Wirkstoff zuerst in den klinischen Phasen seine Wirkung unter Beweis stellen, und zudem verfügt Elan mit ELND-001 (in Phase I) und ELND-002 (Präklinik) ebenfalls 2 derartige Kandidaten, welche einen ähnlichen Wirkungsansatz verfolgen.
Deswegen bleibt Folgendes festzuhalten:
1.) Wirksamkeit von CDP323 ist noch nicht erwiesen
2.) das Konkurrenzprodukt wird nicht vor 2011 eine Marktzulassung erhalten
3.) Elan verfügt in den neurodegenerativen Indikationen über eine anerkannt führende Expertise, so dass Elan-Aktionäre durchaus darauf spekulieren können, dass Elans "kleine Moleküle" im Minimum genau so wirksam sind wie CDP323
...das wurde aber auch Zeit: nun wird Tysabri auch in UK von den KV erstattet, so dass die 85.000 MS-Patienten nicht nur formal sondern auch real ab sofort darüber verfügen können:
http://guidance.nice.org.uk/page.aspx?o=438885
http://guidance.nice.org.uk/page.aspx?o=438885
Antwort auf Beitrag Nr.: 30.463.846 von Cyberhexe am 03.07.07 09:28:46...mit etwas "Nachhilfe" erscheint Elan auch in Branchenkreisen unter den Favoriten:
http://www.depotliga.de/xist4c/web/realdepot-biotech-report_…
http://www.depotliga.de/xist4c/web/realdepot-biotech-report_…
...in den von der britischen Bewertungsstelle über die Wirtschaftlichkeit von Medikamenten publizierten Unterlagen zur Diskussion über eine Behandlung der MS mit Tysabri befinden sich interessante Info u.a. auch über den Vergleich der Wirksamkeit gegenüber anderen Medikamenten:
Tysabri clinical data show that it is far more effective than interferons or glatiramer acetate. It is also safer - see NEJM articles and package inserts. Twice as many people died in the placebo group as in the drug groups during trials, despite the fact that there were twice as many drug patients as placebo patients. A single statistic tells the story: the annualize relapse rate for Gd-Enhanced lesions is as folows: Tysabri = 0.1 lesions per year Avonex= 1.0 lesions per year Placebo= 1.4 lesions per year This data comes straight from the package inserts. Instead of being more expensive, Tysabri is more cost effective for the UK: it costs 30% more that INFb treatments (including infusion costs) but nearly half of patients regain the ability to walk or see or some other lost function, most see an noticable improvement in cognition, and there is a much lower relapse rate and rate of emergency steroid treatment regimen. This adds up to patients being more productive, costing less, and often coming off the dole and returning to society as tax-paying citizens. Please approve Tysabri for the benefit of the UK and UK MS patients.
http://guidance.nice.org.uk/page.aspx?o=438882 p 39/59
Tysabri clinical data show that it is far more effective than interferons or glatiramer acetate. It is also safer - see NEJM articles and package inserts. Twice as many people died in the placebo group as in the drug groups during trials, despite the fact that there were twice as many drug patients as placebo patients. A single statistic tells the story: the annualize relapse rate for Gd-Enhanced lesions is as folows: Tysabri = 0.1 lesions per year Avonex= 1.0 lesions per year Placebo= 1.4 lesions per year This data comes straight from the package inserts. Instead of being more expensive, Tysabri is more cost effective for the UK: it costs 30% more that INFb treatments (including infusion costs) but nearly half of patients regain the ability to walk or see or some other lost function, most see an noticable improvement in cognition, and there is a much lower relapse rate and rate of emergency steroid treatment regimen. This adds up to patients being more productive, costing less, and often coming off the dole and returning to society as tax-paying citizens. Please approve Tysabri for the benefit of the UK and UK MS patients.
http://guidance.nice.org.uk/page.aspx?o=438882 p 39/59
Antwort auf Beitrag Nr.: 30.508.955 von Cyberhexe am 05.07.07 18:16:18Damit hier nicht so einsam ist 
Elan für risikobereite Anleger
Kulmbach (aktiencheck.de AG) - Die Experten vom Anlegermagazin "Der Aktionär" empfehlen die Aktie von Elan (ISIN IE0003072950/ WKN 903801) für risikobereite Anleger.
Werbung
In den vergangenen Monaten habe der Anteilsschein von Elan an der Börse eine bemerkenswerte Renaissance erlebt. Die Aktie habe allein seit Anfang des Jahres um 60 Prozent zugelegt. Für den letzten Aufschwung gebe es mehrere gute Gründe. Die allmähliche Rückkehr des Medikamentes Tysabri, das auch von einigen Fachleuten schon abgemeldet gewesen sei, sei ein Grund.
Tysabri sei ab Ende Februar 2005 nicht mehr auf dem Markt erhältlich gewesen, da drei behandelte Patienten an der seltenen Viruserkrankung des Gehirns, PML, gestorben seien. Man könne das Medikament inzwischen in den USA und Europa wieder erhalten, wenn auch unter strengen Auflagen.
Die Gesellschaft habe auch über Tysabri hinaus in den vergangenen Jahren ihre Entwicklungspipeline sukzessiv erweitert. Ein potenzielles Mittel zur Behandlung von Alzheimer, das Elan in Zusammenarbeit mit Wyeth entwickle, sei besonders interessant. Die Unternehmen hätten nach sehr guten Ergebnissen in der zweiten Phase klinischer Studien schon den Start von finalen Phase-III-Tests gemeldet.
Jedoch würden Analysten munkeln, dass das bislang vorliegende Datenmaterial so gut sein solle, dass eine Beantragung der Zulassung seitens der Unternehmen bereits jetzt möglich sei. Der monoklonale Antikörper mit dem Forschungsnamen AAB-001 wäre dann vielleicht bereits Ende 2008 erhältlich.
Besondere Fantasie erhalte der Titel von Elan von der bevorstehenden Tysabri-Entscheidung für die Indikation Morbus Crohn. Faktoren würden jedoch auch auf mittlere und lange Sicht für ein Engagement sprechen.
Nach Ansicht der Experten von "Der Aktionär" sollten Investoren mit einem gewissen Maß an Risikobereitschaft einige Stücke der Elan-Aktie ins Depot legen. Das Kursziel werde bei 22 Euro gesehen. Zur Absicherung sollte ein Stopp bei 13 Euro platziert werden. (Ausgabe 29) (12.07.2007/ac/a/a)Analyse-Datum: 12.07.2007


Elan für risikobereite Anleger
Kulmbach (aktiencheck.de AG) - Die Experten vom Anlegermagazin "Der Aktionär" empfehlen die Aktie von Elan (ISIN IE0003072950/ WKN 903801) für risikobereite Anleger.
Werbung
In den vergangenen Monaten habe der Anteilsschein von Elan an der Börse eine bemerkenswerte Renaissance erlebt. Die Aktie habe allein seit Anfang des Jahres um 60 Prozent zugelegt. Für den letzten Aufschwung gebe es mehrere gute Gründe. Die allmähliche Rückkehr des Medikamentes Tysabri, das auch von einigen Fachleuten schon abgemeldet gewesen sei, sei ein Grund.
Tysabri sei ab Ende Februar 2005 nicht mehr auf dem Markt erhältlich gewesen, da drei behandelte Patienten an der seltenen Viruserkrankung des Gehirns, PML, gestorben seien. Man könne das Medikament inzwischen in den USA und Europa wieder erhalten, wenn auch unter strengen Auflagen.
Die Gesellschaft habe auch über Tysabri hinaus in den vergangenen Jahren ihre Entwicklungspipeline sukzessiv erweitert. Ein potenzielles Mittel zur Behandlung von Alzheimer, das Elan in Zusammenarbeit mit Wyeth entwickle, sei besonders interessant. Die Unternehmen hätten nach sehr guten Ergebnissen in der zweiten Phase klinischer Studien schon den Start von finalen Phase-III-Tests gemeldet.
Jedoch würden Analysten munkeln, dass das bislang vorliegende Datenmaterial so gut sein solle, dass eine Beantragung der Zulassung seitens der Unternehmen bereits jetzt möglich sei. Der monoklonale Antikörper mit dem Forschungsnamen AAB-001 wäre dann vielleicht bereits Ende 2008 erhältlich.
Besondere Fantasie erhalte der Titel von Elan von der bevorstehenden Tysabri-Entscheidung für die Indikation Morbus Crohn. Faktoren würden jedoch auch auf mittlere und lange Sicht für ein Engagement sprechen.
Nach Ansicht der Experten von "Der Aktionär" sollten Investoren mit einem gewissen Maß an Risikobereitschaft einige Stücke der Elan-Aktie ins Depot legen. Das Kursziel werde bei 22 Euro gesehen. Zur Absicherung sollte ein Stopp bei 13 Euro platziert werden. (Ausgabe 29) (12.07.2007/ac/a/a)Analyse-Datum: 12.07.2007

Elans' neue Patente, wobei die Anzahl mittlerweile nahezu rekordverdächtig ist:
...vom 17. Juli 2007
1.)
Disclosed are compounds of the formula: ##STR00001## where variables Z, X, R.sub.15, R.sub.2, R.sub.3, and R.sub.C are defined herein. Compounds disclosed herein are inhibitors of the beta-secretase enzyme and are therefore useful in the treatment of Alzheimer's disease and other diseases characterized by deposition of A beta peptide in a mammal.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
2.)
Modified BACE
The present invention relates to recombinant human BACE polypeptides. More particularly, the invention relates to recombinant human BACE polypeptides that have a modified amino acid sequence at position 33 of the BACE sequence, as well as to polynucleotides, expression vectors, host cells, and methods for producing the modified recombinant human BACE polypeptides.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
3.)
Methods of making nanoparticulate drug compositions comprising copolymers of vinyl pyrrolidone and vinyl acetate as surface stabilizers
The present invention is directed to nanoparticulate compositions comprising a poorly soluble drug and at least one copolymer of vinyl pyrrolidone and vinyl acetate as a surface stabilizer adsorbed to the surface of the drug. Also encompassed by the invention are pharmaceutical compositions comprising a nanoparticulate composition of the invention, methods of making and using such nanoparticulate and pharmaceutical compositions.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
...vom 12. Juni 2007
1.)
Carbamyloxy compounds which inhibit leukocyte adhesion mediated by VLA-4
Disclosed are compounds which bind VLA-4. Certain of these compounds also inhibit leukocyte adhesion and, in particular, leukocyte adhesion mediated by VLA-4. Such compounds are useful in the treatment of inflammatory diseases in a mammalian patient, e.g., human, such as asthma, Alzheimer's disease, atherosclerosis, AIDS dementia, diabetes, inflammatory bowel disease, rheumatoid arthritis, tissue transplantation, tumor metastasis and myocardial ischemia. The compounds can also be administered for the treatment of inflammatory brain diseases such as multiple sclerosis.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
...vom 17. Juli 2007
1.)
Disclosed are compounds of the formula: ##STR00001## where variables Z, X, R.sub.15, R.sub.2, R.sub.3, and R.sub.C are defined herein. Compounds disclosed herein are inhibitors of the beta-secretase enzyme and are therefore useful in the treatment of Alzheimer's disease and other diseases characterized by deposition of A beta peptide in a mammal.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
2.)
Modified BACE
The present invention relates to recombinant human BACE polypeptides. More particularly, the invention relates to recombinant human BACE polypeptides that have a modified amino acid sequence at position 33 of the BACE sequence, as well as to polynucleotides, expression vectors, host cells, and methods for producing the modified recombinant human BACE polypeptides.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
3.)
Methods of making nanoparticulate drug compositions comprising copolymers of vinyl pyrrolidone and vinyl acetate as surface stabilizers
The present invention is directed to nanoparticulate compositions comprising a poorly soluble drug and at least one copolymer of vinyl pyrrolidone and vinyl acetate as a surface stabilizer adsorbed to the surface of the drug. Also encompassed by the invention are pharmaceutical compositions comprising a nanoparticulate composition of the invention, methods of making and using such nanoparticulate and pharmaceutical compositions.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
...vom 12. Juni 2007
1.)
Carbamyloxy compounds which inhibit leukocyte adhesion mediated by VLA-4
Disclosed are compounds which bind VLA-4. Certain of these compounds also inhibit leukocyte adhesion and, in particular, leukocyte adhesion mediated by VLA-4. Such compounds are useful in the treatment of inflammatory diseases in a mammalian patient, e.g., human, such as asthma, Alzheimer's disease, atherosclerosis, AIDS dementia, diabetes, inflammatory bowel disease, rheumatoid arthritis, tissue transplantation, tumor metastasis and myocardial ischemia. The compounds can also be administered for the treatment of inflammatory brain diseases such as multiple sclerosis.
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Wyeth plans to launch 7 products within 18 months
Thu Jul 19, 2007 8:52AM EDT
Free practice account at FOREX.comNEW YORK, July 19 (Reuters) - Wyeth (WYE.N: Quote, Profile, Research) on Thursday said it expects to launch seven new products within the next 18 months, including introductions this month of its Lybrel contraceptive and Torisel kidney cancer medicine.
The company also said it expects U.S. regulators to extend their review of Viviant, an experimental drug meant to prevent osteoporosis, at least until the end of 2007.
The U.S. Food and Drug Administration in April gave conditional approval to Viviant, the chemical name of which is bazedoxifene, for prevention of post-menopausal osteoporosis. But the agency requested additional fracture-prevention data, which Wyeth said it provided in late June.
Wyeth, meanwhile, is also moving ahead with plans to seek approval of Viviant as a treatment for osteoporosis.
Wyeth also told analysts in a conference call on Thursday that it aims this year to begin late-stage trials on bapineuzumab, a drug for Alzheimer's disease also known as AAB-001. It is designed to attack the A-beta peptide, a component of amyloid plaque that builds up in the brain and is believed to damage brain cells. (Reporting by Ransdell Pierson)
http://www.reuters.com/article/topNews/idUKN1927597420070719…
Thu Jul 19, 2007 8:52AM EDT
Free practice account at FOREX.comNEW YORK, July 19 (Reuters) - Wyeth (WYE.N: Quote, Profile, Research) on Thursday said it expects to launch seven new products within the next 18 months, including introductions this month of its Lybrel contraceptive and Torisel kidney cancer medicine.
The company also said it expects U.S. regulators to extend their review of Viviant, an experimental drug meant to prevent osteoporosis, at least until the end of 2007.
The U.S. Food and Drug Administration in April gave conditional approval to Viviant, the chemical name of which is bazedoxifene, for prevention of post-menopausal osteoporosis. But the agency requested additional fracture-prevention data, which Wyeth said it provided in late June.
Wyeth, meanwhile, is also moving ahead with plans to seek approval of Viviant as a treatment for osteoporosis.
Wyeth also told analysts in a conference call on Thursday that it aims this year to begin late-stage trials on bapineuzumab, a drug for Alzheimer's disease also known as AAB-001. It is designed to attack the A-beta peptide, a component of amyloid plaque that builds up in the brain and is believed to damage brain cells. (Reporting by Ransdell Pierson)
http://www.reuters.com/article/topNews/idUKN1927597420070719…
Antwort auf Beitrag Nr.: 30.761.998 von Cyberhexe am 19.07.07 16:22:16EU drug experts rebuff Elan\'s Tysabri in Crohn\'s
LONDON, July 19 (Reuters) - European drug regulators said on Thursday that multiple sclerosis treatment Tysabri should not be approved as a treatment for Crohn\'s disease.
The European Medicines Agency said the effects of the medicine, from Ireland\'s Elan Corp (ELN.I: Quote, Profile, Research) and Biogen Idec (BIIB.O: Quote, Profile, Research), were modest and there was a risk of serious infections.
The product will be reviewed by U.S. experts for use in Crohn\'s, a serious bowel disorder, at an advisory meeting on July 31.
http://www.reuters.com/article/topNews/idUKWLB911920070719?r…
LONDON, July 19 (Reuters) - European drug regulators said on Thursday that multiple sclerosis treatment Tysabri should not be approved as a treatment for Crohn\'s disease.
The European Medicines Agency said the effects of the medicine, from Ireland\'s Elan Corp (ELN.I: Quote, Profile, Research) and Biogen Idec (BIIB.O: Quote, Profile, Research), were modest and there was a risk of serious infections.
The product will be reviewed by U.S. experts for use in Crohn\'s, a serious bowel disorder, at an advisory meeting on July 31.
http://www.reuters.com/article/topNews/idUKWLB911920070719?r…
Antwort auf Beitrag Nr.: 30.765.760 von surga am 19.07.07 20:24:38CB,
was bedeutet das für ELAN? Der Kurs sieht noch stabil aus
was bedeutet das für ELAN? Der Kurs sieht noch stabil aus

Antwort auf Beitrag Nr.: 30.765.823 von surga am 19.07.07 20:29:54Elan and Biogen Idec Preparing to Appeal Ruling on European Application for Natalizumab for the Treatment of Crohn's Disease
DUBLIN, Ireland & ZUG, Switzerland--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Biogen Idec (NASDAQ: BIIB - News) announced today that they have been informed by the European Medicines Agency (EMEA) that the Committee for Medicinal Products for Human Use (CHMP) has adopted a negative opinion on the marketing application for the use of natalizumab in patients with Crohn's disease. In accordance with European regulations, Elan and Biogen Idec plan to apply for a re-examination of the negative opinion through the appeal procedure. A decision on the appeal is expected by 1Q 2008.
"Without natalizumab, European patients with severely active disease who failed other therapies and who are suffering from continuous symptoms may be offered surgery, with its potential complications, intravenous nutritional therapies or clinical trials with unproven experimental agents, depending upon on the patients' condition," said Professor Jean-Frederick Colombel, University of Lille. "There is a need for new therapies for this very difficult disease."
An application for approval of TYSABRI® (natalizumab) for treatment of moderate to severe Crohn's disease was filed in the US on December 15, 2006. The FDA is holding an advisory committee to discuss the application on July 31, 2007.
.......
http://biz.yahoo.com/bw/070719/20070719005888.html?.v=1
DUBLIN, Ireland & ZUG, Switzerland--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Biogen Idec (NASDAQ: BIIB - News) announced today that they have been informed by the European Medicines Agency (EMEA) that the Committee for Medicinal Products for Human Use (CHMP) has adopted a negative opinion on the marketing application for the use of natalizumab in patients with Crohn's disease. In accordance with European regulations, Elan and Biogen Idec plan to apply for a re-examination of the negative opinion through the appeal procedure. A decision on the appeal is expected by 1Q 2008.
"Without natalizumab, European patients with severely active disease who failed other therapies and who are suffering from continuous symptoms may be offered surgery, with its potential complications, intravenous nutritional therapies or clinical trials with unproven experimental agents, depending upon on the patients' condition," said Professor Jean-Frederick Colombel, University of Lille. "There is a need for new therapies for this very difficult disease."
An application for approval of TYSABRI® (natalizumab) for treatment of moderate to severe Crohn's disease was filed in the US on December 15, 2006. The FDA is holding an advisory committee to discuss the application on July 31, 2007.
.......
http://biz.yahoo.com/bw/070719/20070719005888.html?.v=1
...erstens kommt es anders zweitens als man denk: Ich hatte nicht damit gerechnet, dass das CHMP vor dem Advisory Committee der FDA eine Entscheidung bekannt gibt.
http://www.emea.europa.eu/pdfs/human/opinion/31085607en.pdf
http://www.emea.europa.eu/pdfs/human/opinion/31085607en.pdf
Hi Hexchen!
Wie schätzt du dies ein??
-----------------------------------------------------------------------
By: teknowizz
Finfacts article on the EMEA opinion & the opposing opinion from an expert
This article quotes a EU Professor who has quite a different opinion on Tysabri for CD than the EU CHMP ...
http://www.finfacts.com/irelandbusinessnews/publish/printer_…
Elan's Tysabri drug rejected by European Medicines Agency for treatment of patients with Crohn's disease
By Finfacts Team
Jul 20, 2007, 07:59
Irish drugs firm Elan and its US partner Biogen Idec announced today that they have been informed by the European Medicines Agency (EMEA) that the Committee for Medicinal Products for Human Use (CHMP) has adopted a negative opinion on the marketing application for the use of its Tysabri natalizumab drug in patients with Crohn's disease.
In accordance with European regulations, Elan and Biogen Idec plan to apply for a re-examination of the negative opinion through the appeal procedure. A decision on the appeal is expected by 1Q 2008.
"Without natalizumab, European patients with severely active disease who failed other therapies and who are suffering from continuous symptoms may be offered surgery, with its potential complications, intravenous nutritional therapies or clinical trials with unproven experimental agents, depending upon on the patients' condition," said Professor Jean-Frederick Colombel, University of Lille. "There is a need for new therapies for this very difficult disease."
An application for approval of Tysabri (natalizumab) for treatment of moderate to severe Crohn's disease was filed in the US on December 15, 2006. The FDA is holding an advisory committee to discuss the application on July 31, 2007.
Approximately one million people worldwide have Crohn's disease, a chronic and progressive inflammatory disease of the gastrointestinal tract, which commonly affects both men and women.
The disease usually causes diarrhea and crampy abdominal pain, often associated with fever, and at times rectal bleeding. Loss of appetite and weight loss also may occur. Complications include narrowing of the intestine, obstruction, abscesses, and fistulas (abnormal channels connecting the intestine and other organs, including the skin), and malnutrition. Most patients eventually require surgery, which has both risks and potential short- and long-term complications.
Wie schätzt du dies ein??
-----------------------------------------------------------------------
By: teknowizz
Finfacts article on the EMEA opinion & the opposing opinion from an expert
This article quotes a EU Professor who has quite a different opinion on Tysabri for CD than the EU CHMP ...
http://www.finfacts.com/irelandbusinessnews/publish/printer_…
Elan's Tysabri drug rejected by European Medicines Agency for treatment of patients with Crohn's disease
By Finfacts Team
Jul 20, 2007, 07:59
Irish drugs firm Elan and its US partner Biogen Idec announced today that they have been informed by the European Medicines Agency (EMEA) that the Committee for Medicinal Products for Human Use (CHMP) has adopted a negative opinion on the marketing application for the use of its Tysabri natalizumab drug in patients with Crohn's disease.
In accordance with European regulations, Elan and Biogen Idec plan to apply for a re-examination of the negative opinion through the appeal procedure. A decision on the appeal is expected by 1Q 2008.
"Without natalizumab, European patients with severely active disease who failed other therapies and who are suffering from continuous symptoms may be offered surgery, with its potential complications, intravenous nutritional therapies or clinical trials with unproven experimental agents, depending upon on the patients' condition," said Professor Jean-Frederick Colombel, University of Lille. "There is a need for new therapies for this very difficult disease."
An application for approval of Tysabri (natalizumab) for treatment of moderate to severe Crohn's disease was filed in the US on December 15, 2006. The FDA is holding an advisory committee to discuss the application on July 31, 2007.
Approximately one million people worldwide have Crohn's disease, a chronic and progressive inflammatory disease of the gastrointestinal tract, which commonly affects both men and women.
The disease usually causes diarrhea and crampy abdominal pain, often associated with fever, and at times rectal bleeding. Loss of appetite and weight loss also may occur. Complications include narrowing of the intestine, obstruction, abscesses, and fistulas (abnormal channels connecting the intestine and other organs, including the skin), and malnutrition. Most patients eventually require surgery, which has both risks and potential short- and long-term complications.
Antwort auf Beitrag Nr.: 30.771.838 von Birgit.Tersteegen am 20.07.07 11:43:56@Birgit
...ich glaube, dass das AC der FDA unabhängig von der Entscheidung des CHMP eine Empfehlung zur Behandlung von CD mit Tysabri aussprechen wird, allerdings unter analogen Bedingungen (Risikoplan) wie bei der Behandlung der MS. Die Europäer werden dies von der Seitenlinie aus beobachten und in 2008 erneut über eine Zulassung diskutieren.
Mit Spannung werden hierzu die "Briefing Documents" der FDA erwartet, welche spätestens Morgen (Freitag) veröffentlicht werden.
jmho
...ich glaube, dass das AC der FDA unabhängig von der Entscheidung des CHMP eine Empfehlung zur Behandlung von CD mit Tysabri aussprechen wird, allerdings unter analogen Bedingungen (Risikoplan) wie bei der Behandlung der MS. Die Europäer werden dies von der Seitenlinie aus beobachten und in 2008 erneut über eine Zulassung diskutieren.
Mit Spannung werden hierzu die "Briefing Documents" der FDA erwartet, welche spätestens Morgen (Freitag) veröffentlicht werden.
jmho
Antwort auf Beitrag Nr.: 30.855.820 von Cyberhexe am 26.07.07 10:53:39Danke!

Antwort auf Beitrag Nr.: 30.855.820 von Cyberhexe am 26.07.07 10:53:39Mit Spannung werden hierzu die "Briefing Documents" der FDA erwartet, welche spätestens Morgen (Freitag) veröffentlicht werden.
..dann schauen wir mal...
..dann schauen wir mal...

Antwort auf Beitrag Nr.: 30.878.152 von Cyberhexe am 27.07.07 15:56:06das liest sich doch gut.
Der letzte Absatz:
In summary, natalizumab is a highly effective treatment for inducing and maintaining
clinical benefit in CD patients who have failed conventional therapy. These patients
require treatment with a biological therapy, and natalizumab provides another treatment
choice for physicians and patients. The degree of efficacy and overall safety profile
demonstrated in the clinical studies, combined with the ability to minimize the risk for
PML with the proposed label and risk management plan, lead to a benefit/risk ratio that is
acceptable and will allow physicians and patients to consider natalizumab as a valuable
treatment option for CD.

Der letzte Absatz:
In summary, natalizumab is a highly effective treatment for inducing and maintaining
clinical benefit in CD patients who have failed conventional therapy. These patients
require treatment with a biological therapy, and natalizumab provides another treatment
choice for physicians and patients. The degree of efficacy and overall safety profile
demonstrated in the clinical studies, combined with the ability to minimize the risk for
PML with the proposed label and risk management plan, lead to a benefit/risk ratio that is
acceptable and will allow physicians and patients to consider natalizumab as a valuable
treatment option for CD.

Antwort auf Beitrag Nr.: 30.878.487 von Poppholz am 27.07.07 16:12:35...und was meinst du dazu, poppi?
• From time of re-launch in June 2006 until May 23, 2007, approximately 11,500 patients have received natalizumab in the post-marketing setting worldwide and there have been no new confirmed cases of PML or serious OI’s reported.
...das liest sich auch nicht so schlecht. Bin sehr gespannt auf den Dienstag!
• From time of re-launch in June 2006 until May 23, 2007, approximately 11,500 patients have received natalizumab in the post-marketing setting worldwide and there have been no new confirmed cases of PML or serious OI’s reported.
...das liest sich auch nicht so schlecht. Bin sehr gespannt auf den Dienstag!
Antwort auf Beitrag Nr.: 30.879.092 von Cyberhexe am 27.07.07 16:48:28sehr gut.
Besonders unter dem Gesichtspunkt, dass seit dem 23. Mai bereits ca. 2.500 weitere Patienten hinzu gekommen sind.
Irgendwann kommt der Warnhinweis auf den Packungen auch weg.

Besonders unter dem Gesichtspunkt, dass seit dem 23. Mai bereits ca. 2.500 weitere Patienten hinzu gekommen sind.
Irgendwann kommt der Warnhinweis auf den Packungen auch weg.

Antwort auf Beitrag Nr.: 30.879.092 von Cyberhexe am 27.07.07 16:48:28...und Nebenwirkungen im Plazebobereich:
• Natalizumab was generally well-tolerated by patients for periods of up to 2 years.
Common adverse events were comparable to placebo and the incidence of infections, including serious infections, were similar to patients treated with placebo.
...ich kenne 2 MSler, die mit Tysabri behandelt werden...und beide sind bisher sehr zufrieden!
• Natalizumab was generally well-tolerated by patients for periods of up to 2 years.
Common adverse events were comparable to placebo and the incidence of infections, including serious infections, were similar to patients treated with placebo.
...ich kenne 2 MSler, die mit Tysabri behandelt werden...und beide sind bisher sehr zufrieden!
das liest sich jedoch weniger schön:
With regard to safety in subgroups defined by concomitant medication use or prior medication use, subgroup analyses may help to determine if the risk of infections including serious
infections is increased based on concurrent immunosuppressant use, and help identify a population which may have a more favorable benefit to risk ratio. No clear pattern has emerged.
However, the sponsor has not performed all analyses desired. First, safety analyses by prior medication use were limited. Second, many of the safety analyses by concomitant
medications were for short-term studies, and not longer term studies in CD. Additional analyses were requested to address each of these points.
...das riecht nur nach einem AL!!
With regard to safety in subgroups defined by concomitant medication use or prior medication use, subgroup analyses may help to determine if the risk of infections including serious
infections is increased based on concurrent immunosuppressant use, and help identify a population which may have a more favorable benefit to risk ratio. No clear pattern has emerged.
However, the sponsor has not performed all analyses desired. First, safety analyses by prior medication use were limited. Second, many of the safety analyses by concomitant
medications were for short-term studies, and not longer term studies in CD. Additional analyses were requested to address each of these points.
...das riecht nur nach einem AL!!

in den "Briefings" ist folgende Empfehlung der FDA an das AC festgehalten:
We recommend the Advisory Committee members discuss the following critical issues:
. The best way to monitor the CD population for PML and other OIs
. Identification of the appropriate patient for Tysabri and how in clinical practice these patients
would be identified
. Whether concomitant immunosuppressive and immunomodulatory therapy wil be permitted
and how flares of CD wil be dealt with
The details of this discussion wil be considered in the final design of the CD-TOUCH RiskMAP
program.
...ich bin nicht felsenfest davon überzeugt, aber nach intensiver Durchsicht der Briefing Documents sehe ich dem AC von Morgen optimistisch entgegen: Deswegen mein Tipp , dass maximal 5 der 19 stimmberechtigten AC-Mitglieder eine Zulassung zur Behandlung von CD ablehnen. Dies würde bedeuten, dass die anderen 14 einer Behandlung mit einem "Risikoplan" zustimmen würden.
...und dann sind wir wieder über 20$!
We recommend the Advisory Committee members discuss the following critical issues:
. The best way to monitor the CD population for PML and other OIs
. Identification of the appropriate patient for Tysabri and how in clinical practice these patients
would be identified
. Whether concomitant immunosuppressive and immunomodulatory therapy wil be permitted
and how flares of CD wil be dealt with
The details of this discussion wil be considered in the final design of the CD-TOUCH RiskMAP
program.
...ich bin nicht felsenfest davon überzeugt, aber nach intensiver Durchsicht der Briefing Documents sehe ich dem AC von Morgen optimistisch entgegen: Deswegen mein Tipp , dass maximal 5 der 19 stimmberechtigten AC-Mitglieder eine Zulassung zur Behandlung von CD ablehnen. Dies würde bedeuten, dass die anderen 14 einer Behandlung mit einem "Risikoplan" zustimmen würden.
...und dann sind wir wieder über 20$!
Antwort auf Beitrag Nr.: 30.940.223 von Cyberhexe am 30.07.07 18:11:16...und dann sind wir wieder über 20$! und wenn nicht wo sind wir dann ??
Gruß Noogmann
Gruß Noogmann
Antwort auf Beitrag Nr.: 30.940.257 von noogmann am 30.07.07 18:14:06sollte dann auch nichts machen;haben wir ja heute schon....
Credit Suisse Comments re Tysabri in CD re BIIB
We Expect Panel to Vote for Crohn's Approval;
Size of Opportunity is a Question
■
Conclusion: Following a review of the Tysabri Crohn’s data – ENACT-1, ENACT-2,
and ENCORE – as well as the FDA briefing documents released on Friday, we
believe Tysabri’s clinical efficacy in Crohn’s supports approval, even with the risk of
PML. That said, we do expect the panel to wrestle with the risk/benefit ratio,
particularly given Crohn’s patient’s typical exposure to other immunosuppressive
agents. We also expect the risk management plan to limit the commercial opportunity,
particularly the need to discontinue Tysabri in patients who are still on steroids at 6-
months. Still, even with these challenges, given the prevalence and severity of the
disease, we believe Tysabri sales in Crohn’s could be in the $300 million range.
■
What’s New? A joint meeting of FDA’s Gastrointestinal Drugs Advisory Committee
and Drug Safety and Risk Management Advisory Committee will be held on Tuesday
July 31st to review the potential use of Tysabri for the treatment of patients with
Crohn’s disease. As a reminder, the European regulatory agencies recently denied
approval for Tysabri in Crohns.
■
Implication: Street expectations for Tysabri’s approval and sales for Crohn’s are low.
In fact, we do not include the indication in our estimates and do not expect much
downside if the outcome from the upcoming FDA panel is negative. However, the
upside is also limited, as we believe a risk management program and concern over
PML will cap the market opportunity. Also approval in Crohn’s disease would require
hiring of a new sales force to market to gastroenterologists. Thus, while we believe
that the panel is likely to support approval, we remain Neutral rated with a $53 price
target. Our price target is based on a P/E multiple of 16 times our pro forma 2008
estimated EPS of $3.29, which includes stock option expense.

Credit Suisse Comments re Tysabri in CD re BIIB
We Expect Panel to Vote for Crohn's Approval;
Size of Opportunity is a Question
■
Conclusion: Following a review of the Tysabri Crohn’s data – ENACT-1, ENACT-2,
and ENCORE – as well as the FDA briefing documents released on Friday, we
believe Tysabri’s clinical efficacy in Crohn’s supports approval, even with the risk of
PML. That said, we do expect the panel to wrestle with the risk/benefit ratio,
particularly given Crohn’s patient’s typical exposure to other immunosuppressive
agents. We also expect the risk management plan to limit the commercial opportunity,
particularly the need to discontinue Tysabri in patients who are still on steroids at 6-
months. Still, even with these challenges, given the prevalence and severity of the
disease, we believe Tysabri sales in Crohn’s could be in the $300 million range.
■
What’s New? A joint meeting of FDA’s Gastrointestinal Drugs Advisory Committee
and Drug Safety and Risk Management Advisory Committee will be held on Tuesday
July 31st to review the potential use of Tysabri for the treatment of patients with
Crohn’s disease. As a reminder, the European regulatory agencies recently denied
approval for Tysabri in Crohns.
■
Implication: Street expectations for Tysabri’s approval and sales for Crohn’s are low.
In fact, we do not include the indication in our estimates and do not expect much
downside if the outcome from the upcoming FDA panel is negative. However, the
upside is also limited, as we believe a risk management program and concern over
PML will cap the market opportunity. Also approval in Crohn’s disease would require
hiring of a new sales force to market to gastroenterologists. Thus, while we believe
that the panel is likely to support approval, we remain Neutral rated with a $53 price
target. Our price target is based on a P/E multiple of 16 times our pro forma 2008
estimated EPS of $3.29, which includes stock option expense.
Antwort auf Beitrag Nr.: 30.940.478 von Birgit.Tersteegen am 30.07.07 18:31:50Zeitplan des AC:
8:20 am. Introduction -- David Feigal, MD, SVP Regulatory Affairs, Biometrics and Global Pharmacovigilance & Risk Management at Elan.
8:25 am. Crohn's Disease -- William Sandborn, MD, Prof of Med, Gastro, Mayo Clinic
8:35 am. Efficacy Data -- Stephen Jones, MBBS, Director, Clinical Development, Elan
8:55 am. Safety Data -- Gordon Francis, MD, SVP, Clinical Development, Elan
9:20 am. Risk-Management Plan -- William Maier, MPH, PhD, Senior Director, Epidemiology, Elan
9:40 am. Clinical Perspective -- William Sandborn, MD, Prof of Med, Gastro, Mayo Clinic
9:50 am. Questions to the Sponsor
10:10 am. Break
10:25 am. PML -- Margo Smith, MD, Associate Program Director, Dept of Medicine, Washington Hospital Center
10:40 am. Clinical Review -- Anil Rajpal, MD, Medical Reviewer, Div of Gastro Products, CDER/FDA
11:15 am. Postmarketing Safety and RiskMAP -- Claudia Karwoski, PharmD, Risk Mgmt Team Leader, OSE, CDER/FDA
11:35 am. Questions to the FDA
12:00 pm. Lunch
1:00 pm. Open Public Hearing
2:30 pm. Questions to the Committee and Recommendations (15 min. break at 3:00 pm)
5:00 pm. Adjourn
8:20 am. Introduction -- David Feigal, MD, SVP Regulatory Affairs, Biometrics and Global Pharmacovigilance & Risk Management at Elan.
8:25 am. Crohn's Disease -- William Sandborn, MD, Prof of Med, Gastro, Mayo Clinic
8:35 am. Efficacy Data -- Stephen Jones, MBBS, Director, Clinical Development, Elan
8:55 am. Safety Data -- Gordon Francis, MD, SVP, Clinical Development, Elan
9:20 am. Risk-Management Plan -- William Maier, MPH, PhD, Senior Director, Epidemiology, Elan
9:40 am. Clinical Perspective -- William Sandborn, MD, Prof of Med, Gastro, Mayo Clinic
9:50 am. Questions to the Sponsor
10:10 am. Break
10:25 am. PML -- Margo Smith, MD, Associate Program Director, Dept of Medicine, Washington Hospital Center
10:40 am. Clinical Review -- Anil Rajpal, MD, Medical Reviewer, Div of Gastro Products, CDER/FDA
11:15 am. Postmarketing Safety and RiskMAP -- Claudia Karwoski, PharmD, Risk Mgmt Team Leader, OSE, CDER/FDA
11:35 am. Questions to the FDA
12:00 pm. Lunch
1:00 pm. Open Public Hearing
2:30 pm. Questions to the Committee and Recommendations (15 min. break at 3:00 pm)
5:00 pm. Adjourn
...die Krankheitshäufigkeit bei MC in den US ist riesig, weshalb die heutige Entescheidung nicht bedeutungslos ist.
http://www.ccfa.org/about/press/epidemiologyfacts
About the Epidemiology of IBD
Epidemiology is the study of the frequency and distribution of diseases in the population.
It is estimated that up to one million Americans suffer from Crohn's disease or ulcerative colitis (collectively known as inflammatory bowel diseases, or IBD). The search for risk factors in IBD has been frustrating, and the difficulty in diagnosing these diseases has been a further hindrance. However, epidemiologists have gathered enough information to know a good deal about the distribution of IBD in the United States and Western Europe. Current evidence suggests that both genetic and environmental factors contribute to these diseases.
http://www.ccfa.org/about/press/epidemiologyfacts
About the Epidemiology of IBD
Epidemiology is the study of the frequency and distribution of diseases in the population.
It is estimated that up to one million Americans suffer from Crohn's disease or ulcerative colitis (collectively known as inflammatory bowel diseases, or IBD). The search for risk factors in IBD has been frustrating, and the difficulty in diagnosing these diseases has been a further hindrance. However, epidemiologists have gathered enough information to know a good deal about the distribution of IBD in the United States and Western Europe. Current evidence suggests that both genetic and environmental factors contribute to these diseases.
Antwort auf Beitrag Nr.: 30.953.147 von Cyberhexe am 31.07.07 16:33:02....ich hoffe,Dein Zaubertrunk ist angerichtet!!

Interessant und klug :Elan's RiskMAP will recommend that treatment stop after three months if no improvement


Interessant und klug :Elan's RiskMAP will recommend that treatment stop after three months if no improvement
Ob so etwas WIORKLICH Zufall ist:
-----Original Message-----
From: XXXXXXXXXXX
Sent: Tuesday, July 31, 2007 10:28 AM
To: 'Jennifer.Corbett@dowjones.com'
Subject:
Jennifer
Is there a typo or missing word in the highlighted paragraph considering the quote below??
Officials from Elan Corp. (ELN) said Tuesday the risks of a rare brain infection outweigh the benefits of using Tysabri in patients with Crohn's disease.
David Feigal, Elan's senior vice president for regulatory affairs, said, "Tysabri can offer people with Crohn's disease a new treatment option when others have failed," adding, "We feel Tysabri has a favorable risk to benefit profile to support approval."
----------------------------------------------------------------
Nachdem dieser User es der Jornalistin geschickt hat,kam die Korrektur:
Officials from Elan Corp. (ELN) said Tuesday the benefits of using Tysabri in patients with Crohn's disease outweigh the risks of a rare brain infection.
(A story published at 10:09 a.m. EDT incorrectly stated the risks and benefits.)
> Dow Jones Newswires
07-31-07 1034ET
Copyright (c) 2007 Dow Jones & Company, Inc.
-----Original Message-----
From: XXXXXXXXXXX
Sent: Tuesday, July 31, 2007 10:28 AM
To: 'Jennifer.Corbett@dowjones.com'
Subject:
Jennifer
Is there a typo or missing word in the highlighted paragraph considering the quote below??
Officials from Elan Corp. (ELN) said Tuesday the risks of a rare brain infection outweigh the benefits of using Tysabri in patients with Crohn's disease.
David Feigal, Elan's senior vice president for regulatory affairs, said, "Tysabri can offer people with Crohn's disease a new treatment option when others have failed," adding, "We feel Tysabri has a favorable risk to benefit profile to support approval."
----------------------------------------------------------------
Nachdem dieser User es der Jornalistin geschickt hat,kam die Korrektur:
Officials from Elan Corp. (ELN) said Tuesday the benefits of using Tysabri in patients with Crohn's disease outweigh the risks of a rare brain infection.
(A story published at 10:09 a.m. EDT incorrectly stated the risks and benefits.)
> Dow Jones Newswires
07-31-07 1034ET
Copyright (c) 2007 Dow Jones & Company, Inc.
...das hört/liest sich bisher alles bestens:
Ich glaube ich war mit meinem Tipp (11-5) auf Zulassungsempfehlung wohl zu pessimistisch.
Ich glaube ich war mit meinem Tipp (11-5) auf Zulassungsempfehlung wohl zu pessimistisch.


 ----
----
US panel backs Biogen, Elan MS drug for Crohn's - Reuters
GAITHERSBURG, Md., July 31 (Reuters) - Biogen Idec (BIIB.O: Quote, Profile, Research) and Elan Corp.'s (ELN.I: Quote, Profile, Research) multiple sclerosis drug Tysabri should be approved for treating the bowel disorder Crohn's disease, a U.S. advisory panel said on Tuesday.
The Food and Drug Administration usually follows advisory panel recommendations. A final decision is expected by mid-October.
(Reporting by Lisa Richwine)
Antwort auf Beitrag Nr.: 30.960.384 von Birgit.Tersteegen am 31.07.07 23:57:14Obwohl ich seit Provenge etwas vorsichtiger bin, sollte das Votum
(12-3 für eine Zulassung bei 2 Enthaltungen) ein eindeutiges Signal sein, welches die FDA in Anbetracht der Studiengrösse und des Risikoplanes nicht kippen sollte.
http://www.elan.com/News/full.asp?ID=1034453
31 July 2007
Joint FDA Advisory Committee Recommends Approval of TYSABRI(R) for the Treatment of Moderate to Severe Crohn's Disease
GAITHERSBURG, Md.--(BUSINESS WIRE)--July 31, 2007--Elan Corporation, plc (NYSE: ELN) and Biogen Idec (NASDAQ: BIIB) announced today that the Gastrointestinal Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee of the U.S. Food and Drug Administration (FDA) voted 12 in favor to 3 opposed, with 2 abstaining, to recommend approval of TYSABRI(R) (natalizumab) as a treatment for moderate-to-severe Crohn's disease in patients who have failed or cannot tolerate available therapies.
The recommendation is advisory to the FDA, and the agency is not bound by this recommendation. Elan and Biogen Idec will continue to work closely with the FDA in the weeks ahead with the goal of making TYSABRI available for the treatment of appropriate patients with Crohn's disease. Discussions with the FDA will include adapting the existing TYSABRI risk management plan and addressing any other issues raised during the Committees' deliberations on this new indication.
About TYSABRI(R) (natalizumab)
(12-3 für eine Zulassung bei 2 Enthaltungen) ein eindeutiges Signal sein, welches die FDA in Anbetracht der Studiengrösse und des Risikoplanes nicht kippen sollte.
http://www.elan.com/News/full.asp?ID=1034453
31 July 2007
Joint FDA Advisory Committee Recommends Approval of TYSABRI(R) for the Treatment of Moderate to Severe Crohn's Disease
GAITHERSBURG, Md.--(BUSINESS WIRE)--July 31, 2007--Elan Corporation, plc (NYSE: ELN) and Biogen Idec (NASDAQ: BIIB) announced today that the Gastrointestinal Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee of the U.S. Food and Drug Administration (FDA) voted 12 in favor to 3 opposed, with 2 abstaining, to recommend approval of TYSABRI(R) (natalizumab) as a treatment for moderate-to-severe Crohn's disease in patients who have failed or cannot tolerate available therapies.
The recommendation is advisory to the FDA, and the agency is not bound by this recommendation. Elan and Biogen Idec will continue to work closely with the FDA in the weeks ahead with the goal of making TYSABRI available for the treatment of appropriate patients with Crohn's disease. Discussions with the FDA will include adapting the existing TYSABRI risk management plan and addressing any other issues raised during the Committees' deliberations on this new indication.
About TYSABRI(R) (natalizumab)
...und die EMEA bzw. die von MC-Betroffenen in der EU stehen wieder einmal mehr an der Seitenlinie:
Negative opinion
The CHMP adopted a negative opinion recommending the refusal of a marketing authorisation for Natalizumab (natalizumab), from Elan Pharma. Natalizumab was intended to be used to treat moderate to severe active Crohn’s disease in patients who had an inadequate response to or could not take conventional treatments for the disease and who have evidence of active inflammation. It was to
be used alone or in combination with other medicines for Crohn’s disease. EMEA review began on 18 October 2004 with an active review time of 204 days.
A separate question-and-answer document explaining the grounds for the negative opinion for Natalizumab is available on the EMEA website.
http://www.emea.europa.eu/pdfs/human/press/pr/32370307en.pdf
http://www.emea.europa.eu/pdfs/human/opinion/31085607en.pdf
Negative opinion
The CHMP adopted a negative opinion recommending the refusal of a marketing authorisation for Natalizumab (natalizumab), from Elan Pharma. Natalizumab was intended to be used to treat moderate to severe active Crohn’s disease in patients who had an inadequate response to or could not take conventional treatments for the disease and who have evidence of active inflammation. It was to
be used alone or in combination with other medicines for Crohn’s disease. EMEA review began on 18 October 2004 with an active review time of 204 days.
A separate question-and-answer document explaining the grounds for the negative opinion for Natalizumab is available on the EMEA website.
http://www.emea.europa.eu/pdfs/human/press/pr/32370307en.pdf
http://www.emea.europa.eu/pdfs/human/opinion/31085607en.pdf
...Elans Technologien werden in den F&E-Abteilungen von Pharmaschwergewichten eingesetzt. Es scheint nur eine Frage der Zeit zu sein, bis sich dieser Eisberg auch in den Zahlen niederschlägt.
Oryx Pharmaceuticals announces launch of NAPRELAN(TM)
MISSISSAUGA, ON, July 16 /CNW/ - Oryx Pharmaceuticals Inc. (Oryx) is
pleased to announce the introduction of prescription NAPRELAN(TM) tablets (naproxen sodium tablets) for the management of the signs and symptoms of osteoarthritis and rheumatoid arthritis.
NAPRELAN(TM) is a novel, controlled-release formulation of naproxen sodium, developed by Elan Corporation plc. It uses Elan's Intestinal Protective Drug Absorption System (IPDAS(TM) Technology), which is designed to provide rapid attainment of effective plasma concentrations and to maintain therapeutic concentrations over a 24-hour period. NAPRELAN(TM) was developed
in an attempt to reduce the impact on the mucosa of the stomach and duodenum compared with standard naproxen formulations.
http://www.newswire.ca/en/releases/archive/July2007/16/c6478…
Oryx Pharmaceuticals announces launch of NAPRELAN(TM)
MISSISSAUGA, ON, July 16 /CNW/ - Oryx Pharmaceuticals Inc. (Oryx) is
pleased to announce the introduction of prescription NAPRELAN(TM) tablets (naproxen sodium tablets) for the management of the signs and symptoms of osteoarthritis and rheumatoid arthritis.
NAPRELAN(TM) is a novel, controlled-release formulation of naproxen sodium, developed by Elan Corporation plc. It uses Elan's Intestinal Protective Drug Absorption System (IPDAS(TM) Technology), which is designed to provide rapid attainment of effective plasma concentrations and to maintain therapeutic concentrations over a 24-hour period. NAPRELAN(TM) was developed
in an attempt to reduce the impact on the mucosa of the stomach and duodenum compared with standard naproxen formulations.
http://www.newswire.ca/en/releases/archive/July2007/16/c6478…
Elans grosse Hoffnungsträger zur Therapie von Alzheimer
1.) der Antikörper AAB-001 oder Bapineuzumab
AAB-001 Phase 2
http://www.clinicaltrials.gov/ct/show/NCT00112073
Total Enrollment: 240 Study start: April 2005; Study completion: April 2008
AAB-001 Studie zur Sicherheit bzw. Verträglichkeit Phase 1
http://www.clinicaltrials.gov/ct/show/NCT00397891?order=1
Study start: October 2006; Expected completion: October 2008
2.) das Vakzin ACC-001
ACC-001 in Phase 2
http://www.clinicaltrials.gov/ct/show/NCT00479557?order=8
Total Enrollment: 56 Study start: May 2007; Expected completion: February 2009
und eine weitere Phase2-Studie, welche im Dezember begonnen wird:
http://www.clinicaltrials.gov/ct/show/NCT00498602?order=5
Total Enrollment: 228 Study start: December 2007; Expected completion: March 2009
1.) der Antikörper AAB-001 oder Bapineuzumab
AAB-001 Phase 2
http://www.clinicaltrials.gov/ct/show/NCT00112073
Total Enrollment: 240 Study start: April 2005; Study completion: April 2008
AAB-001 Studie zur Sicherheit bzw. Verträglichkeit Phase 1
http://www.clinicaltrials.gov/ct/show/NCT00397891?order=1
Study start: October 2006; Expected completion: October 2008
2.) das Vakzin ACC-001
ACC-001 in Phase 2
http://www.clinicaltrials.gov/ct/show/NCT00479557?order=8
Total Enrollment: 56 Study start: May 2007; Expected completion: February 2009
und eine weitere Phase2-Studie, welche im Dezember begonnen wird:
http://www.clinicaltrials.gov/ct/show/NCT00498602?order=5
Total Enrollment: 228 Study start: December 2007; Expected completion: March 2009
Fidelity hält knapp 15% -->
http://www.investegate.co.uk/article.aspx?id=200708021739484…
70,250,718 Aktien entsprechen 14.98 %
http://www.investegate.co.uk/article.aspx?id=200708021739484…
70,250,718 Aktien entsprechen 14.98 %
...und die Analysten sind der Meinung , dass...
http://www.newratings.com/companies/headlines.asp?isin=US284…
http://www.newratings.com/companies/headlines.asp?isin=US284…
...die Schlagzeilen häufen sich:
Tests in rats revealed that administering a drug called Bapineuzumab, which is undergoing clinical trials for Alzheimer\'s, stopped the proteins building up, they report in the Proceedings of the National Academy of Sciences.
http://www.guardian.co.uk/science/2007/aug/07/3
Tests in rats revealed that administering a drug called Bapineuzumab, which is undergoing clinical trials for Alzheimer\'s, stopped the proteins building up, they report in the Proceedings of the National Academy of Sciences.
http://www.guardian.co.uk/science/2007/aug/07/3
Überraschung - trotz einstimmiger AC-Empfehlung keine Zulassung für GSKs höher dosierte Formulierung von Advair:
http://biz.yahoo.com/ap/070808/apfn_advair_fda_decision.html…
Ist dies nur ein schlechtes Omen für Tysabri und CD in den Staaten...oder richtungsweisend? Die FDA ist nicht mehr so berechenbar wie auch schon!
http://biz.yahoo.com/ap/070808/apfn_advair_fda_decision.html…
Ist dies nur ein schlechtes Omen für Tysabri und CD in den Staaten...oder richtungsweisend? Die FDA ist nicht mehr so berechenbar wie auch schon!
Antwort auf Beitrag Nr.: 31.100.271 von Cyberhexe am 09.08.07 18:39:30
August 7, 2007, 5:01 pm
Old Drug Takes New Turn in Alzheimer’s Test
Posted by Scott Hensley
http://blogs.wsj.com/health/2007/08/07/old-drug-takes-new-tu…
August 7, 2007, 5:01 pm
Old Drug Takes New Turn in Alzheimer’s Test
Posted by Scott Hensley
http://blogs.wsj.com/health/2007/08/07/old-drug-takes-new-tu…
...der Prozess mit der 4fachen Ausbeute, der "Natalizumab High Titer" process, ist nun in der Klinik:
http://clinicaltrials.gov/ct/show/NCT00516893?order=10
Study ID Numbers: 101MS201
Last Updated: August 15, 2007
Record first received: August 14, 2007
http://clinicaltrials.gov/ct/show/NCT00516893?order=10
Study ID Numbers: 101MS201
Last Updated: August 15, 2007
Record first received: August 14, 2007
...eigentlich sollten nun alle Neurologen ihre Patienten zumindest darüber informieren, welchen Einfluss Natalizumab auf den Verlauf einer MS einschliesslich der Abnahme von kognitiven Fähigkeiten nehmen kann.
http://www3.interscience.wiley.com/cgi-bin/fulltext/11480377…
Despite these limitations, the findings demonstrate that natalizumab therapy resulted in significant improvement of HRQoL compared with placebo in patients with relapsing MS. For many patients, and for many of the dimensions defined by the SF-36 subscales, these findings represent sustained improvement from baseline QoL, rather than a slowing of HRQoL deterioration. In addition, the SF-36 can be further explored as a method to validate and compare the meaning, from the patient's perspective, of changes in clinical and MRI measures of disease within the large number of relapsing MS patients tested in these clinical studies.
http://www3.interscience.wiley.com/cgi-bin/fulltext/11480377…
Despite these limitations, the findings demonstrate that natalizumab therapy resulted in significant improvement of HRQoL compared with placebo in patients with relapsing MS. For many patients, and for many of the dimensions defined by the SF-36 subscales, these findings represent sustained improvement from baseline QoL, rather than a slowing of HRQoL deterioration. In addition, the SF-36 can be further explored as a method to validate and compare the meaning, from the patient's perspective, of changes in clinical and MRI measures of disease within the large number of relapsing MS patients tested in these clinical studies.
Hexchen,BM für Dich...

Doug Wolf, M.D. Atlanta Gastroenterology Associates
beim "Public Hearing" anlässlich des AC am 31.7.2007 zu Tysabri und MorbusCrohn:
http://www.fda.gov/OHRMS/DOCKETS/ac/07/slides/2007-4313oph1-…
Natalizumab-Unmet Need for CD
•Moderate to Severe Crohn’s Disease
•250,000-500,000 of the Crohn’s population fit the definition at some point in their disease course
•Over half of these patients do not have sustained (1 year) benefit with available anti-TNF agents
•125,000 to 250,000 patients have potential need for another agent, e.g. natalizumab
beim "Public Hearing" anlässlich des AC am 31.7.2007 zu Tysabri und MorbusCrohn:
http://www.fda.gov/OHRMS/DOCKETS/ac/07/slides/2007-4313oph1-…
Natalizumab-Unmet Need for CD
•Moderate to Severe Crohn’s Disease
•250,000-500,000 of the Crohn’s population fit the definition at some point in their disease course
•Over half of these patients do not have sustained (1 year) benefit with available anti-TNF agents
•125,000 to 250,000 patients have potential need for another agent, e.g. natalizumab
Das NHS hat Tysabri zur Behandlung der MS empfohlen -->
http://guidance.nice.org.uk/TA127
dies ist vor allem vor dem Hintergrund bemerkenswert, dass das NHS weder beta-Interferone (Avonex, Rebif, Betaseron) noch Glatiramer acetate (Copaxone) empfohlen hat:
http://guidance.nice.org.uk/TA32
Man sollte meinen, dass nun die Tysabri-Restriktionen ein baldiges Ende haben sollten und die therapeutische Überlegenheit zu einer breiten Anwendung führen sollte.
Time will tell!
http://guidance.nice.org.uk/TA127
dies ist vor allem vor dem Hintergrund bemerkenswert, dass das NHS weder beta-Interferone (Avonex, Rebif, Betaseron) noch Glatiramer acetate (Copaxone) empfohlen hat:
http://guidance.nice.org.uk/TA32
Man sollte meinen, dass nun die Tysabri-Restriktionen ein baldiges Ende haben sollten und die therapeutische Überlegenheit zu einer breiten Anwendung führen sollte.
Time will tell!
...die Entscheidung der FDA zu Tysabri und CD wird bis spätestens Mitte Oktober erwartet. Man darf diesbezüglich optimistisch sein.
http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4313s1-01…
http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4313s1-01…
interessant:
12:00 AN UPDATE ON THE SAFETY OF NATALIZUMAB IN PATIENTS WITH RELAPSING MULTIPLE SCLEROSIS: RESULTS FROM TOUCH AND TYGRIS
C. Bozic 1, G. Belcher 1, M. Kooijmans 1, R. Kim 1, F. Lynn 1, M.A. Panzara 1
Cambridge, MA, USA
http://www.kenes.com/efns2007/program/session1.asp
http://www.kenes.com/efns2007/program/ViewAbstract.asp
12:00 AN UPDATE ON THE SAFETY OF NATALIZUMAB IN PATIENTS WITH RELAPSING MULTIPLE SCLEROSIS: RESULTS FROM TOUCH AND TYGRIS
C. Bozic 1, G. Belcher 1, M. Kooijmans 1, R. Kim 1, F. Lynn 1, M.A. Panzara 1
Cambridge, MA, USA
http://www.kenes.com/efns2007/program/session1.asp
http://www.kenes.com/efns2007/program/ViewAbstract.asp
nebeninformation betr. alzheimer:
neurochem´s (nrmx) \\\"alzhemed\\\" dürfte aller wahrscheinlichkeit aus dem rennen sein - der kurs der aktie seit jahresbeginn hat´s angedeutet ...
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
August 26, 2007
Neurochem announces results from tramiprosate (ALZHEMED™) North American Phase III clinical trial
Neurochem will host a conference call tomorrow August 27, 2007, at 8:30 AM EDT.
Neurochem Inc. (NASDAQ: NRMX; TSX: NRM) announces today top-line results from the North American Phase III clinical trial designed to assess the safety, efficacy and disease modification effect of tramiprosate (ALZHEMED™) for the treatment of Alzheimer\\\'s disease (AD). Following a recent meeting with the U.S. Food and Drug Administration (FDA) and subsequent statistical analyses, the Company announces that the North American Phase III clinical trial for tramiprosate (ALZHEMED™), despite the descriptive data showing numerical differences, did not demonstrate a statistically significant difference in favor of tramiprosate (ALZHEMED™) with respect to the primary endpoints over 18 months of treatment. However, a substantial difference observed in hippocampal volume did approach statistical significance. Due to significant interference from high between-site variations that complicated the statistical analyses beyond expectations, it is not possible to draw definitive conclusions with respect to the treatment effect of tramiprosate (ALZHEMED™).
http://www.neurochem.com/PR214.htm
neurochem´s (nrmx) \\\"alzhemed\\\" dürfte aller wahrscheinlichkeit aus dem rennen sein - der kurs der aktie seit jahresbeginn hat´s angedeutet ...
~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
August 26, 2007
Neurochem announces results from tramiprosate (ALZHEMED™) North American Phase III clinical trial
Neurochem will host a conference call tomorrow August 27, 2007, at 8:30 AM EDT.
Neurochem Inc. (NASDAQ: NRMX; TSX: NRM) announces today top-line results from the North American Phase III clinical trial designed to assess the safety, efficacy and disease modification effect of tramiprosate (ALZHEMED™) for the treatment of Alzheimer\\\'s disease (AD). Following a recent meeting with the U.S. Food and Drug Administration (FDA) and subsequent statistical analyses, the Company announces that the North American Phase III clinical trial for tramiprosate (ALZHEMED™), despite the descriptive data showing numerical differences, did not demonstrate a statistically significant difference in favor of tramiprosate (ALZHEMED™) with respect to the primary endpoints over 18 months of treatment. However, a substantial difference observed in hippocampal volume did approach statistical significance. Due to significant interference from high between-site variations that complicated the statistical analyses beyond expectations, it is not possible to draw definitive conclusions with respect to the treatment effect of tramiprosate (ALZHEMED™).
http://www.neurochem.com/PR214.htm
eine weitere Studie mit Natalizumab ist abgeschlossen:
http://clinicaltrials.gov/ct/show/NCT00424788?order=4
Biogen und Elan scheinen folgende Strategie hinsichtlich Tysabri anzustreben:
1.) über den Risikoplan das PML-Risiko klein halten
2.) Monitoring von PML
3.) Therapie: als erste Massnahme ist wohl eine Natalizumab-Eliminierung vorgesehen
http://clinicaltrials.gov/ct/show/NCT00424788?order=4
Biogen und Elan scheinen folgende Strategie hinsichtlich Tysabri anzustreben:
1.) über den Risikoplan das PML-Risiko klein halten
2.) Monitoring von PML
3.) Therapie: als erste Massnahme ist wohl eine Natalizumab-Eliminierung vorgesehen
Dale Schenk - ein zukünftiger Nobelpreisträger? Falls AAB-001 die Zulassung erhält, bin ich davon überzeugt!
http://video.google.com/videoplay?docid=-7826073900349013452…
http://www.elan.com/AboutUs/SeniorManagement/full.asp?ID=135…
http://video.google.com/videoplay?docid=-7826073900349013452…
http://www.elan.com/AboutUs/SeniorManagement/full.asp?ID=135…
...die Spannung steigt wieder einmal, denn bis zum 15.10. hat die FDA über den Zulassungsantrag von Tysabri zur Behandlung von Morbus Crohn (CD Crohns Disease) zu befinden. Und in Anbetracht des Votums der Experten beim AC (13:4 für Zulassung) und der nachfolgenden Argumente, ist von einer positiven Zulassungsentscheidung auszugehen:
- Tysabri soll lediglich in einer Eskalationstherapie denjenigen Patienten zur Verfügung gestellt werden, die mit den anderen Medis austherapiert sind und ausser chirugischen Massnahmen über keine Alternativen verfügen - und die chirurgischen Massnahmen (Entfernung grosser Teile des Darmes) haben seinerseits massiven Einfluss auf Vitalität und Lebensqualität der Betroffenen, zumal damit die Erkrankung nicht gestoppt wird
- Tysabri soll lediglich Patienten mit einem nachweisbaren Marker, und zwar CRP (C reactive protein), welches eine vorliegende Entzündung markiert, gegeben werden - und zwar erst ab einem bestimmten CRP-Level (> 2.87 ?). Bei dieser Subpopulation hat Tysabri in der ersten Induktionsstude (CD301) die besten Ergebnisse gezeigt, weshalb eine weitere P3-Studie (CD307) mit diesem Ausschlusskriterium (CRP kleiner 2.87, d.h. man konnte nur dann an der Studie teilnehmen, wenn der Marker diesen Grenzwert erreichte oder darüber lag, also eine akute Entzündung vorgelegen hat).
Ergebnis CD307:
primärer Endpunkt war eine Reduktion des CDAI (CD activity index) um > 70 Punkte nach 8&12 Wochen:
Natalizumab 48%
Placebo: 32%
p < 0.001
sekundärer Endpunkt: Remission, d.h. einen CDAI < 150 nach 8&12 Wochen
Natalizumab: 26%
Placebo: 16%
p=0.002
Dieses Ergebnis konnte durch den Verlauf des Markers eindeutig bestätigt werden, da sich die Konzentration des Markers der mit dem verum behandelten Patienten halbierte.
Die durchgeführte Erhaltungsstudie (CD303), in welcher alle Teilnehmer aus CD301 mit einem objektiven Response weiterbehandelt wurden, hat zu folgendem Ergebnisse geführt:
primärer Endpunkt: nachhaltiger CDAI (<220 und ein Wiederanstieg des CDAI < 70 Punkte) nach 6 Monaten
nach 6 Monaten:
Tysabri 61%
Placebo 28%
p < 0.001
nach 12 Monaten:
Tysabri 54%
Placebo 20%
nachhaltige klinische Remission (CDAI < 150)
nach 6 Monaten:
Tysabri 44%
Placebo 26%
p < 0.001
nach 12 Monaten:
Tysabri 39%
Placebo 15%
Ein weiteres untersuchtes Kriterium war der Einfluss auf den möglichen Verzicht von entzündungshemmenden Medis, sogen. Steroiden.
Eliminierung von Steroiden
nach 6 Monaten:
Tysabri 58%
Placebo 28%
p < 0.001
nach 12 Monaten:
Tysabri 49%
Placebo 20%
steroidfreie Remission:
nach 6 Monaten:
Tysabri 45%
Placebo 22%
p < 0.014
nach 12 Monaten:
Tysabri 42%
Placebo 14%
...also insgesamt doch sehr viele Gründe für die FDA, Tysabri aus Mangel an Alternativen für austherapierte MC-Patienten zuzulassen. Und dies ist etwa 1/3 bis 1/4 der amerikanischen MC-Gemeinschaft.
- Tysabri soll lediglich in einer Eskalationstherapie denjenigen Patienten zur Verfügung gestellt werden, die mit den anderen Medis austherapiert sind und ausser chirugischen Massnahmen über keine Alternativen verfügen - und die chirurgischen Massnahmen (Entfernung grosser Teile des Darmes) haben seinerseits massiven Einfluss auf Vitalität und Lebensqualität der Betroffenen, zumal damit die Erkrankung nicht gestoppt wird
- Tysabri soll lediglich Patienten mit einem nachweisbaren Marker, und zwar CRP (C reactive protein), welches eine vorliegende Entzündung markiert, gegeben werden - und zwar erst ab einem bestimmten CRP-Level (> 2.87 ?). Bei dieser Subpopulation hat Tysabri in der ersten Induktionsstude (CD301) die besten Ergebnisse gezeigt, weshalb eine weitere P3-Studie (CD307) mit diesem Ausschlusskriterium (CRP kleiner 2.87, d.h. man konnte nur dann an der Studie teilnehmen, wenn der Marker diesen Grenzwert erreichte oder darüber lag, also eine akute Entzündung vorgelegen hat).
Ergebnis CD307:
primärer Endpunkt war eine Reduktion des CDAI (CD activity index) um > 70 Punkte nach 8&12 Wochen:
Natalizumab 48%
Placebo: 32%
p < 0.001
sekundärer Endpunkt: Remission, d.h. einen CDAI < 150 nach 8&12 Wochen
Natalizumab: 26%
Placebo: 16%
p=0.002
Dieses Ergebnis konnte durch den Verlauf des Markers eindeutig bestätigt werden, da sich die Konzentration des Markers der mit dem verum behandelten Patienten halbierte.
Die durchgeführte Erhaltungsstudie (CD303), in welcher alle Teilnehmer aus CD301 mit einem objektiven Response weiterbehandelt wurden, hat zu folgendem Ergebnisse geführt:
primärer Endpunkt: nachhaltiger CDAI (<220 und ein Wiederanstieg des CDAI < 70 Punkte) nach 6 Monaten
nach 6 Monaten:
Tysabri 61%
Placebo 28%
p < 0.001
nach 12 Monaten:
Tysabri 54%
Placebo 20%
nachhaltige klinische Remission (CDAI < 150)
nach 6 Monaten:
Tysabri 44%
Placebo 26%
p < 0.001
nach 12 Monaten:
Tysabri 39%
Placebo 15%
Ein weiteres untersuchtes Kriterium war der Einfluss auf den möglichen Verzicht von entzündungshemmenden Medis, sogen. Steroiden.
Eliminierung von Steroiden
nach 6 Monaten:
Tysabri 58%
Placebo 28%
p < 0.001
nach 12 Monaten:
Tysabri 49%
Placebo 20%
steroidfreie Remission:
nach 6 Monaten:
Tysabri 45%
Placebo 22%
p < 0.014
nach 12 Monaten:
Tysabri 42%
Placebo 14%
...also insgesamt doch sehr viele Gründe für die FDA, Tysabri aus Mangel an Alternativen für austherapierte MC-Patienten zuzulassen. Und dies ist etwa 1/3 bis 1/4 der amerikanischen MC-Gemeinschaft.
Antwort auf Beitrag Nr.: 31.696.426 von Cyberhexe am 22.09.07 12:57:21also insgesamt doch sehr viele Gründe für die FDA, Tysabri aus Mangel an Alternativen für austherapierte MC-Patienten zuzulassen. Und dies ist etwa 1/3 bis 1/4 der amerikanischen MC-Gemeinschaft.
Wie gross ist das Potential bzw. wieviel Patienten kommen in den US für diese Indikation in Frage?
...aus einer Präsentation beim AC zu Ty und CD von W.Sandborn von der Mayo Klinik:
http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4313s1-01…
Folie 4:
>500,000 cases of CD in US
¨70-90% of patients will undergo surgery within 20 years
¨Extra-intestinal symptoms occur –Ocular, articular, cutaneous ¨Major impact on patients’ quality of life
¨Increased mortality rate in patients with Crohn’s disease –SMR 1.5 compared to general population
Folie 12
Unmet Need in Crohn’s Disease¨Many patients fail conventional therapy and require treatment with biologics
(inhibitors of TNF-α)
¨30% of patients treated with inhibitors of TNF-α never respond and another 30-40% resume disease activity within 12 months
¨Need exists for additional therapy, particularly with a different mode of action that might overcome current treatment limitations
Conclusion:
weit über 100.000 MorbusCrohn-Patienten könnten in den US von Tysabri profitieren!
wow
Wie gross ist das Potential bzw. wieviel Patienten kommen in den US für diese Indikation in Frage?
...aus einer Präsentation beim AC zu Ty und CD von W.Sandborn von der Mayo Klinik:
http://www.fda.gov/ohrms/dockets/ac/07/slides/2007-4313s1-01…
Folie 4:
>500,000 cases of CD in US
¨70-90% of patients will undergo surgery within 20 years
¨Extra-intestinal symptoms occur –Ocular, articular, cutaneous ¨Major impact on patients’ quality of life
¨Increased mortality rate in patients with Crohn’s disease –SMR 1.5 compared to general population
Folie 12
Unmet Need in Crohn’s Disease¨Many patients fail conventional therapy and require treatment with biologics
(inhibitors of TNF-α)
¨30% of patients treated with inhibitors of TNF-α never respond and another 30-40% resume disease activity within 12 months
¨Need exists for additional therapy, particularly with a different mode of action that might overcome current treatment limitations
Conclusion:
weit über 100.000 MorbusCrohn-Patienten könnten in den US von Tysabri profitieren!
wow
NEW YORK, Sept. 28 /PRNewswire/

-- The under-treatment of pain is one of the most important public health issues facing the United States today, impacting the lives of an estimated one in three Americans, according to the National Pain Foundation. Unfortunately, many patients who suffer from severe chronic pain do not experience long-term relief from available treatments and key aspects of their daily lives -- including normal physical activity, employment, relationships and a multitude of other capacities -- are often severely compromised.(1)
However, hope exists for those who are suffering in the form of a sea snail. Researchers at Elan Pharmaceuticals, Inc., a wholly-owned subsidiary of Elan Corporation, plc, have harnessed the venom of the Conus magus sea snail into a non-narcotic alternative to traditional pain medications. PRIALT(R) (ziconotide intrathecal infusion), a synthetic form of a naturally occurring peptide, circumvents concerns over addiction and safety linked to opioid use.
"Chronic pain disrupts an individual's life and can add tremendous physical, emotional and economic burden," said Mary Pat Aardrup, executive director, National Pain Foundation. "Chronic pain is one of the great hidden crises of our time, made all the more urgent by the fact that treatment is often limited mainly to opioid medications that may be inadequate or inappropriate for many patients. PRIALT is an important chapter in the management of severe chronic pain that warrants intrathecal therapy."
Physicians and patients nationwide are discovering the pain-relieving capabilities of PRIALT. Dawn Campbell endured 32 surgeries to relieve severe pain in her bladder, back and legs, including removal of her uterus, ovaries and bladder.
"The pain was constant for 23 years and nothing I was using was helping to stop it," said Campbell.
After finally being treated with PRIALT, on the advice of her physician, Dr. Gladstone McDowell at Grant Medical Center at OhioHealth in Columbus, Ohio, Campbell is now living with less pain than before. Though it took time to find the correct dosage to experience relief, both Dawn and her doctor say it was worth it.
"Now I can do so much more, I have my life back," said Campbell.
Approved in 2004 for the management of severe chronic pain, PRIALT is administered through a programmable microinfusion pump that can be implanted or used externally. The pump then releases the drug on a timely basis into the fluid surrounding the spinal cord. Animal research suggests that the mechanism of action for PRIALT works by targeting and blocking N-type calcium channels on nerves in the spinal cord that ordinarily transmit pain signals.
Dr. McDowell explains, "It's a very innovative form of therapy that's very exciting. PRIALT is believed to block the pain by binding to the nerves that transmit pain. It saves you from being exposed to narcotics and you don't develop an addiction or tolerance to it."
Additionally, there are no withdrawal symptoms and patients can abruptly discontinue PRIALT, making it different than all other intrathecal pain medications available to patients.
According to the National Pain Foundation, chronic pain is a leading cause of distress and the primary cause of disability in the US and significantly affects the lives of an estimated 70 million Americans each day. The issue of chronic pain also impacts employers, insurance agencies, healthcare institutions and the U.S. economy, costing an estimated $100 billion annually.
Tragically, chronic pain is undertreated in the healthcare system at all levels, from physician offices, emergency rooms, hospitals and long-term care facilities for a variety of reasons. Pain may be under-diagnosed and under-treated because it is often considered a symptom of another illness or coined as a "get over it" ailment or as part of an injury or weakness, according to Arthur S. Meyerson of Pain Magazine. Additionally, as cited in recent news coverage, fear of legal or disciplinary actions may prevent physicians from using all available treatments, as providers and patients may be concerned about tolerance and addiction problems related to opioid treatments.
About PRIALT
PRIALT, developed by scientists at Elan, is the synthetic equivalent of a naturally occurring conopeptide found in a marine snail known as Conus magus. PRIALT is administered through programmable microinfusion pumps that can be implanted or external and which release the drug into the fluid surrounding the spinal cord. Approved for use in 2004, PRIALT was the first intrathecal analgesic approved in the U.S. in more than two decades.
PRIALT is indicated for the management of severe chronic pain in patients for whom intrathecal (IT) therapy is warranted, and who are tolerant of or refractory to other treatment, such as systemic analgesics, adjunctive therapies, or IT morphine. PRIALT is in a class of non-opioid analgesics known as N-type calcium channel blockers.
WARNING: Severe psychiatric symptoms and neurological impairment may occur during treatment with PRIALT. Patients with a pre-existing history of psychosis should not be treated with PRIALT. All patients should be monitored frequently for evidence of cognitive impairment, hallucinations, or changes in mood or consciousness. PRIALT therapy can be interrupted or discontinued abruptly without evidence of withdrawal effects in the event of serious neurological or psychiatric signs or symptoms.
Dizziness, nausea, confusional state, and nystagmus were the most frequently reported adverse events (greater than or equal to 25%) in clinical trials (N=1,254).
PRIALT(R) (ziconotide intrathecal infusion) is for use only in the Medtronic SynchroMed(R) EL, SynchroMed(R) II Infusion System and the CADD-Micro(R) ambulatory infusion pump.
Information about PRIALT, including prescribing information and comprehensive support services, is available through a toll-free number,
1-888-PRIALT-1, and at http://www.prialt.com/
(1) Testimony of Rollin M. Gallagher, MD, MPH
Congressional Briefing: The Epidemic of Pain in America
June 13, 2006
http://www.painfoundation.org/Action/Briefing061306/Gallaghe…
PRIALT
http://www.earthtimes.org/articles/show/news_press_release,1…

-- The under-treatment of pain is one of the most important public health issues facing the United States today, impacting the lives of an estimated one in three Americans, according to the National Pain Foundation. Unfortunately, many patients who suffer from severe chronic pain do not experience long-term relief from available treatments and key aspects of their daily lives -- including normal physical activity, employment, relationships and a multitude of other capacities -- are often severely compromised.(1)
However, hope exists for those who are suffering in the form of a sea snail. Researchers at Elan Pharmaceuticals, Inc., a wholly-owned subsidiary of Elan Corporation, plc, have harnessed the venom of the Conus magus sea snail into a non-narcotic alternative to traditional pain medications. PRIALT(R) (ziconotide intrathecal infusion), a synthetic form of a naturally occurring peptide, circumvents concerns over addiction and safety linked to opioid use.
"Chronic pain disrupts an individual's life and can add tremendous physical, emotional and economic burden," said Mary Pat Aardrup, executive director, National Pain Foundation. "Chronic pain is one of the great hidden crises of our time, made all the more urgent by the fact that treatment is often limited mainly to opioid medications that may be inadequate or inappropriate for many patients. PRIALT is an important chapter in the management of severe chronic pain that warrants intrathecal therapy."
Physicians and patients nationwide are discovering the pain-relieving capabilities of PRIALT. Dawn Campbell endured 32 surgeries to relieve severe pain in her bladder, back and legs, including removal of her uterus, ovaries and bladder.
"The pain was constant for 23 years and nothing I was using was helping to stop it," said Campbell.
After finally being treated with PRIALT, on the advice of her physician, Dr. Gladstone McDowell at Grant Medical Center at OhioHealth in Columbus, Ohio, Campbell is now living with less pain than before. Though it took time to find the correct dosage to experience relief, both Dawn and her doctor say it was worth it.
"Now I can do so much more, I have my life back," said Campbell.
Approved in 2004 for the management of severe chronic pain, PRIALT is administered through a programmable microinfusion pump that can be implanted or used externally. The pump then releases the drug on a timely basis into the fluid surrounding the spinal cord. Animal research suggests that the mechanism of action for PRIALT works by targeting and blocking N-type calcium channels on nerves in the spinal cord that ordinarily transmit pain signals.
Dr. McDowell explains, "It's a very innovative form of therapy that's very exciting. PRIALT is believed to block the pain by binding to the nerves that transmit pain. It saves you from being exposed to narcotics and you don't develop an addiction or tolerance to it."
Additionally, there are no withdrawal symptoms and patients can abruptly discontinue PRIALT, making it different than all other intrathecal pain medications available to patients.
According to the National Pain Foundation, chronic pain is a leading cause of distress and the primary cause of disability in the US and significantly affects the lives of an estimated 70 million Americans each day. The issue of chronic pain also impacts employers, insurance agencies, healthcare institutions and the U.S. economy, costing an estimated $100 billion annually.
Tragically, chronic pain is undertreated in the healthcare system at all levels, from physician offices, emergency rooms, hospitals and long-term care facilities for a variety of reasons. Pain may be under-diagnosed and under-treated because it is often considered a symptom of another illness or coined as a "get over it" ailment or as part of an injury or weakness, according to Arthur S. Meyerson of Pain Magazine. Additionally, as cited in recent news coverage, fear of legal or disciplinary actions may prevent physicians from using all available treatments, as providers and patients may be concerned about tolerance and addiction problems related to opioid treatments.
About PRIALT
PRIALT, developed by scientists at Elan, is the synthetic equivalent of a naturally occurring conopeptide found in a marine snail known as Conus magus. PRIALT is administered through programmable microinfusion pumps that can be implanted or external and which release the drug into the fluid surrounding the spinal cord. Approved for use in 2004, PRIALT was the first intrathecal analgesic approved in the U.S. in more than two decades.
PRIALT is indicated for the management of severe chronic pain in patients for whom intrathecal (IT) therapy is warranted, and who are tolerant of or refractory to other treatment, such as systemic analgesics, adjunctive therapies, or IT morphine. PRIALT is in a class of non-opioid analgesics known as N-type calcium channel blockers.
WARNING: Severe psychiatric symptoms and neurological impairment may occur during treatment with PRIALT. Patients with a pre-existing history of psychosis should not be treated with PRIALT. All patients should be monitored frequently for evidence of cognitive impairment, hallucinations, or changes in mood or consciousness. PRIALT therapy can be interrupted or discontinued abruptly without evidence of withdrawal effects in the event of serious neurological or psychiatric signs or symptoms.
Dizziness, nausea, confusional state, and nystagmus were the most frequently reported adverse events (greater than or equal to 25%) in clinical trials (N=1,254).
PRIALT(R) (ziconotide intrathecal infusion) is for use only in the Medtronic SynchroMed(R) EL, SynchroMed(R) II Infusion System and the CADD-Micro(R) ambulatory infusion pump.
Information about PRIALT, including prescribing information and comprehensive support services, is available through a toll-free number,
1-888-PRIALT-1, and at http://www.prialt.com/
(1) Testimony of Rollin M. Gallagher, MD, MPH
Congressional Briefing: The Epidemic of Pain in America
June 13, 2006
http://www.painfoundation.org/Action/Briefing061306/Gallaghe…
PRIALT
http://www.earthtimes.org/articles/show/news_press_release,1…
die kommende Woche dürfte äusserst spannend werden, da die FDA bis Montag 15.10. die Entscheidung über die Zulassung von Ty zur Behandlung von Morbus Crohn bekanntgeben wird und dies möglicherweise schon in der nächsten Woche tun wird; und zwar ist Ty sehr wahrscheinlich dann die letzte Alternative vor einem chirurgischen Eingriff, der wiederum bei Crohn-Patienten relativ häufig erforderlich ist:
http://www.ehealthmd.com/library/crohnsdisease/CD_surgery.ht…
Ich bin sehr zuversichtlich, dass die FDA der Empfehlung der Expertenrunde (Advisory Committee) folgen wird.
good luck
ch
http://www.ehealthmd.com/library/crohnsdisease/CD_surgery.ht…
Ich bin sehr zuversichtlich, dass die FDA der Empfehlung der Expertenrunde (Advisory Committee) folgen wird.
good luck
ch
Antwort auf Beitrag Nr.: 31.872.786 von Cyberhexe am 06.10.07 20:49:06....Ich bin sehr zuversichtlich, dass die FDA der Empfehlung der Expertenrunde (Advisory Committee) folgen wird.
..und nicht nur Du, Cybi...
Zum Approval von Tysabri für MC schreibt DAVY:
As we noted last week, a FDA decision looms (around October 15th) on the approval of Tysabri as a treatment for Crohn's Disease. We think that Tysabri will receive approval , albeit in a sub-set of the addressable patient population, i.e. failures and non-responders to anti-TNF therapy.
, albeit in a sub-set of the addressable patient population, i.e. failures and non-responders to anti-TNF therapy.
We also believe that the product will face considerable commercial hurdles given its restrictive labelling and increased competition in the field from other new therapies. Our own forecasts call for 2010 revenues of c.$200m, the bulk of this in the US market.
http://www.davy.ie/other/pubarticles/eqbrief20071010.pdf
..und nicht nur Du, Cybi...

Zum Approval von Tysabri für MC schreibt DAVY:
As we noted last week, a FDA decision looms (around October 15th) on the approval of Tysabri as a treatment for Crohn's Disease. We think that Tysabri will receive approval
 , albeit in a sub-set of the addressable patient population, i.e. failures and non-responders to anti-TNF therapy.
, albeit in a sub-set of the addressable patient population, i.e. failures and non-responders to anti-TNF therapy. We also believe that the product will face considerable commercial hurdles given its restrictive labelling and increased competition in the field from other new therapies. Our own forecasts call for 2010 revenues of c.$200m, the bulk of this in the US market.
http://www.davy.ie/other/pubarticles/eqbrief20071010.pdf
...das ist doch überaus erfereulich: 17.000 Patienten sind nun auf Tysabri und keinweiterer PML-Fall!
As of the end of September 2007:
In the US, approximately 10,500 patients are on TYSABRI commercially and over 2,100 physicians have prescribed the therapy;
In the EU, approximately 5,500 patients are on TYSABRI therapy commercially; and
In global clinical trials, approximately 1,000 patients are on TYSABRI therapy.
TYSABRI is available in the United States through the TOUCH(TM) Prescribing Program. All prescribers, infusion sites and patients receiving TYSABRI are required to enroll in TOUCH. Safety information is also collected through ongoing clinical trials and registries, including TYGRIS and the pregnancy registry, making this the largest long-term patient follow-up effort undertaken for any MS therapy.
According to data available to the companies as of mid-September 2007, there have been no new reports of confirmed cases of progressive multifocal leukoencephalopathy (PML). The safety data to date continue to support a favorable benefit-risk profile for TYSABRI. The companies plan to continue to provide similar updates at future medical meetings.
As of the end of September 2007:
In the US, approximately 10,500 patients are on TYSABRI commercially and over 2,100 physicians have prescribed the therapy;
In the EU, approximately 5,500 patients are on TYSABRI therapy commercially; and
In global clinical trials, approximately 1,000 patients are on TYSABRI therapy.
TYSABRI is available in the United States through the TOUCH(TM) Prescribing Program. All prescribers, infusion sites and patients receiving TYSABRI are required to enroll in TOUCH. Safety information is also collected through ongoing clinical trials and registries, including TYGRIS and the pregnancy registry, making this the largest long-term patient follow-up effort undertaken for any MS therapy.
According to data available to the companies as of mid-September 2007, there have been no new reports of confirmed cases of progressive multifocal leukoencephalopathy (PML). The safety data to date continue to support a favorable benefit-risk profile for TYSABRI. The companies plan to continue to provide similar updates at future medical meetings.
Antwort auf Beitrag Nr.: 31.943.521 von Cyberhexe am 11.10.07 18:24:03Davy (Elan & Pharma)
Elan Corp (USc)
ELN US
No new confirmed cases of PML as of mid-September; good growth in patient numbers as US take-up accelerates
Ahead of ECTRIMS, Elan has outlined the highlights from its safety presentationsand has provided an update on patient numbers on Tysabri.
As of mid-September there have been no new confirmed cases of PML—this is the most recent of a series of regular updates that will be provided at medical meetings. This finding is positive in that it helps to slowly increase neurologists’confidence in prescribing the drug.
Results from the PLEX study also indicate that plasma exchange may be aneffective tool to accelerate the removal of Tysabri from blood serum. This adds tothe armamentarium of potential options available to doctors as more is learned about PML and related intervention methods.
Meanwhile, it was reported that as of end-September some 16,000 patients were on commercial therapy. This represents an increase of 24% since mid-July and compares well with our own mid-October forecast of 17,000. The net addition rate since mid-July was 282 per week, which compares reasonably well with our 300- 330 projection. 16,000 patients exceeds the 15,000 (annualised) number required for operating profit breakeven.
Regionally, US take-up stands at 10,500; the addition rate accelerated from 143 per week to 173 per week and is back up at the levels seen in the first half of the year. Some 5,500 patients are on commercial therapy in the EU. The net addition rate fell from 157 per week to 109 per week since July, but this was heavily impacted by seasonal factors. Tysabri was launched in France at the end of July.
Pharma and healthcare
Davy Healthcheck: Strong revenue growth, margin expansion expected in Q3 results seasonQ3 results in Irish healthcare should deliver strong year-on-year (yoy) revenue growth and PBIT margin expansion. A FDA ruling on Crohn's Disease and BiogenIdec's Q3 results should ensure that Tysabri will be a key highlight for Elan.
But we will also be looking for any updates on the company’s AD programmes,chiefly bapineuzumab's progress towards Phase III initiation. ICON's financial and share price performance in 2007 has been excellent. We expect it to finish the year in style, with the risk to numbers to the upside. As the BioMerieux deal washes out of yoy comparisons, underlying growth will become more visible for Trinity Biotech in Q3. Amarin’s focus is on re-designing its pipeline. Several Phase II studies should be initiated over the course of 2007/2008 for neurological and cardiovascular
candidates.
It has been a good quarter in absolute terms for CROs (upgrades), diagnostics (M&A) and US biotech (product newsflow; Elan has participated). In general,however, the outperforming sectors year-to-date remain service-related rather than product-related. It still pays to be selective on the latter.
For further detail, see the latest issue of Davy Healthcheck , published October 11th.
http://www.investorvillage.com/smbd.asp?mb=160&mn=158923&pt=…
Elan Corp (USc)
ELN US
No new confirmed cases of PML as of mid-September; good growth in patient numbers as US take-up accelerates
Ahead of ECTRIMS, Elan has outlined the highlights from its safety presentationsand has provided an update on patient numbers on Tysabri.
As of mid-September there have been no new confirmed cases of PML—this is the most recent of a series of regular updates that will be provided at medical meetings. This finding is positive in that it helps to slowly increase neurologists’confidence in prescribing the drug.
Results from the PLEX study also indicate that plasma exchange may be aneffective tool to accelerate the removal of Tysabri from blood serum. This adds tothe armamentarium of potential options available to doctors as more is learned about PML and related intervention methods.
Meanwhile, it was reported that as of end-September some 16,000 patients were on commercial therapy. This represents an increase of 24% since mid-July and compares well with our own mid-October forecast of 17,000. The net addition rate since mid-July was 282 per week, which compares reasonably well with our 300- 330 projection. 16,000 patients exceeds the 15,000 (annualised) number required for operating profit breakeven.
Regionally, US take-up stands at 10,500; the addition rate accelerated from 143 per week to 173 per week and is back up at the levels seen in the first half of the year. Some 5,500 patients are on commercial therapy in the EU. The net addition rate fell from 157 per week to 109 per week since July, but this was heavily impacted by seasonal factors. Tysabri was launched in France at the end of July.
Pharma and healthcare
Davy Healthcheck: Strong revenue growth, margin expansion expected in Q3 results seasonQ3 results in Irish healthcare should deliver strong year-on-year (yoy) revenue growth and PBIT margin expansion. A FDA ruling on Crohn's Disease and BiogenIdec's Q3 results should ensure that Tysabri will be a key highlight for Elan.
But we will also be looking for any updates on the company’s AD programmes,chiefly bapineuzumab's progress towards Phase III initiation. ICON's financial and share price performance in 2007 has been excellent. We expect it to finish the year in style, with the risk to numbers to the upside. As the BioMerieux deal washes out of yoy comparisons, underlying growth will become more visible for Trinity Biotech in Q3. Amarin’s focus is on re-designing its pipeline. Several Phase II studies should be initiated over the course of 2007/2008 for neurological and cardiovascular
candidates.
It has been a good quarter in absolute terms for CROs (upgrades), diagnostics (M&A) and US biotech (product newsflow; Elan has participated). In general,however, the outperforming sectors year-to-date remain service-related rather than product-related. It still pays to be selective on the latter.
For further detail, see the latest issue of Davy Healthcheck , published October 11th.
http://www.investorvillage.com/smbd.asp?mb=160&mn=158923&pt=…
Antwort auf Beitrag Nr.: 31.951.159 von bernie55 am 12.10.07 10:15:24...dies liest sich doch gut:
Elan has a 50 percent interest with Biogen in Tysabri, a multiple sclerosis drug that is rebounding after being pulled from the market in 2005 due to safety concerns, and analysts said the potential sale of Biogen put Elan in a strong position.
Shares in Elan were trading 8.9 percent higher at 17.20 euros by 0825 GMT, compared with a 0.5 percent gain on the wider Irish market (.ISEQ: Quote, Profile, Research).
Biogen, one of the world's largest biotechnology companies, best known for its flagship multiple sclerosis drug Avonex, said on Friday it had put itself up for sale.
Elan said that if Biogen was sold, it had various rights, including a right to acquire Biogen's interest for "fair value." It also has the right to sell its Tysabri interest, continue the pact or restructure it, it said.
"If Biogen's evaluation process results in a change of control, Elan will evaluate the forgoing options in the best interest of its shareholders," it said.
http://www.reuters.com/article/marketsNews/idUKL152854812007…
Elan has a 50 percent interest with Biogen in Tysabri, a multiple sclerosis drug that is rebounding after being pulled from the market in 2005 due to safety concerns, and analysts said the potential sale of Biogen put Elan in a strong position.
Shares in Elan were trading 8.9 percent higher at 17.20 euros by 0825 GMT, compared with a 0.5 percent gain on the wider Irish market (.ISEQ: Quote, Profile, Research).
Biogen, one of the world's largest biotechnology companies, best known for its flagship multiple sclerosis drug Avonex, said on Friday it had put itself up for sale.
Elan said that if Biogen was sold, it had various rights, including a right to acquire Biogen's interest for "fair value." It also has the right to sell its Tysabri interest, continue the pact or restructure it, it said.
"If Biogen's evaluation process results in a change of control, Elan will evaluate the forgoing options in the best interest of its shareholders," it said.
http://www.reuters.com/article/marketsNews/idUKL152854812007…
Antwort auf Beitrag Nr.: 31.996.151 von Cyberhexe am 15.10.07 12:07:05Elan and Biogen Idec Announce That FDA Will Extend Regulatory Review Period for TYSABRI(R) for Crohn's Disease
http://www.elan.com/News/full.asp?ID=1062675
...von den Daten her scheint es keine zusätzlichen Input zu benötigen, da die FDA andernfalls einen "Approvable Letter" verschickt hätte.
Es schaut eher danach aus, dass die FDA noch ein weiteres "PML-freies" Quartal abwarten möchte, bevor sie ihre endgültige Zustimmung erteilt.
http://www.elan.com/News/full.asp?ID=1062675
...von den Daten her scheint es keine zusätzlichen Input zu benötigen, da die FDA andernfalls einen "Approvable Letter" verschickt hätte.
Es schaut eher danach aus, dass die FDA noch ein weiteres "PML-freies" Quartal abwarten möchte, bevor sie ihre endgültige Zustimmung erteilt.
Antwort auf Beitrag Nr.: 31.996.883 von Cyberhexe am 15.10.07 12:47:51jetzt rückt die vollmundige aussage von biib-ceo mullen vor einigen wochen ende 2010 100.000 tysabri patienten zu bedienen, für mich in ein anderes licht: im vorfeld zu den übernahmeverhandlungen wird er wohl die braut hübsch gemacht haben wollen.
jeder weiß, dass 100.000 patienten für die nächsten 3 jahre bedeutet: 2008: + 20.000 2009:+ 30.000 2010: +30.000 - zu wünschen wäre dies natürlich - aber aufgrund des allen bekannten procederes und des verdammt hohen preises von ty ein m.e. sehr sportliches ziel!
was bedeutet dies nun für elan?
fall 1.) die beiden behalten ihr jetztiges agreement 50:50 => es ändert sich nichts
fall 2.) elan will die tysabri-rechte kaufen: m.e. negativ für elan, da mullen wohl den potenziellen 2010er umsatz für tysabri wird haben wollen - und da ist die ernte noch nicht eingefahren ...
ich habe mich deshalb vorhin im hype entschlossen, mal ein paar abzudrücken ...
meinungen gerne erwünscht
jeder weiß, dass 100.000 patienten für die nächsten 3 jahre bedeutet: 2008: + 20.000 2009:+ 30.000 2010: +30.000 - zu wünschen wäre dies natürlich - aber aufgrund des allen bekannten procederes und des verdammt hohen preises von ty ein m.e. sehr sportliches ziel!
was bedeutet dies nun für elan?
fall 1.) die beiden behalten ihr jetztiges agreement 50:50 => es ändert sich nichts
fall 2.) elan will die tysabri-rechte kaufen: m.e. negativ für elan, da mullen wohl den potenziellen 2010er umsatz für tysabri wird haben wollen - und da ist die ernte noch nicht eingefahren ...
ich habe mich deshalb vorhin im hype entschlossen, mal ein paar abzudrücken ...
meinungen gerne erwünscht
Antwort auf Beitrag Nr.: 32.003.666 von tippse am 15.10.07 17:43:30....es gibt noch mehere andere Möglichkeiten;eine davon wäre Ty an den Übernehmer von BIIB teuer zu verkaufen,Schulden abzubauen und eine Entwicklungsfirma,spezialisiert auf ALZ (neben Nano etc)zu werden....
my shares are not for sale-----bei 100$ lässt sich drüber reden...
my shares are not for sale-----bei 100$ lässt sich drüber reden...

Antwort auf Beitrag Nr.: 31.996.151 von Cyberhexe am 15.10.07 12:07:05...dies liest sich doch gut:
....BM in deinem Postfach...
....BM in deinem Postfach...
irischer Analystenkommentar von Heute:
Davy Stockbrokers: Starting Points
Abbott's Q3 results issued yesterday (Oct 17th) indicated continued sales growth for its cholesterol therapy, TriCor, with sales up 13% on the same period last year to $300m. Abbott management expects full-year TriCor sales growth of 15-20%.
This is of interest to Elan as it receives a circa 5% royalty on TriCor which uses Elan's NanoCrystal delivery technology. This product accounted for 19% of drug technology revenues at Elan in 2006 (drug technology accounted for 44% of Elan's 2006 total revenues) and 20% in the first half of 2007.
Separately, Wyeth announces Q3 results later today. The main interest from Elan's point of view will be any update on AAB-001 (Bapineuzumab), the Alzheimer’s Disease candidate which the two companies are developing together. Any update on this front is likely to be on the Phase III protocol as more fundamental newsflow is not expected until mid-2008.
http://www.rte.ie/business/2007/morningrep/download/1018davy…
Davy Stockbrokers: Starting Points
Abbott's Q3 results issued yesterday (Oct 17th) indicated continued sales growth for its cholesterol therapy, TriCor, with sales up 13% on the same period last year to $300m. Abbott management expects full-year TriCor sales growth of 15-20%.
This is of interest to Elan as it receives a circa 5% royalty on TriCor which uses Elan's NanoCrystal delivery technology. This product accounted for 19% of drug technology revenues at Elan in 2006 (drug technology accounted for 44% of Elan's 2006 total revenues) and 20% in the first half of 2007.
Separately, Wyeth announces Q3 results later today. The main interest from Elan's point of view will be any update on AAB-001 (Bapineuzumab), the Alzheimer’s Disease candidate which the two companies are developing together. Any update on this front is likely to be on the Phase III protocol as more fundamental newsflow is not expected until mid-2008.
http://www.rte.ie/business/2007/morningrep/download/1018davy…
Ian Hunter von Goodbody Stockbrokers vom 18.10.07:
http://www.rte.ie/business/2007/morningrep/download/1018good…
Elan (Add, Closing Price $22.96); Q307 preview - putting numbers on Tysabri.
Analyst: Ian Hunter T +353-1-6410498 E ian.g.hunter@goodbody.ie
Elan is due to report its Q307 results next Thursday, 25 October. We forecast that the company will report a fully diluted loss per share of $0.17, from revenue of $180.9m. On the commercial side, all eyes will be focussed on the progress of Tysabri. Patient numbers are already known (16,000 commercial patients by the end of September). The unknown is how that has translated into revenue. We had been anticipating 16,900 patients on the drug, giving total worldwide sales of $106.8m in the quarter. Given stated numbers, actual revenue will probably come in light. The overall trend, however, is encouraging, particularly when coupled with the lack of PML in an ever increasing patient population. For the rest of the product mix, we have pencilled in $10.0m for Maxipime, down from $35.6m the previous quarter on generic competition. We have also factored in a dip in Azactam sales (down to $17.3m in Q307 from $20.9m in Q207) on generic pressure. We are expecting the contract manufacturing and royalties side of the business to contribute $76.6m.
On the pipeline, the main focus remains the development of AAB-001 for the treatment of Alzheimer’s Disease. After the excitement generated in May from the announcement that the drug would be moving into Phase III trials one year ahead of schedule, and an interim review of an on-going Phase II trial, all has gone quiet. Elan’s partner in the development, Wyeth, reports today, which should give us some feedback on progress. We do not expect anything startling, as the Phase II trial does not finish until early 2008, with data expected in April/May 2008.
http://www.rte.ie/business/2007/morningrep/download/1018good…
Elan (Add, Closing Price $22.96); Q307 preview - putting numbers on Tysabri.
Analyst: Ian Hunter T +353-1-6410498 E ian.g.hunter@goodbody.ie
Elan is due to report its Q307 results next Thursday, 25 October. We forecast that the company will report a fully diluted loss per share of $0.17, from revenue of $180.9m. On the commercial side, all eyes will be focussed on the progress of Tysabri. Patient numbers are already known (16,000 commercial patients by the end of September). The unknown is how that has translated into revenue. We had been anticipating 16,900 patients on the drug, giving total worldwide sales of $106.8m in the quarter. Given stated numbers, actual revenue will probably come in light. The overall trend, however, is encouraging, particularly when coupled with the lack of PML in an ever increasing patient population. For the rest of the product mix, we have pencilled in $10.0m for Maxipime, down from $35.6m the previous quarter on generic competition. We have also factored in a dip in Azactam sales (down to $17.3m in Q307 from $20.9m in Q207) on generic pressure. We are expecting the contract manufacturing and royalties side of the business to contribute $76.6m.
On the pipeline, the main focus remains the development of AAB-001 for the treatment of Alzheimer’s Disease. After the excitement generated in May from the announcement that the drug would be moving into Phase III trials one year ahead of schedule, and an interim review of an on-going Phase II trial, all has gone quiet. Elan’s partner in the development, Wyeth, reports today, which should give us some feedback on progress. We do not expect anything startling, as the Phase II trial does not finish until early 2008, with data expected in April/May 2008.
http://www.drugpatentwatch.com/premium/preview/detail/index.…
...der Patentschutz für das nanoformulierte Tricor läuft bis 2018!
...der Patentschutz für das nanoformulierte Tricor läuft bis 2018!
...ein bemerkenswertes Umfrageergebnis, vor allem vor dem Hintergrund, dass in den US ca. 500.000 Menschen von CD betroffen sind!
http://www.wrongdiagnosis.com/c/crohns_disease/prevalence.ht…
...approx 1 in 544 or 0.18% or 500,000 people in USA
LOS ANGELES, Sept 6 (Anian) - U.S. gastroenterologists, despite safety concerns,
plan to use Biogen-Idec Inc.'s Tysabri for treating Crohn's disease
once U.S. regulators approve the multiple sclerosis drug for patients with the
bowel disorder, according to a recent survey.
An August survey of 97 U.S. gastroenterologists conducted on behalf of Anian, a
Reuters business that tracks industry trends for institutional investors, found
that respondents planned to use Tysabri in 14 percent of patients within 3
months of the drug's availability.
This contrasts with multiple sclerosis where roughly 1 percent of eligible
patients were prescribed Tysabri in the 6-month period following its U.S. market
reintroduction last year, according to an Anian research report.
Tysabri, developed by Irish drugmaker Elan, was taken off the
market in 2005 after being linked with three cases of progressive multifocal
leukoencephalopathy, a rare and potentially deadly brain infection. No further
cases have emerged and the drug was reintroduced a year ago through a program
under which patients are closely monitored for any signs or symptoms of PML.
Biogen-Idec and Elan are also seeking approval to market Tysabri as a treatment
for moderate to severe Crohn's disease and an advisory panel to the U.S. Food
and Drug Administration last month said the drug should be approved for the new
indication.
The FDA is slated to make a final decision on the companies' application in
mid-October.
The surveyed gastroenterologists, who were paid for their anonymous
participation, estimated that 23 percent of their Crohn's patients are good
candidates for Tysabri.
They described a good candidate as one who is resistant to anti-TNF inhibitors,
mercaptopurine, azathioprine, and steroids, and is not able to tolerate surgery.
Most do not plan to use Tysabri with any other drugs, although 20 percent said
they might use it with steroids.
Crohn's disease is a bowel inflammation that causes potentially disabling
abdominal pain, weight loss, fever and rectal bleeding.
If FDA approval occurs in October, nearly half of U.S. Tysabri sales could be
for Crohn's disease by 2009. However, like neurologists, gastroenterologists
will stop prescribing Tysabri if PML reemerges, said Bennett Weintraub, a
principal at Anian.
In the absence of new PML cases, he estimated that U.S. revenue from Tysabri for
Crohn's disease could exceed $1 billion by 2010, but if even a few new PML cases
occur, Tysabri use in Crohn's would reach just $460 million in 2010.
http://www.wrongdiagnosis.com/c/crohns_disease/prevalence.ht…
...approx 1 in 544 or 0.18% or 500,000 people in USA
LOS ANGELES, Sept 6 (Anian) - U.S. gastroenterologists, despite safety concerns,
plan to use Biogen-Idec Inc.'s Tysabri for treating Crohn's disease
once U.S. regulators approve the multiple sclerosis drug for patients with the
bowel disorder, according to a recent survey.
An August survey of 97 U.S. gastroenterologists conducted on behalf of Anian, a
Reuters business that tracks industry trends for institutional investors, found
that respondents planned to use Tysabri in 14 percent of patients within 3
months of the drug's availability.
This contrasts with multiple sclerosis where roughly 1 percent of eligible
patients were prescribed Tysabri in the 6-month period following its U.S. market
reintroduction last year, according to an Anian research report.
Tysabri, developed by Irish drugmaker Elan, was taken off the
market in 2005 after being linked with three cases of progressive multifocal
leukoencephalopathy, a rare and potentially deadly brain infection. No further
cases have emerged and the drug was reintroduced a year ago through a program
under which patients are closely monitored for any signs or symptoms of PML.
Biogen-Idec and Elan are also seeking approval to market Tysabri as a treatment
for moderate to severe Crohn's disease and an advisory panel to the U.S. Food
and Drug Administration last month said the drug should be approved for the new
indication.
The FDA is slated to make a final decision on the companies' application in
mid-October.
The surveyed gastroenterologists, who were paid for their anonymous
participation, estimated that 23 percent of their Crohn's patients are good
candidates for Tysabri.
They described a good candidate as one who is resistant to anti-TNF inhibitors,
mercaptopurine, azathioprine, and steroids, and is not able to tolerate surgery.
Most do not plan to use Tysabri with any other drugs, although 20 percent said
they might use it with steroids.
Crohn's disease is a bowel inflammation that causes potentially disabling
abdominal pain, weight loss, fever and rectal bleeding.
If FDA approval occurs in October, nearly half of U.S. Tysabri sales could be
for Crohn's disease by 2009. However, like neurologists, gastroenterologists
will stop prescribing Tysabri if PML reemerges, said Bennett Weintraub, a
principal at Anian.
In the absence of new PML cases, he estimated that U.S. revenue from Tysabri for
Crohn's disease could exceed $1 billion by 2010, but if even a few new PML cases
occur, Tysabri use in Crohn's would reach just $460 million in 2010.
...aus der Pressekonferenz von Biogen:
http://seekingalpha.com/article/50984-biogen-idec-q3-2007-ea…
Tysabri, Tysabri und noch einmal...TYSABRI! Biogen scheint Ernst zu machen und hat face-to-face-Studien auf 2008 angekündigt:
We are also moving forward with clinical trials to support the TYSABRI marketing effort. In order to definitively show the efficacy of TYSABRI compared to marketed competitors, we're planning head-to-head switching trials. Additionally, we're concerned that patients may be experiencing delays before moving to the most effective therapy and we are designing trials to potentially address this. We plan to initiate these head-to-head switching trials in early 2008.
...
In the UK, the National Institute for Health and Clinical Excellence has recommended the use of TYSABRI to treat people with highly active relapsing-remitting MS in recognition of its superior cost and efficacy benefits. Reimbursement has been approved on a national level although widespread reimbursement availability will likely start in Q4. TYSABRI is the first treatment for MS to be recommended for use by NICE.
...
Due to this failure of current therapies in CD, therapies such as TYSABRI that have novel biological targets are required. In addition we continue to work on other indications for TYSABRI including exploring potential applications in oncology.
...
We're beginning to see the impact of TYSABRI on the ABCR market in the US, which is now flat to declining on a year-over-year basis.
http://seekingalpha.com/article/50984-biogen-idec-q3-2007-ea…
Tysabri, Tysabri und noch einmal...TYSABRI! Biogen scheint Ernst zu machen und hat face-to-face-Studien auf 2008 angekündigt:
We are also moving forward with clinical trials to support the TYSABRI marketing effort. In order to definitively show the efficacy of TYSABRI compared to marketed competitors, we're planning head-to-head switching trials. Additionally, we're concerned that patients may be experiencing delays before moving to the most effective therapy and we are designing trials to potentially address this. We plan to initiate these head-to-head switching trials in early 2008.
...
In the UK, the National Institute for Health and Clinical Excellence has recommended the use of TYSABRI to treat people with highly active relapsing-remitting MS in recognition of its superior cost and efficacy benefits. Reimbursement has been approved on a national level although widespread reimbursement availability will likely start in Q4. TYSABRI is the first treatment for MS to be recommended for use by NICE.
...
Due to this failure of current therapies in CD, therapies such as TYSABRI that have novel biological targets are required. In addition we continue to work on other indications for TYSABRI including exploring potential applications in oncology.
...
We're beginning to see the impact of TYSABRI on the ABCR market in the US, which is now flat to declining on a year-over-year basis.
we're talking about 50 million people who are gonna call Elan or Wyeth and say, "How do I get in?" I mean it's.... it's big..".
Message #89426
<<For me, this was the highlight of the cc. Thanks to Jive for asking this question!
Cheers to all,
Cynthia ------------------------------------------------------------------
...wie ich finde, hier noch einmal super erklärt, wie komplex die Zusammenhäge von Menge und Dauer von Medikamentation zu betrachten sind...
...und die Jungs von ELAN gehen richtig präzise , gewissenhaft und verantwortungsvoll an die Sache ran....
Jive: What you have mentioned, the ph3 issue for aab, any chance we can file for a BLA at that time?
KM: It would depend on the data... What we're trying to do with AAB-001, just to be clear... is we have an opportunity to have the ph2 be a pivotal trial. At the same time, we have to do a ph3 because the size of ph2 isn't big enough from a safety point of view.
We're trying to understand 2 things in as much specificity as we can-- one is the dose, and one is the length of the dose. Those 2 things are incredibly important for 2 reasons -- one is obvious, one is less obvious... The dose and the length of the dose-- you need to understand the clinical response... How much time do you need at what dose... and, you know, this is an ascending study. So we have different doses at different times, so we have different sets of data, there's different sets of patients that have had different doses for different periods of time.
Why the AN-1792 data is so important, cause that gives you all the clues of what to look for, cause that is an immunotheraputic approach to Alzheimer's... so that is rich with data that we look at all the time, because that gives us clues as far as how to look at.... if we're looking at ph2, having these people look at it, what you're supposed to look at....
So doses are important because when you go for a disease-modifying drug or even a symptomatic drug, you need to understand what are the endpoints that the regulator needs to see in order to give you approval... And the endpoints move at different times for different reasons, and to add to that, you have Alzheimer's patients who are all slightly different...
So dose is important for that reason, and dose is very important for manufacturing-- because there's a gigantic difference between lowest dose and highest dose from a manufacturing point of view-- then --and you have 10 million patients-- so you couldn't build enough biological capacity fast enough if you got the highest dose at the shortest interval, and it's a fantastic drug from an alzheimer's point of view, you're gonna have a hard time meeting capacity fairly shortly.
So, you know, there's no other 2 companies on Earth, Wyeth and Elan, that want to move this forward, but again, we've been working on this from Elan point of view since the late '80s, in research, and Wyeth's a great partner, but getting it right-- it's certainly in our collective, including shareholders' best interest to get it right as precisely as we can.
We will move to ph3 when we have enough confirmatory data to do that and to make reasonable judgements about those 2 things, and we need the ph3 because we need more safety data... So ph2, it may stand on its own with the data, the data will drive it, and if it does we will file... and we will file, but we'll also need a ph3 going, because you'll need more patients from a safety point of view for the FDA... The FDA is not gonna make a decision on 10 million patients based on a 240 patient trial, this is not gonna do it, I don't think they should do it...
So that's were we are.... Immunotherapy works... It's not new information... AN-1792 works-- after 2 doses-- so, we're fine-tuning what we already know, in the public domain... and when we reach the 95th to 100th percentile, in comfort, because of those reasons, then Wyeth and ourselves will proceed... but we could file with ph2 if the data allows us to do that.
Jive: And when will you look at the ph2 data?
KM: Mid-year... and the mid-year correlates to where the doses are with different dose groups... the mid-year is gonna be rich with data... and again, what we know is the immunotheraputic approach to Alzheimer's works... That's not a debate, the debate is what's the dose, and what's the time interval....
And you're making a decision for 10 million patients... and 10 million patients, by the way, have on average 3 caregivers... so when we say we're gonna move forward, we're talking about 50 million people who are gonna call Elan or Wyeth and say, "How do I get in?" I mean it's.... it's big...
And, we understand, from a shareholder point of view it's important... and we're not, in the big scheme of things timeline-wise, you know, we're not that far away from having a lot, all the clarity we need to move forward...
If we get up and stand up here and say, "We're in ph3" people are gonna say, "How'd you get to ph3?"
I wanna be able to say here's how we got here, and here's what we're doing, and here's why, and that credibility is very important to us...
There's lots of companies in ph2 or 3 in Alzheimer's, haven't seen any data, but they're in ph2, ph3... We don't wanna be like that... That's a stock market drug development strategy...>>
http://www.investorvillage.com/smbd.asp?mb=160&pt=msg&mn=163…
Message #89426
<<For me, this was the highlight of the cc. Thanks to Jive for asking this question!
Cheers to all,
Cynthia ------------------------------------------------------------------
...wie ich finde, hier noch einmal super erklärt, wie komplex die Zusammenhäge von Menge und Dauer von Medikamentation zu betrachten sind...
...und die Jungs von ELAN gehen richtig präzise , gewissenhaft und verantwortungsvoll an die Sache ran....
Jive: What you have mentioned, the ph3 issue for aab, any chance we can file for a BLA at that time?
KM: It would depend on the data... What we're trying to do with AAB-001, just to be clear... is we have an opportunity to have the ph2 be a pivotal trial. At the same time, we have to do a ph3 because the size of ph2 isn't big enough from a safety point of view.
We're trying to understand 2 things in as much specificity as we can-- one is the dose, and one is the length of the dose. Those 2 things are incredibly important for 2 reasons -- one is obvious, one is less obvious... The dose and the length of the dose-- you need to understand the clinical response... How much time do you need at what dose... and, you know, this is an ascending study. So we have different doses at different times, so we have different sets of data, there's different sets of patients that have had different doses for different periods of time.
Why the AN-1792 data is so important, cause that gives you all the clues of what to look for, cause that is an immunotheraputic approach to Alzheimer's... so that is rich with data that we look at all the time, because that gives us clues as far as how to look at.... if we're looking at ph2, having these people look at it, what you're supposed to look at....
So doses are important because when you go for a disease-modifying drug or even a symptomatic drug, you need to understand what are the endpoints that the regulator needs to see in order to give you approval... And the endpoints move at different times for different reasons, and to add to that, you have Alzheimer's patients who are all slightly different...
So dose is important for that reason, and dose is very important for manufacturing-- because there's a gigantic difference between lowest dose and highest dose from a manufacturing point of view-- then --and you have 10 million patients-- so you couldn't build enough biological capacity fast enough if you got the highest dose at the shortest interval, and it's a fantastic drug from an alzheimer's point of view, you're gonna have a hard time meeting capacity fairly shortly.
So, you know, there's no other 2 companies on Earth, Wyeth and Elan, that want to move this forward, but again, we've been working on this from Elan point of view since the late '80s, in research, and Wyeth's a great partner, but getting it right-- it's certainly in our collective, including shareholders' best interest to get it right as precisely as we can.
We will move to ph3 when we have enough confirmatory data to do that and to make reasonable judgements about those 2 things, and we need the ph3 because we need more safety data... So ph2, it may stand on its own with the data, the data will drive it, and if it does we will file... and we will file, but we'll also need a ph3 going, because you'll need more patients from a safety point of view for the FDA... The FDA is not gonna make a decision on 10 million patients based on a 240 patient trial, this is not gonna do it, I don't think they should do it...
So that's were we are.... Immunotherapy works... It's not new information... AN-1792 works-- after 2 doses-- so, we're fine-tuning what we already know, in the public domain... and when we reach the 95th to 100th percentile, in comfort, because of those reasons, then Wyeth and ourselves will proceed... but we could file with ph2 if the data allows us to do that.
Jive: And when will you look at the ph2 data?
KM: Mid-year... and the mid-year correlates to where the doses are with different dose groups... the mid-year is gonna be rich with data... and again, what we know is the immunotheraputic approach to Alzheimer's works... That's not a debate, the debate is what's the dose, and what's the time interval....
And you're making a decision for 10 million patients... and 10 million patients, by the way, have on average 3 caregivers... so when we say we're gonna move forward, we're talking about 50 million people who are gonna call Elan or Wyeth and say, "How do I get in?" I mean it's.... it's big...
And, we understand, from a shareholder point of view it's important... and we're not, in the big scheme of things timeline-wise, you know, we're not that far away from having a lot, all the clarity we need to move forward...
If we get up and stand up here and say, "We're in ph3" people are gonna say, "How'd you get to ph3?"
I wanna be able to say here's how we got here, and here's what we're doing, and here's why, and that credibility is very important to us...
There's lots of companies in ph2 or 3 in Alzheimer's, haven't seen any data, but they're in ph2, ph3... We don't wanna be like that... That's a stock market drug development strategy...>>
http://www.investorvillage.com/smbd.asp?mb=160&pt=msg&mn=163…
AUDIO
- Elan Corporation, plc Earnings Conference Call (Q3 2007)
Scheduled to start Thu, Oct 25, 2007, 8:30 am Eastern
http://biz.yahoo.com/cc/8/85778.html
- Elan Corporation, plc Earnings Conference Call (Q3 2007)
Scheduled to start Thu, Oct 25, 2007, 8:30 am Eastern
http://biz.yahoo.com/cc/8/85778.html
Hi Cybie, was hat es mit dem - vasogenic-oedema - bei den Alzheimer Patienten auf sich ????
Diese Vorfälle treten ja, so wie ich es verstanden habe, in Kombination mit dem " ApoE gene " gehäufter auf......
Kann sich dieses als " largely asymptomatic " und " tansient MRI finding " ???? bezeichnete Phänomen negativ auf eine erfolgreiche ZULA von AAB01 auswirken ???
NCB
The FDA in the US and the CHMP in Europe have agreed that Elan/Wyeth can proceed to Phase III studies with AAB-001. The FDA has agreed to a proposed Phase III trial design (after reviewing data from AN1792, the Phase I data and the interim data from the Phase II trial) and the CHMP has agreed to AAB-001 proceeding to Phase III with minor adjustments. There will be 4 separate pivotal placebo-controlled studies, with a total of c.4,000 patients involving 2 populations (ApoE carriers and non-ApoE carrierssee below*) of Alzheimer’s Disease patients with mild to moderate disease. The studies (2 global pivotal US and European studies in ApoE carriers and 2 global pivotal US and
European studies in non-ApoE carriers) will be of 18-month duration and each of the Phase III studies are pivotal stand alone studies.
• In an analysis of the clinical data with AAB-001 vasogenic oedema was observed (this is fluid accumulation in the brain which was noted as “largely asymptomatic” and as a transient MRI finding). While the incidence of vasogenic oedema in the total treated AAB-001 population was low, it was relatively higher in AD patients that carried the ApoE gene. In the Phase III studies, patients carrying the ApoE gene will receive a single low dose (concentration) of AAB-001 (to minimize the oedema risk) and non- ApoE carriers will receive the same dose and multiple higher doses. The doses of AAB- 001 that were used in the Phase II study will also be used in the Phase III studies.
• The decision to initiate the Phase III AAB-001 studies in the US and Europe in parallel (considering study costs, side effect risk etc) underlies Elan/Wyeth’s confidence in the data that has been generated with AAB-001 to date. Although still possible, given the complexity of the Phase III studies and the lack of specifics at this point on potential interim analyses of the Phase III data, it is less apparent if Elan/Wyeth will file for marketing approval of AAB-001 in the US based on the Phase II efficacy and safety data from a Phase III study. The Phase III studies are on track to enroll the first patients in the US by the end of the year. Further details on interim looks etc are expected to be available when the study protocols have been communicated to investigators.
Considering the design of the Phase II study it is likely that interim analyses will be incorporated into the Phase III protocols. The Phase II AAB-001 data will be released in
mid-2008. It is expected that the headline Phase II data will be released initially and that the entire dataset will then be released at a scientific conference.
• Elsewhere, Elan noted yesterday that they have started to see some acceleration in Tysabri run rate in the US with an uptick noted in Europe post the holiday season. We have revised our forecasts based on the geographic mix of the patient no’s/revenues reported in Q3 and a higher discount to pharmacies/wholesalers in US than previously forecast. Our number of patients on treatment by year end is now 20,300 compared to 20,550 previously. Our revised 2007 in-market sales forecast is $339m compared to our previous forecast of $355m.
• * The presence of the ApoE(4) gene is a risk factor for Alzheimer’s Disease. Approximately 60% of the Alzheimer's Disease patient population are non-carriers with the remainder of the population ApoE carriers
http://www.investorvillage.com/smbd.asp?mb=160&mn=165241&pt=…
Diese Vorfälle treten ja, so wie ich es verstanden habe, in Kombination mit dem " ApoE gene " gehäufter auf......
Kann sich dieses als " largely asymptomatic " und " tansient MRI finding " ???? bezeichnete Phänomen negativ auf eine erfolgreiche ZULA von AAB01 auswirken ???
NCB
The FDA in the US and the CHMP in Europe have agreed that Elan/Wyeth can proceed to Phase III studies with AAB-001. The FDA has agreed to a proposed Phase III trial design (after reviewing data from AN1792, the Phase I data and the interim data from the Phase II trial) and the CHMP has agreed to AAB-001 proceeding to Phase III with minor adjustments. There will be 4 separate pivotal placebo-controlled studies, with a total of c.4,000 patients involving 2 populations (ApoE carriers and non-ApoE carrierssee below*) of Alzheimer’s Disease patients with mild to moderate disease. The studies (2 global pivotal US and European studies in ApoE carriers and 2 global pivotal US and
European studies in non-ApoE carriers) will be of 18-month duration and each of the Phase III studies are pivotal stand alone studies.
• In an analysis of the clinical data with AAB-001 vasogenic oedema was observed (this is fluid accumulation in the brain which was noted as “largely asymptomatic” and as a transient MRI finding). While the incidence of vasogenic oedema in the total treated AAB-001 population was low, it was relatively higher in AD patients that carried the ApoE gene. In the Phase III studies, patients carrying the ApoE gene will receive a single low dose (concentration) of AAB-001 (to minimize the oedema risk) and non- ApoE carriers will receive the same dose and multiple higher doses. The doses of AAB- 001 that were used in the Phase II study will also be used in the Phase III studies.
• The decision to initiate the Phase III AAB-001 studies in the US and Europe in parallel (considering study costs, side effect risk etc) underlies Elan/Wyeth’s confidence in the data that has been generated with AAB-001 to date. Although still possible, given the complexity of the Phase III studies and the lack of specifics at this point on potential interim analyses of the Phase III data, it is less apparent if Elan/Wyeth will file for marketing approval of AAB-001 in the US based on the Phase II efficacy and safety data from a Phase III study. The Phase III studies are on track to enroll the first patients in the US by the end of the year. Further details on interim looks etc are expected to be available when the study protocols have been communicated to investigators.
Considering the design of the Phase II study it is likely that interim analyses will be incorporated into the Phase III protocols. The Phase II AAB-001 data will be released in
mid-2008. It is expected that the headline Phase II data will be released initially and that the entire dataset will then be released at a scientific conference.
• Elsewhere, Elan noted yesterday that they have started to see some acceleration in Tysabri run rate in the US with an uptick noted in Europe post the holiday season. We have revised our forecasts based on the geographic mix of the patient no’s/revenues reported in Q3 and a higher discount to pharmacies/wholesalers in US than previously forecast. Our number of patients on treatment by year end is now 20,300 compared to 20,550 previously. Our revised 2007 in-market sales forecast is $339m compared to our previous forecast of $355m.
• * The presence of the ApoE(4) gene is a risk factor for Alzheimer’s Disease. Approximately 60% of the Alzheimer's Disease patient population are non-carriers with the remainder of the population ApoE carriers
http://www.investorvillage.com/smbd.asp?mb=160&mn=165241&pt=…
Für diejenigen die sich für meine Tipps interessieren, habe jetzt einen eigenen Thread 
http://www.wallstreet-online.de/community/thread/1134690-1.h…

http://www.wallstreet-online.de/community/thread/1134690-1.h…
...und wieder gibts ganz aktuell neue "elanvolle" Patente:
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=H…
Elan's Vortrag von der European Laarge CAp Pharmaceutical Conference
http://www.csfb.com/conferences/pharma2007/pdf/elan.pdf
Elan's Vortrag von der European Laarge CAp Pharmaceutical Conference
http://www.csfb.com/conferences/pharma2007/pdf/elan.pdf
Elan's Vortrag von der European Laarge CAp Pharmaceutical Conference
Antwort auf Beitrag Nr.: 32.355.931 von bernie55 am 09.11.07 08:26:30
CREDIT SUISSE 7th ANNUAL EUROPEAN LARGE CAP PHARMACEUTICAL CONFERENCE
ELAN VORTRAG vom 6 November 2007
http://www.csfb.com/conferences/pharma2007/pdf/elan.pdf

CREDIT SUISSE 7th ANNUAL EUROPEAN LARGE CAP PHARMACEUTICAL CONFERENCE
ELAN VORTRAG vom 6 November 2007
http://www.csfb.com/conferences/pharma2007/pdf/elan.pdf
..auf Seite 26 des Vortrages - Memory Improvement in Patients - zeigen die Werte der 0,5 und 1,5 Dosen bei der Verabreichung von Bapineuzumab " positive Veränderungen "....
...bei der 5 mg Dosis zeigen sich keine " positive Veränderungen "...
@ Cyberhexe
Hast du eine ERKLÄRUNG dafür ????
...bei der 5 mg Dosis zeigen sich keine " positive Veränderungen "...
@ Cyberhexe
Hast du eine ERKLÄRUNG dafür ????
Antwort auf Beitrag Nr.: 32.359.031 von bernie55 am 09.11.07 11:53:22...der untersuchte Parameter ist nicht Plaquebildung bzw. Verhinderung derselben, sondern „Clinical worsening“ bezgl. Memory.
Eine Erklärung wäre, dass ein Zuviel an MABs eine stärkere Immunresponse auslöst, und wenn diese aus neutralisierenden Antikörpern besteht, können die therapeutischen AK nicht an den Rezeptor gelangen, sondern werden vorher von den Anti-Antikörpern abgefangen und neutralisiert. Aber diese Beurteilung aus der Ferne ist lediglich eine "Schreibtischerklärung" und kann selbstverständlich auch andere Ursachen haben...
Eine Erklärung wäre, dass ein Zuviel an MABs eine stärkere Immunresponse auslöst, und wenn diese aus neutralisierenden Antikörpern besteht, können die therapeutischen AK nicht an den Rezeptor gelangen, sondern werden vorher von den Anti-Antikörpern abgefangen und neutralisiert. Aber diese Beurteilung aus der Ferne ist lediglich eine "Schreibtischerklärung" und kann selbstverständlich auch andere Ursachen haben...
Antwort auf Beitrag Nr.: 32.394.012 von Cyberhexe am 12.11.07 15:30:21....ist lediglich eine "Schreibtischerklärung...
.. , die wir ELANITES und ELANIACS alle sehr schätzen....
.. , die wir ELANITES und ELANIACS alle sehr schätzen....

Crohn's Disease Drug Market Will More Than Double to $4 Billion by 2016, with Near-Term Growth Driven by Remicade and Humira
Press Release Source: Decision Resources
Tuesday November 13, 8:00 am ET
Market Growth Will be Driven by Launches of Several Therapies From 2011 to 2016, According to a New Report from Decision Resources
WALTHAM, Mass., Nov. 13 /PRNewswire/ -- Decision Resources, one of the world's leading research and advisory firms for pharmaceutical and healthcare issues, finds that the Crohn's disease drug market will more than double, from $1.5 billion in 2006 to $4 billion in 2016, in the United States, France, Germany, Italy, Spain, the United Kingdom and Japan.
The new Pharmacor report entitled Crohn's Disease finds that market growth through 2011 will be driven by the continued use of Centocor/Schering- Plough/Mitsubishi Tanabe's biologic agent Remicade, as well as by robust uptake of Abbott/Eisai's TNF-alpha inhibitor Humira. From 2011 through 2016, growth will be sustained by Humira and the launches of UCB/Nektar's Cimzia, Centocor/Janssen-Cilag's ustekinumab, Abbott's ABT-874, and Bristol-Myers Squibb's Orencia.
The report also finds that, although prescriptions for TNF-alphas will increase, oral aminosalicylates will remain the most widely prescribed agents across all major markets, owing to their advantageous safety profile. However, the 5-ASA class of aminosalicylates will see a steady decline in patient share as physicians begin to move away from these agents and use more aggressive biologics and conventional immunosuppressant therapies.
"The development of effective therapies for Crohn's disease has been hindered by a poor understanding of the etiology of the disease," said Kathryn Benton, analyst at Decision Resources. "A significant unmet need continues to exist for novel agents that can be applied early in the treatment of the disease to prevent surgery and hospitalization and to maintain remission."
About Crohn's Disease
Over the past decade, Remicade has significantly expanded the Crohn's disease market. Additionally, robust expansion of this market through 2016 will be driven by the rapid uptake of Humira and novel biologics currently in late-stage clinical development, as well as by more aggressive medical practices. Despite high unmet need and significant market growth expected over the next decade, barriers to entry remain high, as evidenced by recent failures and regulatory setbacks experienced by several drugs.
About Decision Resources
Decision Resources (www.decisionresources.com) is a world leader in market research publications, advisory services, and consulting designed to help clients shape strategy, allocate resources, and master their chosen markets.
All company, brand, or product names contained in this document may be trademarks or registered trademarks of their respective holders.
For more information, contact:
Elizabeth Marshall
Decision Resources, Inc.
781-296-2563
emarshall@dresources.com
http://www.investorvillage.com/smbd.asp?mb=160&mn=169443&pt=…
Press Release Source: Decision Resources
Tuesday November 13, 8:00 am ET
Market Growth Will be Driven by Launches of Several Therapies From 2011 to 2016, According to a New Report from Decision Resources
WALTHAM, Mass., Nov. 13 /PRNewswire/ -- Decision Resources, one of the world's leading research and advisory firms for pharmaceutical and healthcare issues, finds that the Crohn's disease drug market will more than double, from $1.5 billion in 2006 to $4 billion in 2016, in the United States, France, Germany, Italy, Spain, the United Kingdom and Japan.
The new Pharmacor report entitled Crohn's Disease finds that market growth through 2011 will be driven by the continued use of Centocor/Schering- Plough/Mitsubishi Tanabe's biologic agent Remicade, as well as by robust uptake of Abbott/Eisai's TNF-alpha inhibitor Humira. From 2011 through 2016, growth will be sustained by Humira and the launches of UCB/Nektar's Cimzia, Centocor/Janssen-Cilag's ustekinumab, Abbott's ABT-874, and Bristol-Myers Squibb's Orencia.
The report also finds that, although prescriptions for TNF-alphas will increase, oral aminosalicylates will remain the most widely prescribed agents across all major markets, owing to their advantageous safety profile. However, the 5-ASA class of aminosalicylates will see a steady decline in patient share as physicians begin to move away from these agents and use more aggressive biologics and conventional immunosuppressant therapies.
"The development of effective therapies for Crohn's disease has been hindered by a poor understanding of the etiology of the disease," said Kathryn Benton, analyst at Decision Resources. "A significant unmet need continues to exist for novel agents that can be applied early in the treatment of the disease to prevent surgery and hospitalization and to maintain remission."
About Crohn's Disease
Over the past decade, Remicade has significantly expanded the Crohn's disease market. Additionally, robust expansion of this market through 2016 will be driven by the rapid uptake of Humira and novel biologics currently in late-stage clinical development, as well as by more aggressive medical practices. Despite high unmet need and significant market growth expected over the next decade, barriers to entry remain high, as evidenced by recent failures and regulatory setbacks experienced by several drugs.
About Decision Resources
Decision Resources (www.decisionresources.com) is a world leader in market research publications, advisory services, and consulting designed to help clients shape strategy, allocate resources, and master their chosen markets.
All company, brand, or product names contained in this document may be trademarks or registered trademarks of their respective holders.
For more information, contact:
Elizabeth Marshall
Decision Resources, Inc.
781-296-2563
emarshall@dresources.com
http://www.investorvillage.com/smbd.asp?mb=160&mn=169443&pt=…
Antwort auf Beitrag Nr.: 32.450.217 von Cyberhexe am 16.11.07 12:24:35...und dieses Patent dürfte zukünftig eine Perle sein im Geschaäft von EDT (ElanDrugTechnology):
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
Nanoparticulate fibrate formulations
Abstract
The present invention is directed to fibrate compositions having improved pharmacokinetic profiles and reduced fed/fasted variability. The fibrate particles of the composition have an effective average particle size of less than about 2000 nm.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
Nanoparticulate fibrate formulations
Abstract
The present invention is directed to fibrate compositions having improved pharmacokinetic profiles and reduced fed/fasted variability. The fibrate particles of the composition have an effective average particle size of less than about 2000 nm.
EU Crohn's
CHMP Adopts Negative Opinion on Appeal on European Application For natalizumab for the Treatment of Crohn's Disease
--------------------------------------------------------------------------------
Fri Nov 16 09:58:00 2007 EST
DUBLIN, IRELAND & CAMBRIDGE, MASS., Nov 16, 2007 (Canada NewsWire Elan Corporation, plc (NYSE: ELN) and Biogen Idec (NASDAQ: BIIB) today announced
that the Committee for Medicinal Products for Human Use (CHMP), the scientific committee
of the European Medicines Agency (EMEA), has adopted a negative opinion on the marketing
authorization for natalizumab as a treatment for Crohn's disease. This decision
is on the appeal the companies filed following a previous negative opinion adopted
by the CHMP earlier in 2007.
This negative opinion is now referred to the European Commission (EC), which
grants marketing authorizations in the EU. Final EC decisions customarily follow
the CHMP's recommendation, and the companies expect to hear from the EC during the
first quarter of 2008.
About Crohn's Disease
Approximately one million people worldwide have Crohn's disease, a chronic
and progressive inflammatory disease of the gastrointestinal tract, which commonly
affects both men and women.
The disease usually causes diarrhea and crampy abdominal pain, often associated
with fever, and at times rectal bleeding. Loss of appetite and weight loss also
may occur. Complications include narrowing of the intestine, obstruction, abscesses,
and fistulas (abnormal channels connecting the intestine and other organs, including
the skin), and malnutrition. Most patients eventually require surgery, which has
both risks and potential short- and long-term complications.
Crohn's disease can have a devastating impact on the lifestyle of patients,
many of whom are young and active. Currently there is no medical or surgical cure
for Crohn's disease. Many patients fail to respond to current therapies, including
biological therapies such as agents that inhibit tumor necrosis factor alpha (TNF-alpha).
Due to this failure of current therapies in CD, therapies that have novel biological
targets are required.
About TYSABRI(R) (natalizumab)
TYSABRI is a treatment approved for relapsing forms of MS in the United States
and relapsing-remitting MS in the European Union. According to data that have been
published in the New England Journal of Medicine, after two years, TYSABRI treatment
led to a 68% relative reduction (p less than0.001) in the annualized relapse rate
compared to placebo and reduced the relative risk of disability progression by 42-54%
(p less than0.001).
TYSABRI increases the risk of progressive multifocal leukoencephalopathy
(PML), an opportunistic viral infection of the brain that usually leads to death
or severe disability. Other serious adverse events that have occurred in TYSABRI-treated
patients included hypersensitivity reactions (e.g., anaphylaxis) and infections.
Serious opportunistic and other atypical infections have been observed in TYSABRI-treated
patients, some of whom were receiving concurrent immunosuppressants. Herpes infections
were slightly more common in patients treated with TYSABRI. In MS trials, the incidence
and rate of other serious and common adverse events, including the overall incidence
and rate of infections, were balanced between treatment groups. Common adverse events
reported in TYSABRI-treated patients include headache, fatigue, infusion reactions,
urinary tract infections, joint and limb pain, and rash.
In addition to the United States and European Union, TYSABRI is also approved
for MS in Switzerland, Canada, Australia, New Zealand and Israel. TYSABRI was discovered
by Elan and is co-developed with Biogen Idec.
http://www.investorvillage.com/smbd.asp?mb=160&mn=170547&pt=…
CHMP Adopts Negative Opinion on Appeal on European Application For natalizumab for the Treatment of Crohn's Disease

--------------------------------------------------------------------------------
Fri Nov 16 09:58:00 2007 EST
DUBLIN, IRELAND & CAMBRIDGE, MASS., Nov 16, 2007 (Canada NewsWire Elan Corporation, plc (NYSE: ELN) and Biogen Idec (NASDAQ: BIIB) today announced
that the Committee for Medicinal Products for Human Use (CHMP), the scientific committee
of the European Medicines Agency (EMEA), has adopted a negative opinion on the marketing
authorization for natalizumab as a treatment for Crohn's disease. This decision
is on the appeal the companies filed following a previous negative opinion adopted
by the CHMP earlier in 2007.
This negative opinion is now referred to the European Commission (EC), which
grants marketing authorizations in the EU. Final EC decisions customarily follow
the CHMP's recommendation, and the companies expect to hear from the EC during the
first quarter of 2008.
About Crohn's Disease
Approximately one million people worldwide have Crohn's disease, a chronic
and progressive inflammatory disease of the gastrointestinal tract, which commonly
affects both men and women.
The disease usually causes diarrhea and crampy abdominal pain, often associated
with fever, and at times rectal bleeding. Loss of appetite and weight loss also
may occur. Complications include narrowing of the intestine, obstruction, abscesses,
and fistulas (abnormal channels connecting the intestine and other organs, including
the skin), and malnutrition. Most patients eventually require surgery, which has
both risks and potential short- and long-term complications.
Crohn's disease can have a devastating impact on the lifestyle of patients,
many of whom are young and active. Currently there is no medical or surgical cure
for Crohn's disease. Many patients fail to respond to current therapies, including
biological therapies such as agents that inhibit tumor necrosis factor alpha (TNF-alpha).
Due to this failure of current therapies in CD, therapies that have novel biological
targets are required.
About TYSABRI(R) (natalizumab)
TYSABRI is a treatment approved for relapsing forms of MS in the United States
and relapsing-remitting MS in the European Union. According to data that have been
published in the New England Journal of Medicine, after two years, TYSABRI treatment
led to a 68% relative reduction (p less than0.001) in the annualized relapse rate
compared to placebo and reduced the relative risk of disability progression by 42-54%
(p less than0.001).
TYSABRI increases the risk of progressive multifocal leukoencephalopathy
(PML), an opportunistic viral infection of the brain that usually leads to death
or severe disability. Other serious adverse events that have occurred in TYSABRI-treated
patients included hypersensitivity reactions (e.g., anaphylaxis) and infections.
Serious opportunistic and other atypical infections have been observed in TYSABRI-treated
patients, some of whom were receiving concurrent immunosuppressants. Herpes infections
were slightly more common in patients treated with TYSABRI. In MS trials, the incidence
and rate of other serious and common adverse events, including the overall incidence
and rate of infections, were balanced between treatment groups. Common adverse events
reported in TYSABRI-treated patients include headache, fatigue, infusion reactions,
urinary tract infections, joint and limb pain, and rash.
In addition to the United States and European Union, TYSABRI is also approved
for MS in Switzerland, Canada, Australia, New Zealand and Israel. TYSABRI was discovered
by Elan and is co-developed with Biogen Idec.
http://www.investorvillage.com/smbd.asp?mb=160&mn=170547&pt=…
Phase 3 für Bapineuzumab - es scheint loszugehen:
"The Boston University Alzheimer’s Disease Center (BU ADC) has been selected to participate in a new nationwide clinical trial for the treatment of mild to moderate Alzheimer’s disease (AD). BU ADC investigators will be partnering with Elan and Wyeth pharmaceutical companies, the sponsors of this clinical trial, who developed the new vaccine called bapineuzumab."
http://www.bu.edu/alzresearch/newsletter/2007/fall/new-trail…
"The Boston University Alzheimer’s Disease Center (BU ADC) has been selected to participate in a new nationwide clinical trial for the treatment of mild to moderate Alzheimer’s disease (AD). BU ADC investigators will be partnering with Elan and Wyeth pharmaceutical companies, the sponsors of this clinical trial, who developed the new vaccine called bapineuzumab."
http://www.bu.edu/alzresearch/newsletter/2007/fall/new-trail…
ebenfalls von Interesse:
eine neue Studie zu Tysabri mit subkutaner und intramuskulärer Applikation!
http://www.clinicaltrials.gov/ct/show/NCT00559702?order=33
eine neue Studie zu Tysabri mit subkutaner und intramuskulärer Applikation!
http://www.clinicaltrials.gov/ct/show/NCT00559702?order=33
Antwort auf Beitrag Nr.: 32.468.909 von Cyberhexe am 18.11.07 11:55:58...Tysabri wird in diesem Versuch für die Indikation "Secondary Progressive Multiple Sclerosis" (SPMS) getestet, für die bisher keine wirkungsvolle Therapiemöglichkeiten zur Verfügung stehen:
http://www.amsel.de/chat/index.php?kategorie=chatprotokolle&…
N: sehr geehrter Herr Dr. Schreiber, gibt es neue Informationen zu möglichen SPMS-Therapien? vielen Dank für Ihre Antwort im Voraus !
Dr. Herbert Schreiber: Leider gibt es nichts wesentlich Neues. Bei SPMS (sekundär progredient) steht weiterhin Betaferon zur Verfügung, als Eskalationstherapie auch Mitoxantron. Tysabri ist für die sekundär progrediente Form derzeit nicht zugelassen, wohl aber für schwerwiegende schubförmige MS. Möglicherweise erweitert sich das Spektrum jedoch in den kommenden Jahren.
...und der Nutzen von Betaferon und Mitoxantron ist ja bestens bekannt!
http://www.amsel.de/chat/index.php?kategorie=chatprotokolle&…
N: sehr geehrter Herr Dr. Schreiber, gibt es neue Informationen zu möglichen SPMS-Therapien? vielen Dank für Ihre Antwort im Voraus !
Dr. Herbert Schreiber: Leider gibt es nichts wesentlich Neues. Bei SPMS (sekundär progredient) steht weiterhin Betaferon zur Verfügung, als Eskalationstherapie auch Mitoxantron. Tysabri ist für die sekundär progrediente Form derzeit nicht zugelassen, wohl aber für schwerwiegende schubförmige MS. Möglicherweise erweitert sich das Spektrum jedoch in den kommenden Jahren.
...und der Nutzen von Betaferon und Mitoxantron ist ja bestens bekannt!
Tysabri for Crohn`s Disease
BRIEFING BOOK vom 21.06.07
..auf den Seiten 93-96 geht es um die " Tysabri/Crohn's RISKMap".
http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4313b1-…
BRIEFING BOOK vom 21.06.07
..auf den Seiten 93-96 geht es um die " Tysabri/Crohn's RISKMap".
http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4313b1-…

Health-Related Quality of Life During Natalizumab Maintenance Therapy for Crohn's Disease
Volume 102 Issue 12 Page 2737-2746, December 2007
Brian G. Feagan, M.D.11
Robarts Research Institute, University of Western Ontario, London, Canada, William J. Sandborn, M.D.22
Mayo Clinic, Rochester, Minnesota, Steven Hass, Ph.D.33Elan Pharmaceuticals, Inc., San Diego, California, Timothy Niecko, M.S.44Niecko Health Economics, LLC, Escondido, California, and
Jeffrey White, M.S. - Elan Pharmaceuticals, Inc., San Diego, California
OBJECTIVES: We evaluated the effects of treatment on health-related quality of life (HRQoL) during a randomized controlled trial of natalizumab maintenance therapy (ENACT-2) using both disease-specific and generic measures.
METHODS: Crohn's disease patients who received natalizumab as induction therapy in ENACT-1 (N = 724) and responded (N = 339) were re-randomized to ENACT-2 in which they received natalizumab 300 mg (N = 168) or placebo (N = 171) every 4 wk for 48 additional wk. Outcome measures were the change from baseline on the inflammatory bowel disease questionnaire (IBDQ), the short form-36 (SF-36), the EuroQol-5D (EQ-5D), and a subject global assessment.
RESULTS: At entry into ENACT-1, scores indicated substantially impaired HRQoL for both the disease-specific and general measures. Natalizumab responders showed clinically meaningful improvement in HRQoL over the course of the ENACT-1 study. During maintenance therapy, IBDQ and SF-36 scale scores of patients who responded to natalizumab induction and were re-randomized to receive the drug in ENACT-2 (N = 168) remained stable, while those re-randomized to placebo (N = 171) worsened. At week 60, 48 wk after the initiation of maintenance therapy, the mean change from ENACT-1 baseline of all scales of the IBDQ and the SF-36 was significantly higher for those who continued to receive natalizumab (P < 0.001 for all scales). The scores of patients who received maintenance natalizumab treatment were not statistically different from those of a cross-section of the U.S. population for 6 of 8 scales of the SF-36.
CONCLUSIONS: The substantial improvement in HRQoL experienced by patients who responded to natalizumab induction therapy was maintained during an additional 48 wk of maintenance therapy.
http://www.blackwell-synergy.com/doi/abs/10.1111/j.1572-0241…
Elan Pharmaceuticals Licenses Ariadne's Pathway Studio® with MedScan®
Ariadne announced today that Elan Pharmaceuticals Inc. has licensed its Pathway Studio software. Elan is a biotechnology company focused on discovery and development of therapies for neurology and autoimmune diseases. They chose Pathway Studio for its advanced text-mining tools and powerful pathway analysis capability.
Rockville, MD (PRWEB) November 30, 2007 -- Ariadne announced today that Elan Pharmaceuticals Inc. has licensed its Pathway Studio software. Elan is a biotechnology company focused on discovery and development of therapies for neurology and autoimmune diseases. They chose Pathway Studio for its advanced text-mining tools and powerful pathway analysis capability.
Ariadne's Pathway Studio includes the ResNet database of functional relationships that can be used for assembly of biological pathways, exploring signaling networks, and to interpret microarray experimental data. MedScan's natural language processing technology enables customers to extend ResNet though extraction of pathway-related information from biomedical literature.
"Ariadne's Pathway Studio, MedScan, and ResNet complement the tools already in use at Elan for pathway investigation and gene expression analysis," commented Jim Miller, Principal Scientist at Elan Pharmaceuticals. "The MedScan module, in particular, gives a scientist the capability to quickly create pathways from the newest literature sources in a matter of minutes."
"Ariadne worked with several research groups at Elan to meet their common and unique requirements. Pathway Studio's flexible support for multiple workflows and data types was a key factor in acceptance of the product across the company," commented John Clouston, Senior Director of Sales at Ariadne.
For more information about Pathway Studio and MedScan Technology, please contact Ariadne at 240-453-6296, or visit www.ariadnegenomics.com.
About Ariadne
ARIADNE (www.ariadnegenomics.com) Pathway Studio software is used worldwide as a comprehensive solution for analysis and searching of pathways and molecular interaction information. Ariadne offers desktop and enterprise editions of Pathway Studio which are supported by several commercial and publicly available molecular interaction databases including ResNet Mammalian and ResNet Plant. Pathway Studio powered by MedScan Technology can be used to edit and enhance database content from the current literature and from experimental data.
http://www.emediawire.com/releases/2007/11/emw572906.htm
Ariadne announced today that Elan Pharmaceuticals Inc. has licensed its Pathway Studio software. Elan is a biotechnology company focused on discovery and development of therapies for neurology and autoimmune diseases. They chose Pathway Studio for its advanced text-mining tools and powerful pathway analysis capability.
Rockville, MD (PRWEB) November 30, 2007 -- Ariadne announced today that Elan Pharmaceuticals Inc. has licensed its Pathway Studio software. Elan is a biotechnology company focused on discovery and development of therapies for neurology and autoimmune diseases. They chose Pathway Studio for its advanced text-mining tools and powerful pathway analysis capability.
Ariadne's Pathway Studio includes the ResNet database of functional relationships that can be used for assembly of biological pathways, exploring signaling networks, and to interpret microarray experimental data. MedScan's natural language processing technology enables customers to extend ResNet though extraction of pathway-related information from biomedical literature.
"Ariadne's Pathway Studio, MedScan, and ResNet complement the tools already in use at Elan for pathway investigation and gene expression analysis," commented Jim Miller, Principal Scientist at Elan Pharmaceuticals. "The MedScan module, in particular, gives a scientist the capability to quickly create pathways from the newest literature sources in a matter of minutes."
"Ariadne worked with several research groups at Elan to meet their common and unique requirements. Pathway Studio's flexible support for multiple workflows and data types was a key factor in acceptance of the product across the company," commented John Clouston, Senior Director of Sales at Ariadne.
For more information about Pathway Studio and MedScan Technology, please contact Ariadne at 240-453-6296, or visit www.ariadnegenomics.com.
About Ariadne
ARIADNE (www.ariadnegenomics.com) Pathway Studio software is used worldwide as a comprehensive solution for analysis and searching of pathways and molecular interaction information. Ariadne offers desktop and enterprise editions of Pathway Studio which are supported by several commercial and publicly available molecular interaction databases including ResNet Mammalian and ResNet Plant. Pathway Studio powered by MedScan Technology can be used to edit and enhance database content from the current literature and from experimental data.
http://www.emediawire.com/releases/2007/11/emw572906.htm
...aus der Patentabteilung:
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
...langsam aber sicher scheint Elan Fahrt aufzunehmen. Wenn mit Tysabri nichts schief geht (PML) und Bapineuzumab die Erwartungen erfüllen sollte, wird Elan, auch in Verbindung mit der vielversprechenden EDT - Bernie super Link! -, seinen Aktionären noch sehr, sehr viel Freude bereiten.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
...langsam aber sicher scheint Elan Fahrt aufzunehmen. Wenn mit Tysabri nichts schief geht (PML) und Bapineuzumab die Erwartungen erfüllen sollte, wird Elan, auch in Verbindung mit der vielversprechenden EDT - Bernie super Link! -, seinen Aktionären noch sehr, sehr viel Freude bereiten.
ELND-005 in Patients With Mild to Moderate Alzheimer's Disease
Start: December 2007 - May 2010
This study is not yet open for participant recruitment.
Verified by Elan Pharmaceuticals, December 2007
The purpose of this study is to evaluate the dose-related safety and efficacy of multiple oral dosages of
ELND-005 as treatment for Alzheimer's disease.
http://clinicaltrials.gov/ct2/show/NCT00568776?term=alzheime…" target="_blank" rel="nofollow ugc noopener">http://clinicaltrials.gov/ct2/show/NCT00568776?term=alzheime…
Start: December 2007 - May 2010
This study is not yet open for participant recruitment.
Verified by Elan Pharmaceuticals, December 2007
The purpose of this study is to evaluate the dose-related safety and efficacy of multiple oral dosages of
ELND-005 as treatment for Alzheimer's disease.
http://clinicaltrials.gov/ct2/show/NCT00568776?term=alzheime…" target="_blank" rel="nofollow ugc noopener">http://clinicaltrials.gov/ct2/show/NCT00568776?term=alzheime…
Another ALZ immuneotherapy
Eisai To Develop BioArctic's Antibody For Alzheimer's Disease
Nikkei English News - Dec. 03, 2007
TOKYO (Dow Jones)--Eisai Co. (4523.TO) said Tuesday it will exclusively develop an antibody drug discovered by Sweden's BioArctic Neuroscience AB to treat Alzheimer's disease.
The two companies agreed on Eisai's global rights to develop and market BioArctic's BAN2401 monoclonal antibody following their joint research since 2005 to find a potential immunotherapy for Alzheimer's disease.
BAN2401 is in a pre-clinical stage, Eisai said in a statement. Eisai and BioArctic didn't disclose financial terms for the agreement.
The antibody is expected to reduce amyloid plaques in the brain that are believed to play a key role in the development of Alzheimer's disease.
Eisai is also developing E2012 which is designed to inhibit amyloid production.
Other companies developing an antibody to the plaques include Elan Corp. Plc, Wyeth and Eli Lilly & Co.
-By Kazuhiro Shimamura, Dow Jones Newswires; 813-5255-2929; kazuhiro.shimamura@dowjones.com
http://www.investorvillage.com/smbd.asp?mb=160&mn=175904&pt=…
Eisai To Develop BioArctic's Antibody For Alzheimer's Disease
Nikkei English News - Dec. 03, 2007
TOKYO (Dow Jones)--Eisai Co. (4523.TO) said Tuesday it will exclusively develop an antibody drug discovered by Sweden's BioArctic Neuroscience AB to treat Alzheimer's disease.
The two companies agreed on Eisai's global rights to develop and market BioArctic's BAN2401 monoclonal antibody following their joint research since 2005 to find a potential immunotherapy for Alzheimer's disease.
BAN2401 is in a pre-clinical stage, Eisai said in a statement. Eisai and BioArctic didn't disclose financial terms for the agreement.
The antibody is expected to reduce amyloid plaques in the brain that are believed to play a key role in the development of Alzheimer's disease.
Eisai is also developing E2012 which is designed to inhibit amyloid production.
Other companies developing an antibody to the plaques include Elan Corp. Plc, Wyeth and Eli Lilly & Co.
-By Kazuhiro Shimamura, Dow Jones Newswires; 813-5255-2929; kazuhiro.shimamura@dowjones.com
http://www.investorvillage.com/smbd.asp?mb=160&mn=175904&pt=…
Press Release Source: Medidata Solutions
Elan Selects Medidata Rave(R) to Support Clinical Development Activities
Monday December 17, 9:00 am ET
Neuroscience-Based Biotechnology Company Implements Industry's Leading EDC Solution to Improve Study Efficiency and Gain Instant Access to Study Data
NEW YORK--(BUSINESS WIRE)--Medidata Solutions today announced that Elan Pharmaceuticals, Inc. has selected Medidata Rave as its electronic data capture (EDC), management and reporting solution.
Elan plans to begin using Rave in mid- and late-stage clinical studies in North America.
Elan had limited previous experience using EDC and conducted mainly paper-based trials. After a careful evaluation of the market landscape, Elan’s management team identified EDC as a critical component in clinical research and began looking for an EDC solution that could address two major concerns. First, the product had to easily integrate the data collected in an EDC system with other systems across the company, as well as systems used by external vendors and partners. Second, because much of Elan’s data management is outsourced, the company needed a solution that could be readily adopted by a contract research organization (CRO) without prior experience with the technology.
Elan ultimately selected Medidata as its EDC provider from a field of competitors, and by fully integrating Medidata Rave across Elan’s systems and clinical processes, the company expects to achieve greater access to clinical and operational data that will enable earlier decision-making throughout each trial.
Medidata Rave offers several key benefits:
A robust architecture that would easily integrate with their existing systems in order to securely and efficiently share data;
High acceptance by investigators;
An easy learning curve, making the implementation of an additional data system non-disruptive to researchers;
Instant access to clinical data, making it easier to predict and prioritize monitoring efforts to make site visits more effective;
Intuitive case report forms (CRFs) that may help Elan data managers to reduce “petty queries,” keeping studies on time more often;
The ability to access data in real time, allowing monitors to catch and respond to problems as early as possible.
“Each customer’s clinical requirements differ, and Medidata is unique in our ability to critically evaluate the technical and clinical needs of researchers to understand how Rave can properly complement existing systems and enhance the experience of each individual customer,” said Tarek Sherif, CEO of Medidata Solutions. “Medidata is proud to work with Elan as it moves toward its goal of delivering promising therapeutics as safely and effectively possible to serve patients and the health care providers caring for them.”
About Medidata Solutions Worldwide
Medidata Solutions helps the world’s leading pharmaceutical, biotechnology, medical device and research organizations maximize the value of their clinical research investments. Innovative process design, technology and services streamline clinical trials by providing early visibility to reliable clinical data – the lifeblood of every research organization. Working with companies and institutions both large and small, Medidata Solutions helps clinical researchers safely accelerate the process of bringing life-enhancing treatments to market – on six continents and in more than 80 countries. Medidata Solutions brings significant value to its broad client base with deep clinical experience and expertise in more than 20 therapeutic areas, projects in Phase I, II, III, IV, registries and surveillance, and studies with thousands of investigators and tens-of-thousands of subjects. For more information, please visit www.mdsol.co
Contact:
Lois Paul & Partners
Susan Lombardo, 781-782-5767
susan_lombardo@lpp.com
http://www.investorvillage.com/smbd.asp?mb=160&mn=178282&pt=…
Elan Selects Medidata Rave(R) to Support Clinical Development Activities
Monday December 17, 9:00 am ET
Neuroscience-Based Biotechnology Company Implements Industry's Leading EDC Solution to Improve Study Efficiency and Gain Instant Access to Study Data
NEW YORK--(BUSINESS WIRE)--Medidata Solutions today announced that Elan Pharmaceuticals, Inc. has selected Medidata Rave as its electronic data capture (EDC), management and reporting solution.
Elan plans to begin using Rave in mid- and late-stage clinical studies in North America.

Elan had limited previous experience using EDC and conducted mainly paper-based trials. After a careful evaluation of the market landscape, Elan’s management team identified EDC as a critical component in clinical research and began looking for an EDC solution that could address two major concerns. First, the product had to easily integrate the data collected in an EDC system with other systems across the company, as well as systems used by external vendors and partners. Second, because much of Elan’s data management is outsourced, the company needed a solution that could be readily adopted by a contract research organization (CRO) without prior experience with the technology.
Elan ultimately selected Medidata as its EDC provider from a field of competitors, and by fully integrating Medidata Rave across Elan’s systems and clinical processes, the company expects to achieve greater access to clinical and operational data that will enable earlier decision-making throughout each trial.
Medidata Rave offers several key benefits:
A robust architecture that would easily integrate with their existing systems in order to securely and efficiently share data;
High acceptance by investigators;
An easy learning curve, making the implementation of an additional data system non-disruptive to researchers;
Instant access to clinical data, making it easier to predict and prioritize monitoring efforts to make site visits more effective;
Intuitive case report forms (CRFs) that may help Elan data managers to reduce “petty queries,” keeping studies on time more often;
The ability to access data in real time, allowing monitors to catch and respond to problems as early as possible.
“Each customer’s clinical requirements differ, and Medidata is unique in our ability to critically evaluate the technical and clinical needs of researchers to understand how Rave can properly complement existing systems and enhance the experience of each individual customer,” said Tarek Sherif, CEO of Medidata Solutions. “Medidata is proud to work with Elan as it moves toward its goal of delivering promising therapeutics as safely and effectively possible to serve patients and the health care providers caring for them.”
About Medidata Solutions Worldwide
Medidata Solutions helps the world’s leading pharmaceutical, biotechnology, medical device and research organizations maximize the value of their clinical research investments. Innovative process design, technology and services streamline clinical trials by providing early visibility to reliable clinical data – the lifeblood of every research organization. Working with companies and institutions both large and small, Medidata Solutions helps clinical researchers safely accelerate the process of bringing life-enhancing treatments to market – on six continents and in more than 80 countries. Medidata Solutions brings significant value to its broad client base with deep clinical experience and expertise in more than 20 therapeutic areas, projects in Phase I, II, III, IV, registries and surveillance, and studies with thousands of investigators and tens-of-thousands of subjects. For more information, please visit www.mdsol.co
Contact:
Lois Paul & Partners
Susan Lombardo, 781-782-5767
susan_lombardo@lpp.com
http://www.investorvillage.com/smbd.asp?mb=160&mn=178282&pt=…
Bapineuzumab Phase3: der Studienentwurf ist nun verfügbar -->
http://clinicaltrials.gov/ct2/show/NCT00575055?term=aab-001&…
http://clinicaltrials.gov/ct2/show/NCT00575055?term=aab-001&…
Antwort auf Beitrag Nr.: 32.797.229 von Cyberhexe am 17.12.07 22:40:21Bapineuzumab Fortsetzung:
http://clinicaltrials.gov/ct2/show/NCT00575055?term=elan&rec…
http://clinicaltrials.gov/ct2/show/NCT00575055?term=elan&rec…
Antwort auf Beitrag Nr.: 32.797.255 von Cyberhexe am 17.12.07 22:42:58sorry, dies ist die andere Studie:
NCT00574132
AAB-001-301
n=1250 Teilnehmer
Start Date: December 2007
Expected Completion Date: December 2010
4 Arme davon 3 Verum und 1 Plazebo
http://clinicaltrials.gov/ct2/show/NCT00574132?term=elan&rec…
NCT00574132
AAB-001-301
n=1250 Teilnehmer
Start Date: December 2007
Expected Completion Date: December 2010
4 Arme davon 3 Verum und 1 Plazebo
http://clinicaltrials.gov/ct2/show/NCT00574132?term=elan&rec…
Antwort auf Beitrag Nr.: 32.797.377 von Cyberhexe am 17.12.07 22:56:14
"Patients will be randomized to receive either bapineuzumab or placebo. Each patient's participation will last approximately 1.5 years."
In Anbetracht der riesigen Prävalenz der Indikation sollte es doch keine 15 Monate dauern, bis die Rekrutierung der Studienteilnehmer abgeschlossen ist - hätte ich einen Betroffenen in meiner Verwandtschaft/Freundeskreis, ich würde alles daran setzen, dass eine Studienteilnahme schnellstens möglich wäre!
"Patients will be randomized to receive either bapineuzumab or placebo. Each patient's participation will last approximately 1.5 years."
In Anbetracht der riesigen Prävalenz der Indikation sollte es doch keine 15 Monate dauern, bis die Rekrutierung der Studienteilnehmer abgeschlossen ist - hätte ich einen Betroffenen in meiner Verwandtschaft/Freundeskreis, ich würde alles daran setzen, dass eine Studienteilnahme schnellstens möglich wäre!
Antwort auf Beitrag Nr.: 32.797.454 von Cyberhexe am 17.12.07 23:05:56BM, Cybie...

Antwort auf Beitrag Nr.: 32.791.677 von bernie55 am 17.12.07 15:29:0317.12.2007 15:01
Elan entscheidet sich für Medidata Rave(R) für den Support klinischer Entwicklungsaktivitäten
Medidata Solutions gab heute bekannt, dass sich Elan Pharmaceuticals, (News) Inc. für Medidata Rave entschieden habe als seine Lösung für die Bereiche Electronic Data Capture (EDC), Management und Reporting. Elan plant, Rave anfangs in Nordamerika bei klinischen Studien im mittleren und späten Stadium einzusetzen.
Elan hatte mit dem Einsatz von EDC bisher nur wenig Erfahrung und führte im Wesentlichen auf Papier basierende Untersuchungen durch. Nach einer sorgfältigen Einschätzung des Marktumfelds hat das Managementteam EDC jedoch als wesentliche Komponente für die klinischer Forschung erkannt und damit angefangen, nach einer EDC-Lösung für zwei bedeutende Anforderungen zu suchen. Erstens sollte das Produkt eine einfache Integration mit den gesammelten Daten in einem EDC-System mit anderen Systemen quer durch das Unternehmen sowie mit Systemen von externen Lieferanten und Partnern ermöglichen. Zweitens benötigte das Unternehmen eine Lösung, die leicht von einer Vertragsforschungsorganisation (Contract Research Organization - CRO) ohne vorherige Erfahrung mit der Technologie übernommen werden kann, da ein Großteil des Datenmanagements von Elan outgesourct ist.
Elan hat sich letztendlich aus einer Reihe von Wettbewerbern für Medidata als seinen EDC-Provider entschieden. Durch die komplette Integration von Medidata Rave auf alle Systeme und klinischen Prozesse von Elan erwartet das Unternehmen einen größeren Zugang zu klinischen und operativen Daten, wodurch eine frühere Entscheidungsfindung bei jeder Untersuchung ermöglicht werden soll.
Medidata Rave hat verschiedene wichtige Vorteile zu bieten:
Eine robuste Architektur, die leicht mit bestehenden Systemen integriert werden kann, um Daten sicher und effizient zu teilen;
hohe Akzeptanz bei Forschern;
eine einfache Lernkurve, wodurch die Implementierung eines zusätzlichen Datensystems von Forschern ohne Störungen verläuft;
Sofortiger Zugang zu klinischen Daten für die leichtere Vorhersage und Priorisierung von Überwachungsanstrengungen, um Besuche in Prüfzentren effektiver zu gestalten;
Intuitive Case Report Forms (CRFs), die Datenmanager von Elan unterstützen können, "triviale Anfragen" zu reduzieren und Untersuchungen somit öfter im beabsichtigtem Zeitrahmen zu halten;
Die Möglichkeit des Datenzugangs in Echtzeit, wodurch bei Überwachungen Probleme so früh wie möglich erkannt und darauf reagiert werden kann.
"Die klinischen Erfordernisse eines jeden Kunden sind unterschiedlich, und Medidata ist einzigartig bei der kritischen Bewertung der technischen und klinischen Bedürfnisse von Forschern, um zu verstehen, wie Rave bestehende Systeme richtig ergänzt und die Erfahrung eines jeden individuellen Kunden verbessert", so Tarek Sherif, CEO von Medidata Solutions. "Medidata ist stolz auf die Zusammenarbeit mit Elan, während das Unternehmen seine Ziele angeht, vielversprechende Therapeutika so sicher und effektiv wie möglich zu liefern, um sowohl den Patienten als auch den Gesundheitsdienstleistern zu dienen, die sich um diese sorgen."
http://www.finanznachrichten.de/nachrichten-2007-12/artikel-…
Elan entscheidet sich für Medidata Rave(R) für den Support klinischer Entwicklungsaktivitäten
Medidata Solutions gab heute bekannt, dass sich Elan Pharmaceuticals, (News) Inc. für Medidata Rave entschieden habe als seine Lösung für die Bereiche Electronic Data Capture (EDC), Management und Reporting. Elan plant, Rave anfangs in Nordamerika bei klinischen Studien im mittleren und späten Stadium einzusetzen.
Elan hatte mit dem Einsatz von EDC bisher nur wenig Erfahrung und führte im Wesentlichen auf Papier basierende Untersuchungen durch. Nach einer sorgfältigen Einschätzung des Marktumfelds hat das Managementteam EDC jedoch als wesentliche Komponente für die klinischer Forschung erkannt und damit angefangen, nach einer EDC-Lösung für zwei bedeutende Anforderungen zu suchen. Erstens sollte das Produkt eine einfache Integration mit den gesammelten Daten in einem EDC-System mit anderen Systemen quer durch das Unternehmen sowie mit Systemen von externen Lieferanten und Partnern ermöglichen. Zweitens benötigte das Unternehmen eine Lösung, die leicht von einer Vertragsforschungsorganisation (Contract Research Organization - CRO) ohne vorherige Erfahrung mit der Technologie übernommen werden kann, da ein Großteil des Datenmanagements von Elan outgesourct ist.
Elan hat sich letztendlich aus einer Reihe von Wettbewerbern für Medidata als seinen EDC-Provider entschieden. Durch die komplette Integration von Medidata Rave auf alle Systeme und klinischen Prozesse von Elan erwartet das Unternehmen einen größeren Zugang zu klinischen und operativen Daten, wodurch eine frühere Entscheidungsfindung bei jeder Untersuchung ermöglicht werden soll.
Medidata Rave hat verschiedene wichtige Vorteile zu bieten:
Eine robuste Architektur, die leicht mit bestehenden Systemen integriert werden kann, um Daten sicher und effizient zu teilen;
hohe Akzeptanz bei Forschern;
eine einfache Lernkurve, wodurch die Implementierung eines zusätzlichen Datensystems von Forschern ohne Störungen verläuft;
Sofortiger Zugang zu klinischen Daten für die leichtere Vorhersage und Priorisierung von Überwachungsanstrengungen, um Besuche in Prüfzentren effektiver zu gestalten;
Intuitive Case Report Forms (CRFs), die Datenmanager von Elan unterstützen können, "triviale Anfragen" zu reduzieren und Untersuchungen somit öfter im beabsichtigtem Zeitrahmen zu halten;
Die Möglichkeit des Datenzugangs in Echtzeit, wodurch bei Überwachungen Probleme so früh wie möglich erkannt und darauf reagiert werden kann.
"Die klinischen Erfordernisse eines jeden Kunden sind unterschiedlich, und Medidata ist einzigartig bei der kritischen Bewertung der technischen und klinischen Bedürfnisse von Forschern, um zu verstehen, wie Rave bestehende Systeme richtig ergänzt und die Erfahrung eines jeden individuellen Kunden verbessert", so Tarek Sherif, CEO von Medidata Solutions. "Medidata ist stolz auf die Zusammenarbeit mit Elan, während das Unternehmen seine Ziele angeht, vielversprechende Therapeutika so sicher und effektiv wie möglich zu liefern, um sowohl den Patienten als auch den Gesundheitsdienstleistern zu dienen, die sich um diese sorgen."
http://www.finanznachrichten.de/nachrichten-2007-12/artikel-…
19 studies with search of: alzheimer's | Open Studies | Phase III | Industry
Include studies which are not seeking new volunteers.
Display Options
1 Recruiting Efficacy and Safety of Rivastigmine Transdermal Patch in Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: rivastigmine transdermal patch
2 Recruiting Safety and Tolerability of Long-Term Treatment by Rivastigmine Transdermal Patch in Patients With Probable Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: rivastigmine transdermal patch
http://www.investorvillage.com/smbd.asp?mb=160&mn=178778&pt=…
3 Not yet recruiting Open-Label Extension Study of Rosiglitazone XR in Subjects With Mild-to-Moderate Alzheimer's
Condition: Alzheimer's Disease
Intervention: Drug: Rosiglitazone XR
4 Recruiting Comparison of 23 mg Donepezil Sustained Release (SR) to 10 mg Donepezil Immediate Release (IR) in Patients With Moderate to Severe Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Aricept (donepezil SR)
5 Recruiting Rosiglitazone (Extended Release Tablets) Monotherapy In Subjects With Mild To Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Rosiglitazone XR (extended release) oral tablets
6 Recruiting Rosiglitazone (Extended Release Tablets) As Adjunctive Therapy In Subjects With Mild To Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Rosiglitazone XR (extended release) oral tablets
7 Recruiting Open-Label Extension Study of Rosiglitazone XR as Adjunctive Therapy in Subjects With Mild-to-Moderate Alzheimer's
Condition: Alzheimers Disease
Intervention: Drug: Rosiglitazone XR
8 Recruiting Rosiglitazone (Extended Release Tablets) As Adjunctive Therapy For Subjects With Mild To Moderate Alzheimer's Disease
Condition: Alzheimer Disease
Intervention: Drug: Rosiglitazone XR (extended release) oral tablets
9 Recruiting Open-Label Treatment With MPC-7869 for Patients With Alzheimer's Who Previously Participated in an MPC-7869 Protocol
Condition: Alzheimer's Disease
Intervention: Drug: MPC-7869
10 Recruiting Study to Evaluate the Effect of Megestrol Acetate in the Weight Loss in Alzheimer Disease
Condition: Alzheimer Disease
Intervention: Drug: Megestrol acetate
11 Recruiting Comparative Efficacy, Safety, and Tolerability of Rivastigmine 10 and 15 cm2 Patch in Patients With Alzheimer's Disease (AD) Showing Cognitive Decline
Condition: Alzheimer Disease
Intervention: Drug: Rivastigmine transdermal patch
12 Not yet recruiting Open-Label Extension Study of 23mg Donepezil SR in Patients With Moderate to Severe Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Aricept
13 Not yet recruiting Bapineuzumab in Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Interventions: Drug: Bapineuzumab; Drug: Placebo Control
14 Not yet recruiting Bapineuzumab in Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Interventions: Drug: Bapineuzumab; Drug: Placebo Control
15 Recruiting Treatment of Elderly Subjects With Psychosis and Behavioral Disturbances Associated With Dementia of the Alzheimer's Type
Condition: Psychosis and Behavioral Disturbances Associated With Dementia of the Alzheimer's Type
Intervention: Drug: bifeprunox
16 Recruiting Vitamin E and Namenda (Memantine) for the Treatment of Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Interventions: Drug: Vitamin E; Drug: Memantine; Drug: Vitamin E and Memantine
17 Recruiting Hormone and Information Processing Study
Conditions: Mild Cognitive Impairment; Alzheimer's Disease
Interventions: Drug: testosterone gel; Drug: placebo gel
18 Recruiting Vaspect Study - An Open-Label Trial Of Donepezil in Vascular and Mixed Dementia
Conditions: Dementia, Vascular; Dementia, Mixed
Intervention: Drug: Donepezil
19 Recruiting Memantine for Disability in Amyotrophic Lateral Sclerosis (MEDALS)
Condition: Amyotrophic Lateral Sclerosis
Intervention: Drug: Memantine
Include studies which are not seeking new volunteers.
Display Options
1 Recruiting Efficacy and Safety of Rivastigmine Transdermal Patch in Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: rivastigmine transdermal patch
2 Recruiting Safety and Tolerability of Long-Term Treatment by Rivastigmine Transdermal Patch in Patients With Probable Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: rivastigmine transdermal patch
http://www.investorvillage.com/smbd.asp?mb=160&mn=178778&pt=…
3 Not yet recruiting Open-Label Extension Study of Rosiglitazone XR in Subjects With Mild-to-Moderate Alzheimer's
Condition: Alzheimer's Disease
Intervention: Drug: Rosiglitazone XR
4 Recruiting Comparison of 23 mg Donepezil Sustained Release (SR) to 10 mg Donepezil Immediate Release (IR) in Patients With Moderate to Severe Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Aricept (donepezil SR)
5 Recruiting Rosiglitazone (Extended Release Tablets) Monotherapy In Subjects With Mild To Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Rosiglitazone XR (extended release) oral tablets
6 Recruiting Rosiglitazone (Extended Release Tablets) As Adjunctive Therapy In Subjects With Mild To Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Rosiglitazone XR (extended release) oral tablets
7 Recruiting Open-Label Extension Study of Rosiglitazone XR as Adjunctive Therapy in Subjects With Mild-to-Moderate Alzheimer's
Condition: Alzheimers Disease
Intervention: Drug: Rosiglitazone XR
8 Recruiting Rosiglitazone (Extended Release Tablets) As Adjunctive Therapy For Subjects With Mild To Moderate Alzheimer's Disease
Condition: Alzheimer Disease
Intervention: Drug: Rosiglitazone XR (extended release) oral tablets
9 Recruiting Open-Label Treatment With MPC-7869 for Patients With Alzheimer's Who Previously Participated in an MPC-7869 Protocol
Condition: Alzheimer's Disease
Intervention: Drug: MPC-7869
10 Recruiting Study to Evaluate the Effect of Megestrol Acetate in the Weight Loss in Alzheimer Disease
Condition: Alzheimer Disease
Intervention: Drug: Megestrol acetate
11 Recruiting Comparative Efficacy, Safety, and Tolerability of Rivastigmine 10 and 15 cm2 Patch in Patients With Alzheimer's Disease (AD) Showing Cognitive Decline
Condition: Alzheimer Disease
Intervention: Drug: Rivastigmine transdermal patch
12 Not yet recruiting Open-Label Extension Study of 23mg Donepezil SR in Patients With Moderate to Severe Alzheimer's Disease
Condition: Alzheimer's Disease
Intervention: Drug: Aricept
13 Not yet recruiting Bapineuzumab in Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Interventions: Drug: Bapineuzumab; Drug: Placebo Control
14 Not yet recruiting Bapineuzumab in Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Interventions: Drug: Bapineuzumab; Drug: Placebo Control
15 Recruiting Treatment of Elderly Subjects With Psychosis and Behavioral Disturbances Associated With Dementia of the Alzheimer's Type
Condition: Psychosis and Behavioral Disturbances Associated With Dementia of the Alzheimer's Type
Intervention: Drug: bifeprunox
16 Recruiting Vitamin E and Namenda (Memantine) for the Treatment of Patients With Mild to Moderate Alzheimer's Disease
Condition: Alzheimer's Disease
Interventions: Drug: Vitamin E; Drug: Memantine; Drug: Vitamin E and Memantine
17 Recruiting Hormone and Information Processing Study
Conditions: Mild Cognitive Impairment; Alzheimer's Disease
Interventions: Drug: testosterone gel; Drug: placebo gel
18 Recruiting Vaspect Study - An Open-Label Trial Of Donepezil in Vascular and Mixed Dementia
Conditions: Dementia, Vascular; Dementia, Mixed
Intervention: Drug: Donepezil
19 Recruiting Memantine for Disability in Amyotrophic Lateral Sclerosis (MEDALS)
Condition: Amyotrophic Lateral Sclerosis
Intervention: Drug: Memantine

Elan to Present at the 26th Annual JP Morgan Healthcare Conference
Tuesday December 18, 11:37 am ET
DUBLIN, Ireland--(BUSINESS WIRE)--Elan Corporation, plc announces that it will present at the 26th Annual JP Morgan Healthcare Conference in San Francisco on Tuesday, January 8th, 2008 at 8.30 a.m. Pacific Standard Time, 11.30 a.m. Eastern Standard Time and 4.30 p.m. Greenwich Mean Time.
Interested parties may access a live audio webcast of the presentation by visiting Elan’s website at www.elan.com and clicking on the Investor Relations section, then on the event icon.
http://www.elan.com/News/full.asp?ID=1088388
NCB
EPIX Pharmaceuticals (EPIX US, market cap of $145m) announced strong top-line
results yesterday from a Phase IIa two-week clinical trial of its Alzheimer's disease
product. These results showed that patients receiving the highest dose of the drug,
PRX-03140, achieved a 5.7pt improvement on ADAS-Cog (this is the current standard
for evaluating efficacy for cognition in Alzheimer's disease) compared to a 0.2 pt
worsening in patients on placebo. An effect on ADAS-cog is not typically observed in
less than 12 weeks of treatment.
• This Phase IIa study assessed the effects of PRX-03140 following two weeks of
treatment as monotherapy (oral, once daily) and separately in combination with Aricept
(Pfizer/Eisai) in patients with mild Alzheimer's disease. The one noteworthy observation
from this study was that results showed that patients on PRX-03140 alone achieved a
statistically significant improvement in ADAS-cog although the ADAS-cog data in
patients on a combination of PRX-03140 and Aricept was not statistically significant.
Further Phase II studies, which are expected to begin in H1 2008, will be required in a
larger patient population to explore these preliminary cognitive effects and to assess
the long term potential of this product in improving cognition and memory.
• Elsewhere, the Phase III trial details for AAB-001 were posted on the FDA website
yesterday (http://clinicaltrials.gov/ct2/show/NCT00575055?term=aab001&r…
http://clinicaltrials.gov/ct2/show/NCT00574132?term=aab001&r… As previously
disclosed, Elan is initiating two studies in the US/Canada of 18-month duration
(followed by a safety review) iwith 800 ApoE+ patients in a single dose study and 1250
ApoE- patients in a multi-dose study. Two further studies are expected to be initiated in
Europe. AAB-001 Phase II data is expected to be released in mid-2008
http://www.investorvillage.com/smbd.asp?mb=160&mn=179240&pt=…
EPIX Pharmaceuticals (EPIX US, market cap of $145m) announced strong top-line
results yesterday from a Phase IIa two-week clinical trial of its Alzheimer's disease
product. These results showed that patients receiving the highest dose of the drug,
PRX-03140, achieved a 5.7pt improvement on ADAS-Cog (this is the current standard
for evaluating efficacy for cognition in Alzheimer's disease) compared to a 0.2 pt
worsening in patients on placebo. An effect on ADAS-cog is not typically observed in
less than 12 weeks of treatment.
• This Phase IIa study assessed the effects of PRX-03140 following two weeks of
treatment as monotherapy (oral, once daily) and separately in combination with Aricept
(Pfizer/Eisai) in patients with mild Alzheimer's disease. The one noteworthy observation
from this study was that results showed that patients on PRX-03140 alone achieved a
statistically significant improvement in ADAS-cog although the ADAS-cog data in
patients on a combination of PRX-03140 and Aricept was not statistically significant.
Further Phase II studies, which are expected to begin in H1 2008, will be required in a
larger patient population to explore these preliminary cognitive effects and to assess
the long term potential of this product in improving cognition and memory.
• Elsewhere, the Phase III trial details for AAB-001 were posted on the FDA website
yesterday (http://clinicaltrials.gov/ct2/show/NCT00575055?term=aab001&r…
http://clinicaltrials.gov/ct2/show/NCT00574132?term=aab001&r… As previously
disclosed, Elan is initiating two studies in the US/Canada of 18-month duration
(followed by a safety review) iwith 800 ApoE+ patients in a single dose study and 1250
ApoE- patients in a multi-dose study. Two further studies are expected to be initiated in
Europe. AAB-001 Phase II data is expected to be released in mid-2008
http://www.investorvillage.com/smbd.asp?mb=160&mn=179240&pt=…
@Cyberhexe
ACHTUNG !!!!!!!
BM für Dich !!!!
ACHTUNG !!!!!!!
BM für Dich !!!!
ELND005 in Patients With Mild to Moderate Alzheimer's Disease
This study is currently recruiting participants.
Verified by Elan Pharmaceuticals, December 2007
Sponsors and Collaborators: Elan Pharmaceuticals
Transition Therapeutics
Information provided by: Elan Pharmaceuticals
ClinicalTrials.gov Identifier: NCT00568776
Purpose
The purpose of this study is to evaluate the dose-related safety and efficacy of multiple oral dosages of ELND005 as treatment for Alzheimer's disease.
Condition Intervention Phase
Alzheimer Disease
Drug: ELND005
Drug: Placebo Control
Phase II
Genetics Home Reference related topics: Alzheimer disease
MedlinePlus related topics: Alzheimer's Caregivers Alzheimer's Disease
Study Type: Interventional
Study Design: Treatment, Randomized, Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor), Placebo Control, Parallel Assignment, Safety/Efficacy Study
Official Title: A Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging, Safety and Efficacy Study of Oral ELND005 (AZD-103) in Alzheimer's Disease
Primary Outcome Measures:
Safety and Tolerability; Cognitive and Functional Measures [ Time Frame: 18 months ] [ Designated as safety issue: No ]
Enrollment: 340
Start Date: December 2007
Expected Completion Date: May 2010
http://clinicaltrials.gov/ct2/show/NCT00568776?spons=%22Elan…
This study is currently recruiting participants.
Verified by Elan Pharmaceuticals, December 2007
Sponsors and Collaborators: Elan Pharmaceuticals
Transition Therapeutics
Information provided by: Elan Pharmaceuticals
ClinicalTrials.gov Identifier: NCT00568776
Purpose
The purpose of this study is to evaluate the dose-related safety and efficacy of multiple oral dosages of ELND005 as treatment for Alzheimer's disease.
Condition Intervention Phase
Alzheimer Disease
Drug: ELND005
Drug: Placebo Control
Phase II
Genetics Home Reference related topics: Alzheimer disease
MedlinePlus related topics: Alzheimer's Caregivers Alzheimer's Disease
Study Type: Interventional
Study Design: Treatment, Randomized, Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor), Placebo Control, Parallel Assignment, Safety/Efficacy Study
Official Title: A Randomized, Double-Blind, Placebo-Controlled, Dose-Ranging, Safety and Efficacy Study of Oral ELND005 (AZD-103) in Alzheimer's Disease
Primary Outcome Measures:
Safety and Tolerability; Cognitive and Functional Measures [ Time Frame: 18 months ] [ Designated as safety issue: No ]
Enrollment: 340
Start Date: December 2007
Expected Completion Date: May 2010
http://clinicaltrials.gov/ct2/show/NCT00568776?spons=%22Elan…
2 - eigentlich 3 -Pressemitteilungen am Freitag nach Börsenschluss:
Den ersten Studienteilnehmer in Phase 3 wurden nun Bapineuzumab und ELND-005 appliziert - bleibt zu hoffen, dass die Rekrutierung relativ zügig voran geht:
http://www.elan.com/news/full.asp?ID=1089760
http://www.elan.com/news/full.asp?ID=1089758
Darüber hinaus hat die FDA für Luvox CR einen "Approvable Letter" verschickt, der jedoch lediglich das CMC betrifft, also nicht die Sicherheit und die Wirkung in Frage stellt sondern lediglich die Produktion betrifft, weshalb Elan direkt davon betroffen sein könnte:
The FDA requested information related to the companies' response concerning the previously disclosed CMC issue. The companies are seeking clarification from FDA and look forward to working with FDA to resolve this as quickly as possible. The approvable letter did not raise any questions related to safety or efficacy of LUVOX® CR. The approvable letter included the FDA's proposed labeling.
Separately, the FDA approved LUVOX® (fluvoxamine maleate) for the treatment of OCD on December 20, 2007.
http://biz.yahoo.com/prnews/071221/aqf081.html?.v=13
Den ersten Studienteilnehmer in Phase 3 wurden nun Bapineuzumab und ELND-005 appliziert - bleibt zu hoffen, dass die Rekrutierung relativ zügig voran geht:
http://www.elan.com/news/full.asp?ID=1089760
http://www.elan.com/news/full.asp?ID=1089758
Darüber hinaus hat die FDA für Luvox CR einen "Approvable Letter" verschickt, der jedoch lediglich das CMC betrifft, also nicht die Sicherheit und die Wirkung in Frage stellt sondern lediglich die Produktion betrifft, weshalb Elan direkt davon betroffen sein könnte:
The FDA requested information related to the companies' response concerning the previously disclosed CMC issue. The companies are seeking clarification from FDA and look forward to working with FDA to resolve this as quickly as possible. The approvable letter did not raise any questions related to safety or efficacy of LUVOX® CR. The approvable letter included the FDA's proposed labeling.
Separately, the FDA approved LUVOX® (fluvoxamine maleate) for the treatment of OCD on December 20, 2007.
http://biz.yahoo.com/prnews/071221/aqf081.html?.v=13
...spätestens am 11.1.2008 hat die FDA über die Zulassung von Ty zur Behandlung von CD (Crohns Disease) zu entscheiden. Anbei die "briefing documents", welche den Mitgliedern des AC als Entscheidungsgrundlage für deren Empfehlung gedient haben - und mit 12:3 ist das Experten-Votum eindeutig für eine Zulassung ausgefallen:
http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4313b1-…
...das neue Jahr beginnt spannend!
http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4313b1-…
...das neue Jahr beginnt spannend!
...und wieder wurden viel versprechende Patente gesichert - die Innovationen dieses Unternehmens sind unglaublich:
December 27, 2007
20070298115
Nanoparticulate fibrate formulations
Abstract
The present invention is directed to fibrate compositions having improved pharmacokinetic profiles and reduced fed/fasted variability. The fibrate particles of the composition have an effective average particle size of less than about 2000 nm.
20070299263
Kind Code A1
Freskos; John ; et al. December 27, 2007
Hydroxy Alkyl Amines
Abstract
Disclosed are compounds of formula X, which are useful in treating Alzheimer's disease and other similar diseases. Pharmaceutical compositions comprising compounds of formula X and methods of preparing the compounds of formula X are also disclosed.
20070298098
Kind Code A1
Jenkins; Scott A. ; et al. December 27, 2007
Controlled Release Compositions Comprising Levetiracetam
Abstract
The invention relates to a controlled release composition comprising levetiracetam for the treatment of epilepsy. The controlled release composition comprises an immediate release component and a modified release component or formulation. The immediate release component comprises a first population of levetiracetam and the modified release component or formulation preferably comprises a second population of levetiracetam and a controlled release constituent. The modified release formulation is preferably in the form of an erodable formulation, a diffusion controlled formulation or an osmotic controlled formulation. The combination of the immediate release component and the modified release component or formulation in operation deliver the active ingredient in a pulsed or bimodal manner.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
December 27, 2007
20070298115
Nanoparticulate fibrate formulations
Abstract
The present invention is directed to fibrate compositions having improved pharmacokinetic profiles and reduced fed/fasted variability. The fibrate particles of the composition have an effective average particle size of less than about 2000 nm.
20070299263
Kind Code A1
Freskos; John ; et al. December 27, 2007
Hydroxy Alkyl Amines
Abstract
Disclosed are compounds of formula X, which are useful in treating Alzheimer's disease and other similar diseases. Pharmaceutical compositions comprising compounds of formula X and methods of preparing the compounds of formula X are also disclosed.
20070298098
Kind Code A1
Jenkins; Scott A. ; et al. December 27, 2007
Controlled Release Compositions Comprising Levetiracetam
Abstract
The invention relates to a controlled release composition comprising levetiracetam for the treatment of epilepsy. The controlled release composition comprises an immediate release component and a modified release component or formulation. The immediate release component comprises a first population of levetiracetam and the modified release component or formulation preferably comprises a second population of levetiracetam and a controlled release constituent. The modified release formulation is preferably in the form of an erodable formulation, a diffusion controlled formulation or an osmotic controlled formulation. The combination of the immediate release component and the modified release component or formulation in operation deliver the active ingredient in a pulsed or bimodal manner.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…

What We Know, What We Don't Know about AD
http://www.alzforum.org/res/for/journal/centennial/whatwekno…
Antwort auf Beitrag Nr.: 32.846.000 von Cyberhexe am 22.12.07 01:14:29
Elan, Transition Begin Alzheimer's Trial
Ireland’s Elan and Canadian drugmaker Transition Therapeutics have dosed the first patient in a Phase II study of ELND005 in patients with Alzheimer's disease.
The randomized, double-blind, placebo-controlled, dose-ranging study enrolled approximately 340 patients with mild to moderate Alzheimer's disease.
The study will evaluate cognitive and functional endpoints, and patients will participate in the trial for approximately 18 months, according to Elan.
http://fdanews.com/newsletter/article?issueId=11169&articleI…

Elan, Transition Begin Alzheimer's Trial
Ireland’s Elan and Canadian drugmaker Transition Therapeutics have dosed the first patient in a Phase II study of ELND005 in patients with Alzheimer's disease.
The randomized, double-blind, placebo-controlled, dose-ranging study enrolled approximately 340 patients with mild to moderate Alzheimer's disease.
The study will evaluate cognitive and functional endpoints, and patients will participate in the trial for approximately 18 months, according to Elan.
http://fdanews.com/newsletter/article?issueId=11169&articleI…
AAB001 & ELN005 Dosing range on clinicaltrial
DETAILS
AAB001
Estimated Enrollment: 1250
Study Start Date: December 2007
Estimated Primary Completion Date: December 2010 (Final data collection date for primary outcome measure)
Arms Assigned Interventions
A: Active Comparator Drug: Bapineuzumab
0.5 mg/kg administered over approximately 60 minutes at a constant rate. Infusions given Q 13 weeks
B: Active Comparator Drug: Bapineuzumab
1.0 mg/kg administered approximately 60 minutes at a constant rate. Infusions given Q 13 weeks
C: Active Comparator Drug: Bapineuzumab
2.0 mg/kg administered over approximately 60 minutes at a constant rate. Infusions given Q 13 weeks
D: Placebo Comparator Drug: Placebo Control
Placebo administered over approximately 60 minutes at a constant rate. Infusions given Q 13 wks.
Estimated Enrollment: 800
Study Start Date: December 2007
Estimated Primary Completion Date: December 2010 (Final data collection date for primary outcome measure)
Arms Assigned Interventions
A: Active Comparator Drug: Bapineuzumab
0.5 mg/kg administered over approximately 60 minutes at a constant rate. Infusions given Q 13 wks.
B: Placebo Comparator Drug: Placebo Control
Placebo administered over approximately 60 minutes at a constant rate. Infusions given Q 13 wks.
ELND 005
Estimated Enrollment: 340
Study Start Date: December 2007
Estimated Study Completion Date: May 2010
Estimated Primary Completion Date: May 2010 (Final data collection date for primary outcome measure)
Arms Assigned Interventions
1: Active Comparator Drug: ELND005
250mg BID [total daily dose (TDD) = 500mg]administered orally.
2: Active Comparator Drug: ELND005
1000mg BID [total daily dose (TDD) = 2000mg] administered orally.
3: Active Comparator Drug: ELND005
2000mg BID [total daily dose (TDD) = 4000mg]administered orally.
4: Placebo Comparator Drug: Placebo Control
Placebo control administered to patients.
http://www.investorvillage.com/smbd.asp?mb=160&mn=182425&pt=…
DETAILS
AAB001
Estimated Enrollment: 1250
Study Start Date: December 2007
Estimated Primary Completion Date: December 2010 (Final data collection date for primary outcome measure)
Arms Assigned Interventions
A: Active Comparator Drug: Bapineuzumab
0.5 mg/kg administered over approximately 60 minutes at a constant rate. Infusions given Q 13 weeks
B: Active Comparator Drug: Bapineuzumab
1.0 mg/kg administered approximately 60 minutes at a constant rate. Infusions given Q 13 weeks
C: Active Comparator Drug: Bapineuzumab
2.0 mg/kg administered over approximately 60 minutes at a constant rate. Infusions given Q 13 weeks
D: Placebo Comparator Drug: Placebo Control
Placebo administered over approximately 60 minutes at a constant rate. Infusions given Q 13 wks.
Estimated Enrollment: 800
Study Start Date: December 2007
Estimated Primary Completion Date: December 2010 (Final data collection date for primary outcome measure)
Arms Assigned Interventions
A: Active Comparator Drug: Bapineuzumab
0.5 mg/kg administered over approximately 60 minutes at a constant rate. Infusions given Q 13 wks.
B: Placebo Comparator Drug: Placebo Control
Placebo administered over approximately 60 minutes at a constant rate. Infusions given Q 13 wks.
ELND 005
Estimated Enrollment: 340
Study Start Date: December 2007
Estimated Study Completion Date: May 2010
Estimated Primary Completion Date: May 2010 (Final data collection date for primary outcome measure)
Arms Assigned Interventions
1: Active Comparator Drug: ELND005
250mg BID [total daily dose (TDD) = 500mg]administered orally.
2: Active Comparator Drug: ELND005
1000mg BID [total daily dose (TDD) = 2000mg] administered orally.
3: Active Comparator Drug: ELND005
2000mg BID [total daily dose (TDD) = 4000mg]administered orally.
4: Placebo Comparator Drug: Placebo Control
Placebo control administered to patients.
http://www.investorvillage.com/smbd.asp?mb=160&mn=182425&pt=…
 .......jetzt wird für die AD Marketing-Strategie schon ein Senior Director gesucht......
.......jetzt wird für die AD Marketing-Strategie schon ein Senior Director gesucht......
Elan Job Listing
Job Title: Director/Senior Director, Strategic Marketing
Req #: elan-00001227
Location: Dublin DUB
"Director/Senior Director, Strategic Marketing
Primary Objective:
The primary role of this key leadership position will be to provide a balance of both strategic marketing expertise, as well as, the requisite commercial operational support to prepare the company for its first new product launch in the International AD marketplace.
Duties and Responibilites:
-Partner with the VP's to develop and implement an innovative strategic sales and marketing plan designed to drive revenue for the company and build the requisite internal commercial infrastructure to support the upcoming launch of Élan's new AD product..."
Develop, a comprehensive understanding of the global Alzheimer's marketplace, including an opportunity assessment which can serve as the basis for creation of Elan's major market strategy in AD.
-Work collaboratively with Elan commercial strategic marketing counterparts, as well as other key departments, to provide valuable commercial input, analytical support and strategic thought leadership on the future development of new products in Élan's AD portfolio.
-Formulate and execute a strategy to enhance Élan's position in the biopharmaceutical marketplace as the premier research and development organization in AD.
-Assume responsibility for directing and monitoring all third party contractors and vendors to ensure maximum ROI.
-Provide direction to field-based personnel to assure optimal customer interaction and seamless execution of communication programs.
-Ensure that the strategic marketing function has the highest level of visibility both internally, as well as with external corporate customers.
-Work collaboratively with human resources to recruit and manage a team of high performance marketing executives.
http://sh.webhire.com/servlet/av/jd?ai=195&ji=2151932&sn=I
Biogen Idec and Elan Provide Update on Utilization, Safety and Total Patient Exposure of TYSABRI(R) in Patients with Multiple Sclerosis
Monday January 7, 2:00 am ET
-- More than 21,000 patients on commercial and clinical therapy worldwide --
http://www.investorvillage.com/smbd.asp?mb=160&pt=qn
Monday January 7, 2:00 am ET
-- More than 21,000 patients on commercial and clinical therapy worldwide --
http://www.investorvillage.com/smbd.asp?mb=160&pt=qn
@Cyberhexe
Was hälst du von diesem Artikel ?????
Drug 'can reverse Alzheimer's symptoms in minutes'
Last updated at 22:07pm on 10.01.08
Add your view
Alzheimer's affects 700,000 Britons
A drug used for arthritis can reverse the symptoms of Alzheimer's "in minutes".
It appears to tackle one of the main features of the disease - inflammation in the brain.
The drug, called Enbrel, is injected into the spine where it blocks a chemical responsible for damaging the brain and other organs.
A pilot study carried out by U.S. researchers found one patient had his symptoms reversed "in minutes". Other patients have shown some improvements in symptoms such as forgetfulness and confusion after weekly injections over six months.
The study of 15 patients with moderate to severe Alzheimer's has just been published in the Journal of Neuroinflammation by online publishers Biomed Central.
The experiment showed that Enbrel can deactivate TNF (tumour necrosis factor) - a chemical in the fluid surrounding the brain that is found in Alzheimer's sufferers.
When used by arthritis sufferers, the drug is self-administered by injection and researchers had to develop a way of injecting it into the spine to affect the brain cells.
Sue Griffin, a researcher at the University of Arkansas for Medical Sciences, said: 'It is unprecedented to see cognitive and behavioural improvement in a patient with established dementia within minutes of therapeutic intervention.
'This gives all of us in Alzheimer research a tremendous new clue
about new avenues of research.' Enbrel is not approved for treating Alzheimer's in the U.S. or the UK and is regarded as highly experimental, said Dr Griffin.
'Even though this report predominantly discusses a single patient it is of significant scientific interest because of the potential insight it may give into the processes involved in the brain dysfunction of Alzheimer's,' she added.
Lead author of the study Edward Tobinick, of the University of California and Director of the Institute for Neurological Research, said the drug had 'a very rapid effect that's never been reported in a human being before'.
He added: 'It makes practical changes that are significant and perceptible, making a difference to his daily living.
'Some patients have been able to start driving again. They don't come back to normal but the change is good enough for patients to want to continue treatment.'
He said top-up injections were necessary but some patients had them a month apart.
Alzheimer's is the most common cause of dementia, affecting more than 700,000 Britons with about 500 cases diagnosed every day.
Neil Hunt, of the Alzheimer's Society charity, said: 'The pursuit of a miracle cure for Alzheimer's continues to drive research into a variety of potential treatment targets.
These include a possible link between inflammatory reactions in the brain and Alzheimer's.'
Children exposed to lead in old paint, Victorian pipes and toys could be at risk of Alzheimer's later in life, scientists said yesterday.
A study shows that even small amounts of the metal in the first few years can build up plaques around the brain.
Scientists at the University of Rhode Island told the New Scientist that they fed infant formula milk laced with low doses of lead to baby monkeys, then followed their progress for 23 years. A post mortem of the brains revealed plaques - harmful deposits of protein found in Alzheimer's patients
http://www.thisislondon.co.uk/news/article-23431610-details/…
Was hälst du von diesem Artikel ?????
Drug 'can reverse Alzheimer's symptoms in minutes'
Last updated at 22:07pm on 10.01.08
Add your view
Alzheimer's affects 700,000 Britons
A drug used for arthritis can reverse the symptoms of Alzheimer's "in minutes".
It appears to tackle one of the main features of the disease - inflammation in the brain.
The drug, called Enbrel, is injected into the spine where it blocks a chemical responsible for damaging the brain and other organs.
A pilot study carried out by U.S. researchers found one patient had his symptoms reversed "in minutes". Other patients have shown some improvements in symptoms such as forgetfulness and confusion after weekly injections over six months.
The study of 15 patients with moderate to severe Alzheimer's has just been published in the Journal of Neuroinflammation by online publishers Biomed Central.
The experiment showed that Enbrel can deactivate TNF (tumour necrosis factor) - a chemical in the fluid surrounding the brain that is found in Alzheimer's sufferers.
When used by arthritis sufferers, the drug is self-administered by injection and researchers had to develop a way of injecting it into the spine to affect the brain cells.
Sue Griffin, a researcher at the University of Arkansas for Medical Sciences, said: 'It is unprecedented to see cognitive and behavioural improvement in a patient with established dementia within minutes of therapeutic intervention.
'This gives all of us in Alzheimer research a tremendous new clue
about new avenues of research.' Enbrel is not approved for treating Alzheimer's in the U.S. or the UK and is regarded as highly experimental, said Dr Griffin.
'Even though this report predominantly discusses a single patient it is of significant scientific interest because of the potential insight it may give into the processes involved in the brain dysfunction of Alzheimer's,' she added.
Lead author of the study Edward Tobinick, of the University of California and Director of the Institute for Neurological Research, said the drug had 'a very rapid effect that's never been reported in a human being before'.
He added: 'It makes practical changes that are significant and perceptible, making a difference to his daily living.
'Some patients have been able to start driving again. They don't come back to normal but the change is good enough for patients to want to continue treatment.'
He said top-up injections were necessary but some patients had them a month apart.
Alzheimer's is the most common cause of dementia, affecting more than 700,000 Britons with about 500 cases diagnosed every day.
Neil Hunt, of the Alzheimer's Society charity, said: 'The pursuit of a miracle cure for Alzheimer's continues to drive research into a variety of potential treatment targets.
These include a possible link between inflammatory reactions in the brain and Alzheimer's.'
Children exposed to lead in old paint, Victorian pipes and toys could be at risk of Alzheimer's later in life, scientists said yesterday.
A study shows that even small amounts of the metal in the first few years can build up plaques around the brain.
Scientists at the University of Rhode Island told the New Scientist that they fed infant formula milk laced with low doses of lead to baby monkeys, then followed their progress for 23 years. A post mortem of the brains revealed plaques - harmful deposits of protein found in Alzheimer's patients
http://www.thisislondon.co.uk/news/article-23431610-details/…
Antwort auf Beitrag Nr.: 33.014.174 von bernie55 am 10.01.08 23:41:20...es ist äusserst schwierig aus der Ferne derartige Nachrichten richtig einzuordnen, ich bin mir jedoch sicher, dass eine erweiterte Anwendung von Enbrel zuerst einer wissenschaftlichen Überprüfung in umfangreichen klinischen Studien Stand halten müsste - ich vermute dahinter eher taktische Störmanöver, weil Elan bei Alzheimer gegenüber Wyeth eindeutig besser aufgestellt ist...und früher oder später wird Wyeth versuchen, den "Juniorpartner" zu schlucken.
Antwort auf Beitrag Nr.: 33.015.137 von Cyberhexe am 11.01.08 08:22:42@ Cyberhexe
Myriad Genetics scheint da schon ein bisschen weiter zu sein.......
.... der große Konkurrent für ELAN / WYETH ????
Could Elan (ELN) Be A Blockbuster Biotech Company in 2008?
Also Keep An Eye On Myriad Genetics Inc. (MYGN).
Submitted by Biotechman and Optiondragon
http://www.investorvillage.com/smbd.asp?mb=160&mn=185674&pt=…
unter anderem steht in diesem Artikel:
On a competition note and alert, Myriad Genetics(MYGN) is experimenting with Flurizan which also targets the amyloid plaque. They are considered the front runner in this disease space due to their lead time with completion of their Phase III by March 2008 and the data release of top-line results in late 2nd quarter, around mid 2008. The study is the largest study ever for Alzheimer’s and has nearly 1,700 patients. Since this trial is a Phase III, MYGN can file for FDA approval on positive results and a very low P-value (less than 0.001) on the results, which indicates that the data is statistically significant. Ian Sanderson, analyst for Cowen and Co., said that Flurizan will probably be the first among the next generation of Alzheimer’s drugs to go before the FDA. He believes it could reach $600 million in annual sales by 2012 and would eventually peak at $1.2 billion.
Myriad Genetics scheint da schon ein bisschen weiter zu sein.......
.... der große Konkurrent für ELAN / WYETH ????
Could Elan (ELN) Be A Blockbuster Biotech Company in 2008?
Also Keep An Eye On Myriad Genetics Inc. (MYGN).
Submitted by Biotechman and Optiondragon
http://www.investorvillage.com/smbd.asp?mb=160&mn=185674&pt=…
unter anderem steht in diesem Artikel:
On a competition note and alert, Myriad Genetics(MYGN) is experimenting with Flurizan which also targets the amyloid plaque. They are considered the front runner in this disease space due to their lead time with completion of their Phase III by March 2008 and the data release of top-line results in late 2nd quarter, around mid 2008. The study is the largest study ever for Alzheimer’s and has nearly 1,700 patients. Since this trial is a Phase III, MYGN can file for FDA approval on positive results and a very low P-value (less than 0.001) on the results, which indicates that the data is statistically significant. Ian Sanderson, analyst for Cowen and Co., said that Flurizan will probably be the first among the next generation of Alzheimer’s drugs to go before the FDA. He believes it could reach $600 million in annual sales by 2012 and would eventually peak at $1.2 billion.
Antwort auf Beitrag Nr.: 33.018.389 von bernie55 am 11.01.08 12:32:50@Cyberhexe
..hier wieder ein Artikel, der ELAN / WYETH " vorne " sieht....
CSFB Report -leaders in this approach are WYE/ELN’s bapineuzumab
02 January 2008
unter anderem steht in diesem Artikel:
http://www.investorvillage.com/smbd.asp?mb=160&mn=185272&pt=…
■ Our panelists were also optimistic about the potential for gamma secretase modulators and other drugs that alter the Aβ 42/40 ratio, but felt that Myriad Genetic’s Flurizan may not be potent enough to have a significant benefit. Each panelist expressed some concern about the safety of therapies that completely inhibit enzymes involved in generating Aβ (e.g. gamma and beta secretase inhihitors) because these enzymes have other important functions in the body, but they anxiously await more data.
=======================
some of the discussion follows
Catherine Arnold:
Dr. Aisen, why don't we move to passive immunotherapy. And obviously bapineuzumab is what's coming most here. As far as results in hand for Phase II by mid '08, starting a Phase III program. Contrast your view, active versus passive, and what you think of bapineuzumab's success.
Paul Aisen:
Well I'm also very interested in passive immunotherapy. Again if you believe the amyloid hypothesis has merit, and you see the results of active vaccination, you want to build on those results. And that includes passive immunotherapy with monoclonals, passive immunotherapy with pulled human immunoglobulin, and active vaccination. And I'm pleased that all of those programs are moving forward vigorously. Specifically with bapineuzumab, it does mimic what is likely to have been the amyloid clearing type of antibody produced in the active vaccination. And that's in it's favor. The signal that was seen in Phase 1 was very interesting and intriguing. It supports the idea that Rudy put forward, that amyloid is a synaptic toxin. And tying up amyloid will have an acute beneficial effect.
My guess is that although the numbers are so tiny. It's certainly - one can't be confident. But my guess is there was a real systematic improvement. That is being confirmed in Phase II. And will be shown to be real in Phase III. So of all the anti amyloid approaches, perhaps the strongest evidence of clinical benefit today comes from bapineuzumab. So I think there are safety issues related to vaccination. And the angioedema episodes, which fortunately don't seem to be so serious, but are of some concern. But also good evidence of the possibility of a favorable clinical effect. So I would give bapineuzumab a 4.3
..hier wieder ein Artikel, der ELAN / WYETH " vorne " sieht....
CSFB Report -leaders in this approach are WYE/ELN’s bapineuzumab
02 January 2008
unter anderem steht in diesem Artikel:
http://www.investorvillage.com/smbd.asp?mb=160&mn=185272&pt=…
■ Our panelists were also optimistic about the potential for gamma secretase modulators and other drugs that alter the Aβ 42/40 ratio, but felt that Myriad Genetic’s Flurizan may not be potent enough to have a significant benefit. Each panelist expressed some concern about the safety of therapies that completely inhibit enzymes involved in generating Aβ (e.g. gamma and beta secretase inhihitors) because these enzymes have other important functions in the body, but they anxiously await more data.
=======================
some of the discussion follows
Catherine Arnold:
Dr. Aisen, why don't we move to passive immunotherapy. And obviously bapineuzumab is what's coming most here. As far as results in hand for Phase II by mid '08, starting a Phase III program. Contrast your view, active versus passive, and what you think of bapineuzumab's success.
Paul Aisen:
Well I'm also very interested in passive immunotherapy. Again if you believe the amyloid hypothesis has merit, and you see the results of active vaccination, you want to build on those results. And that includes passive immunotherapy with monoclonals, passive immunotherapy with pulled human immunoglobulin, and active vaccination. And I'm pleased that all of those programs are moving forward vigorously. Specifically with bapineuzumab, it does mimic what is likely to have been the amyloid clearing type of antibody produced in the active vaccination. And that's in it's favor. The signal that was seen in Phase 1 was very interesting and intriguing. It supports the idea that Rudy put forward, that amyloid is a synaptic toxin. And tying up amyloid will have an acute beneficial effect.
My guess is that although the numbers are so tiny. It's certainly - one can't be confident. But my guess is there was a real systematic improvement. That is being confirmed in Phase II. And will be shown to be real in Phase III. So of all the anti amyloid approaches, perhaps the strongest evidence of clinical benefit today comes from bapineuzumab. So I think there are safety issues related to vaccination. And the angioedema episodes, which fortunately don't seem to be so serious, but are of some concern. But also good evidence of the possibility of a favorable clinical effect. So I would give bapineuzumab a 4.3
FDA Approves TYSABRI(R) for the Treatment of Moderate-to-Severe Crohn's Disease
Elan Corporation, plc (NYSE: ELN) and Biogen Idec (NASDAQ: BIIB) today announced the approval of a supplemental Biologics License Application (sBLA) by the U.S. Food and Drug Administration (FDA) for TYSABRI® (natalizumab). TYSABRI is now approved for inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn’s disease (CD) with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional CD therapies and inhibitors of TNF-alpha. TYSABRI will be available for the treatment of CD upon the completion of key implementation activities related to the approved risk management plan. The companies anticipate TYSABRI will be available to Crohn’s patients by the end of February 2008.
"The FDA's approval of TYSABRI is an important step forward in the treatment of Crohn's disease," said Dr. Stephen Hanauer, Professor of Medicine & Clinical Pharmacology & Chief of the Section of Gastroenterology at the University of Chicago Pritzker School of Medicine. "A significant number of patients either fail or cannot tolerate current therapies. The unique mechanism of action of TYSABRI affords us a new class of therapy in our fight against this debilitating disease."
The FDA granted approval based on its review of TYSABRI CD clinical trial data and overall safety data. The approval is accompanied by robust labeling with safety warnings; and a CD-specific risk management plan (including the mandatory TOUCH™ Prescribing Program) designed to inform prescribers, patients and infusion centers about the use of TYSABRI and to minimize potential risk of progressive multifocal leukoencephalopathy (PML) and other opportunistic infections.
“We are delighted that TYSABRI will be available for Crohn’s patients and their physicians, who continue to need new therapeutic options with novel mechanisms of action,” said Gordon Francis, MD, Senior Vice President, Global Clinical Development, Elan. “We are committed to providing therapeutic choice to those patients who can benefit from TYSABRI, and will continue to work with the FDA and the medical community to implement the TOUCH™ Prescribing Program for Crohn’s patients.”
“We are pleased with the FDA’s decision to make TYSABRI available to Crohn’s patients suffering from this chronic, debilitating disease," said Evan Beckman, MD, Senior Vice President, Immunology Research and Development, Biogen Idec. “Despite the therapeutic advances of the TNF-alpha inhibitors in CD, there remains a significant unmet need for Crohn’s patients who have inadequate responses to, or are unable to tolerate, current CD therapies.”
TOUCH™ Prescribing Program
The TOUCH™ (TYSABRI Outreach: Unified Commitment to Health) Prescribing Program was developed in conjunction with the FDA to facilitate appropriate use of TYSABRI and to assess, on an ongoing basis, the incidence and risk factors for PML and other serious opportunistic infections associated with TYSABRI treatment. This program represents Elan and Biogen Idec’s commitment to making the unique benefits of TYSABRI available in a responsible manner. The program already has been implemented for patients receiving TYSABRI therapy for MS.
About Crohn’s Disease
An estimated 500,000 people in the United States have Crohn's disease, a chronic and progressive inflammatory disease of the gastrointestinal tract, which commonly affects both men and women.
The disease usually causes diarrhea and crampy abdominal pain, often associated with fever, and at times rectal bleeding. Loss of appetite and weight loss also may occur. Complications include narrowing of the intestine, obstruction, abscesses, and fistulas (abnormal channels connecting the intestine and other organs, including the skin), and malnutrition. Most patients eventually require surgery, which has both risks and potential short- and long-term complications.
Crohn’s disease can have a devastating impact on the lifestyle of patients, many of whom are young and active. Currently there is no medical or surgical cure for Crohn’s disease. Many patients fail to respond to current therapies, including biological therapies such as agents that inhibit tumor necrosis factor alpha (TNF-alpha). Due to this failure of current therapies in CD, therapies that have alternate biological targets provide patients and physicians with therapeutic options.
About TYSABRI
Data from the ENCORE trial showed that TYSABRI induced response and remission among patients with moderately to severely active Crohn’s disease, and objective evidence of inflammation, as measured by elevated C-reactive protein. After 12 weeks of therapy, 60% of TYSABRI-treated patients attained response, compared to 44% of placebo treated patients, and 48% of patients had sustained response at both weeks 8 and 12, compared to 32% of placebo treated patients (p<0.005 for both). Among the patients who had inadequate response to prior treatment with inhibitors of TNF-alpha, 38% achieved sustained response at weeks 8 and 12.
Data from the ENACT-2 showed that an additional year of TYSABRI therapy sustained response and remission among patients with an initial response to TYSABRI after 3 months in ENACT-1. Of patients with response in ENACT-1, sustained response during ENACT-2 was seen in 61% of patients treated with TYSABRI at every visit through an additional 6 months of therapy, compared to 29% for placebo. This treatment difference was also sustained through 12 months of additional therapy (54% vs. 20%). Remission was sustained at every visit with an additional 6 months or 12 months of TYSABRI in 45% and 40% of patients, respectively, compared to 26% and 15% of placebo treated patients (p<0.005 for all comparisons). Among the patients that had previously failed TNF-inhibitors, response and remission was sustained at every visit through an additional 6 months of TYSABRI in 52% and 30% of patients, respectively. Among patients on steroids and in whom a clinical response was achieved, approximately two-thirds were able to discontinue steroids within 10 weeks of beginning to taper steroids.
TYSABRI increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability. Other serious adverse events that have occurred in TYSABRI-treated patients included hypersensitivity reactions (e.g., anaphylaxis) and infections. Serious opportunistic and other atypical infections have been observed in TYSABRI-treated patients, some of whom were receiving concurrent immunosuppressants. Herpes infections were slightly more common in patients treated with TYSABRI. In MS and CD clinical trials, the incidence and rate of other serious adverse events, including serious infections, were similar in patients receiving TYSABRI and those receiving placebo. Common adverse events reported in TYSABRI-treated MS patients include headache, fatigue, infusion reactions, urinary tract infections, joint and limb pain, and rash. Other common adverse events reported in TYSABRI-treated CD patients include respiratory tract infections and nausea. Clinically significant liver injury has been reported in patients treated with TYSABRI in the post-marketing setting.
TYSABRI has previously been approved for relapsing forms of MS in the United States and relapsing-remitting MS in the European Union. According to data that have been published in the New England Journal of Medicine, after two years, TYSABRI treatment led to a 68% relative reduction (p<0.001) in the annualized relapse rate compared to placebo and reduced the relative risk of disability progression by 42-54% (p<0.001). In addition to the United States and European Union, TYSABRI is also approved for MS in Switzerland, Canada, Australia, New Zealand and Israel. TYSABRI was discovered by Elan and is co-developed with Biogen Idec.
For more information about TYSABRI please visit www.tysabri.com, www.biogenidec.com or www.elan.com, or call 1-800-456-2255.
About Elan
Elan Corporation, plc is a neuroscience-based biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. Elan shares trade on the New York, London and Dublin Stock Exchanges. For additional information about the company, please visit www.elan.com.
About Biogen Idec
Biogen Idec creates new standards of care in therapeutic areas with high unmet medical needs. Founded in 1978, Biogen Idec is a global leader in the discovery, development, manufacturing, and commercialization of innovative therapies. Patients in more than 90 countries benefit from Biogen Idec’s significant products that address diseases such as lymphoma, multiple sclerosis, and rheumatoid arthritis. For product labeling, press releases and additional information about the company, please visit www.biogenidec.com.
Safe Harbor/Forward-Looking Statements
This press release contains forward-looking statements regarding TYSABRI. These statements are based on the companies’ current beliefs and expectations. The commercial potential of TYSABRI is subject to a number of risks and uncertainties. Factors which could cause actual results to differ materially from the companies’ current expectations include the risk that we may be unable to adequately address concerns or questions raised by FDA or other regulatory authorities, that concerns may arise from additional data, that the incidence and/or risk of PML or other opportunistic infections in patients treated with TYSABRI may be higher than observed in clinical trials, or that the companies may encounter other unexpected hurdles. Drug development and commercialization involves a high degree of risk.
For more detailed information on the risks and uncertainties associated with the companies’ drug development and other activities, see the periodic and current reports that Biogen Idec and Elan have filed with the Securities and Exchange Commission. The companies assume no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Media:
Elan
Jonathan Birt, 212 850 5664
Elizabeth Headon, 353 1 498 0300
or
Biogen Idec
Amy Reilly, 617 914 6524
or
Investors:
Elan
Chris Burns, 353 1 709 4444
800 252 3526
or
Biogen Idec
Eric Hoffman, 617 679 2812
http://www.investorvillage.com/smbd.asp?mb=160&pt=qn
Elan Corporation, plc (NYSE: ELN) and Biogen Idec (NASDAQ: BIIB) today announced the approval of a supplemental Biologics License Application (sBLA) by the U.S. Food and Drug Administration (FDA) for TYSABRI® (natalizumab). TYSABRI is now approved for inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn’s disease (CD) with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional CD therapies and inhibitors of TNF-alpha. TYSABRI will be available for the treatment of CD upon the completion of key implementation activities related to the approved risk management plan. The companies anticipate TYSABRI will be available to Crohn’s patients by the end of February 2008.
"The FDA's approval of TYSABRI is an important step forward in the treatment of Crohn's disease," said Dr. Stephen Hanauer, Professor of Medicine & Clinical Pharmacology & Chief of the Section of Gastroenterology at the University of Chicago Pritzker School of Medicine. "A significant number of patients either fail or cannot tolerate current therapies. The unique mechanism of action of TYSABRI affords us a new class of therapy in our fight against this debilitating disease."
The FDA granted approval based on its review of TYSABRI CD clinical trial data and overall safety data. The approval is accompanied by robust labeling with safety warnings; and a CD-specific risk management plan (including the mandatory TOUCH™ Prescribing Program) designed to inform prescribers, patients and infusion centers about the use of TYSABRI and to minimize potential risk of progressive multifocal leukoencephalopathy (PML) and other opportunistic infections.
“We are delighted that TYSABRI will be available for Crohn’s patients and their physicians, who continue to need new therapeutic options with novel mechanisms of action,” said Gordon Francis, MD, Senior Vice President, Global Clinical Development, Elan. “We are committed to providing therapeutic choice to those patients who can benefit from TYSABRI, and will continue to work with the FDA and the medical community to implement the TOUCH™ Prescribing Program for Crohn’s patients.”
“We are pleased with the FDA’s decision to make TYSABRI available to Crohn’s patients suffering from this chronic, debilitating disease," said Evan Beckman, MD, Senior Vice President, Immunology Research and Development, Biogen Idec. “Despite the therapeutic advances of the TNF-alpha inhibitors in CD, there remains a significant unmet need for Crohn’s patients who have inadequate responses to, or are unable to tolerate, current CD therapies.”
TOUCH™ Prescribing Program
The TOUCH™ (TYSABRI Outreach: Unified Commitment to Health) Prescribing Program was developed in conjunction with the FDA to facilitate appropriate use of TYSABRI and to assess, on an ongoing basis, the incidence and risk factors for PML and other serious opportunistic infections associated with TYSABRI treatment. This program represents Elan and Biogen Idec’s commitment to making the unique benefits of TYSABRI available in a responsible manner. The program already has been implemented for patients receiving TYSABRI therapy for MS.
About Crohn’s Disease
An estimated 500,000 people in the United States have Crohn's disease, a chronic and progressive inflammatory disease of the gastrointestinal tract, which commonly affects both men and women.
The disease usually causes diarrhea and crampy abdominal pain, often associated with fever, and at times rectal bleeding. Loss of appetite and weight loss also may occur. Complications include narrowing of the intestine, obstruction, abscesses, and fistulas (abnormal channels connecting the intestine and other organs, including the skin), and malnutrition. Most patients eventually require surgery, which has both risks and potential short- and long-term complications.
Crohn’s disease can have a devastating impact on the lifestyle of patients, many of whom are young and active. Currently there is no medical or surgical cure for Crohn’s disease. Many patients fail to respond to current therapies, including biological therapies such as agents that inhibit tumor necrosis factor alpha (TNF-alpha). Due to this failure of current therapies in CD, therapies that have alternate biological targets provide patients and physicians with therapeutic options.
About TYSABRI
Data from the ENCORE trial showed that TYSABRI induced response and remission among patients with moderately to severely active Crohn’s disease, and objective evidence of inflammation, as measured by elevated C-reactive protein. After 12 weeks of therapy, 60% of TYSABRI-treated patients attained response, compared to 44% of placebo treated patients, and 48% of patients had sustained response at both weeks 8 and 12, compared to 32% of placebo treated patients (p<0.005 for both). Among the patients who had inadequate response to prior treatment with inhibitors of TNF-alpha, 38% achieved sustained response at weeks 8 and 12.
Data from the ENACT-2 showed that an additional year of TYSABRI therapy sustained response and remission among patients with an initial response to TYSABRI after 3 months in ENACT-1. Of patients with response in ENACT-1, sustained response during ENACT-2 was seen in 61% of patients treated with TYSABRI at every visit through an additional 6 months of therapy, compared to 29% for placebo. This treatment difference was also sustained through 12 months of additional therapy (54% vs. 20%). Remission was sustained at every visit with an additional 6 months or 12 months of TYSABRI in 45% and 40% of patients, respectively, compared to 26% and 15% of placebo treated patients (p<0.005 for all comparisons). Among the patients that had previously failed TNF-inhibitors, response and remission was sustained at every visit through an additional 6 months of TYSABRI in 52% and 30% of patients, respectively. Among patients on steroids and in whom a clinical response was achieved, approximately two-thirds were able to discontinue steroids within 10 weeks of beginning to taper steroids.
TYSABRI increases the risk of progressive multifocal leukoencephalopathy (PML), an opportunistic viral infection of the brain that usually leads to death or severe disability. Other serious adverse events that have occurred in TYSABRI-treated patients included hypersensitivity reactions (e.g., anaphylaxis) and infections. Serious opportunistic and other atypical infections have been observed in TYSABRI-treated patients, some of whom were receiving concurrent immunosuppressants. Herpes infections were slightly more common in patients treated with TYSABRI. In MS and CD clinical trials, the incidence and rate of other serious adverse events, including serious infections, were similar in patients receiving TYSABRI and those receiving placebo. Common adverse events reported in TYSABRI-treated MS patients include headache, fatigue, infusion reactions, urinary tract infections, joint and limb pain, and rash. Other common adverse events reported in TYSABRI-treated CD patients include respiratory tract infections and nausea. Clinically significant liver injury has been reported in patients treated with TYSABRI in the post-marketing setting.
TYSABRI has previously been approved for relapsing forms of MS in the United States and relapsing-remitting MS in the European Union. According to data that have been published in the New England Journal of Medicine, after two years, TYSABRI treatment led to a 68% relative reduction (p<0.001) in the annualized relapse rate compared to placebo and reduced the relative risk of disability progression by 42-54% (p<0.001). In addition to the United States and European Union, TYSABRI is also approved for MS in Switzerland, Canada, Australia, New Zealand and Israel. TYSABRI was discovered by Elan and is co-developed with Biogen Idec.
For more information about TYSABRI please visit www.tysabri.com, www.biogenidec.com or www.elan.com, or call 1-800-456-2255.
About Elan
Elan Corporation, plc is a neuroscience-based biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. Elan shares trade on the New York, London and Dublin Stock Exchanges. For additional information about the company, please visit www.elan.com.
About Biogen Idec
Biogen Idec creates new standards of care in therapeutic areas with high unmet medical needs. Founded in 1978, Biogen Idec is a global leader in the discovery, development, manufacturing, and commercialization of innovative therapies. Patients in more than 90 countries benefit from Biogen Idec’s significant products that address diseases such as lymphoma, multiple sclerosis, and rheumatoid arthritis. For product labeling, press releases and additional information about the company, please visit www.biogenidec.com.
Safe Harbor/Forward-Looking Statements
This press release contains forward-looking statements regarding TYSABRI. These statements are based on the companies’ current beliefs and expectations. The commercial potential of TYSABRI is subject to a number of risks and uncertainties. Factors which could cause actual results to differ materially from the companies’ current expectations include the risk that we may be unable to adequately address concerns or questions raised by FDA or other regulatory authorities, that concerns may arise from additional data, that the incidence and/or risk of PML or other opportunistic infections in patients treated with TYSABRI may be higher than observed in clinical trials, or that the companies may encounter other unexpected hurdles. Drug development and commercialization involves a high degree of risk.
For more detailed information on the risks and uncertainties associated with the companies’ drug development and other activities, see the periodic and current reports that Biogen Idec and Elan have filed with the Securities and Exchange Commission. The companies assume no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Media:
Elan
Jonathan Birt, 212 850 5664
Elizabeth Headon, 353 1 498 0300
or
Biogen Idec
Amy Reilly, 617 914 6524
or
Investors:
Elan
Chris Burns, 353 1 709 4444
800 252 3526
or
Biogen Idec
Eric Hoffman, 617 679 2812
http://www.investorvillage.com/smbd.asp?mb=160&pt=qn
Crohn's Disease:
http://www.digestive.niddk.nih.gov/ddiseases/pubs/crohns/ind…
FDA Approves Tysabri to Treat Moderate-to-Severe Crohn's Disease
Drug currently approved for use in treating some forms of multiple sclerosis
http://www.fda.gov/bbs/topics/NEWS/2008/NEW01775.html
http://www.digestive.niddk.nih.gov/ddiseases/pubs/crohns/ind…
FDA Approves Tysabri to Treat Moderate-to-Severe Crohn's Disease
Drug currently approved for use in treating some forms of multiple sclerosis
http://www.fda.gov/bbs/topics/NEWS/2008/NEW01775.html
Antwort auf Beitrag Nr.: 33.014.174 von bernie55 am 10.01.08 23:41:20
E N B R E L
Breakthrough or False Hope?
Etanercept [Enbrel for AD] Case Report [by Edward Tobinick] Draws Scrutiny
Resummee aus dem Artikel....sogar AMGEN der " MAcher von ENbrel " distanziert sich von dieser " Pseudostudie "..
“This study was not supported nor endorsed by Amgen. While Amgen and others have long recognized the potential for TNF inhibitors to have an effect on neurological conditions, we have carefully examined this study and believe that at this time there is insufficient scientific data to support the use of a TNF inhibitor [e.g. Enbrel] as a means of treating Alzheimer's disease.”
http://www.alzforum.org/new/detail.asp?id=1738
E N B R E L
Breakthrough or False Hope?
Etanercept [Enbrel for AD] Case Report [by Edward Tobinick] Draws Scrutiny
Resummee aus dem Artikel....sogar AMGEN der " MAcher von ENbrel " distanziert sich von dieser " Pseudostudie "..
“This study was not supported nor endorsed by Amgen. While Amgen and others have long recognized the potential for TNF inhibitors to have an effect on neurological conditions, we have carefully examined this study and believe that at this time there is insufficient scientific data to support the use of a TNF inhibitor [e.g. Enbrel] as a means of treating Alzheimer's disease.”
http://www.alzforum.org/new/detail.asp?id=1738
Effect of LY451039 on the Long Term Progression of Alzheimer's Disease
This study is not yet open for patient recruitment.
Verified by Eli Lilly and Company January 2008
Sponsored by: Eli Lilly and Company
Information provided by: Eli Lilly and Company
ClinicalTrials.gov Identifier: NCT00594568
Study start : March 2008
Study completion : March 2012
http://www.clinicaltrialsplus.com/dms/index.jsp?RQ_mode=view…
This study is not yet open for patient recruitment.
Verified by Eli Lilly and Company January 2008
Sponsored by: Eli Lilly and Company
Information provided by: Eli Lilly and Company
ClinicalTrials.gov Identifier: NCT00594568
Study start : March 2008
Study completion : March 2012
http://www.clinicaltrialsplus.com/dms/index.jsp?RQ_mode=view…
Antwort auf Beitrag Nr.: 33.196.700 von bernie55 am 28.01.08 22:58:12Effect of LY451039 on the Long Term Progression of Alzheimer's Disease
http://www.clinicaltrials.gov/ct2/show/NCT00594568?cond=alzh…
http://www.clinicaltrials.gov/ct2/show/NCT00594568?cond=alzh…
Antwort auf Beitrag Nr.: 32.901.405 von Cyberhexe am 30.12.07 18:57:34..und weiter geht es mit neuen Patenten:
January 17, 2008
20080014194
Prevention and Treatment of Synucleinopathic and Amyloidogenic Disease
Abstract
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
January 31, 2008
20080025807
SYSTEM AND METHOD FOR MILLING MATERIALS
Abstract
A system for milling at least one material, e.g., a drug. The system includes a milling apparatus and at least one milling medium. The milling apparatus includes a chamber having a rotary milling head located in it. The milling head is rotated within the chamber by a magnetic drive system.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
January 17, 2008
20080014194
Prevention and Treatment of Synucleinopathic and Amyloidogenic Disease
Abstract
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body. The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
January 31, 2008
20080025807
SYSTEM AND METHOD FOR MILLING MATERIALS
Abstract
A system for milling at least one material, e.g., a drug. The system includes a milling apparatus and at least one milling medium. The milling apparatus includes a chamber having a rotary milling head located in it. The milling head is rotated within the chamber by a magnetic drive system.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
moin moin , das baby entwickelt sich ja prächtig! 

 Northern Ireland Gives OK to Tysabri
Northern Ireland Gives OK to Tysabri 
Vital MS drug given green light in Ulster
Tuesday, February 12, 2008
By Claire Regan
Fresh hope is being offered to hundreds of Ulster multiple sclerosis sufferers today after the Government ruled that a promising new drug which could slow progression will be available within months.
Health Minister Michael McGimpsey has announced that Tysabri will be available to MS patients from April onwards.
MS is a relapsing or progressive disabling condition in which the body's immune system attacks the central nervous system, disrupting signals from the brain. Northern Ireland has the second highest rate of the disease in the world, slightly behind Scotland.
Tysabri is used in the treatment of severe 'relapsing remitting' MS - when a sufferer has more than two relapses in a year. Clinical trials have shown it can reduce the relapse rate by 67%. It reduced disability progression by 42% over a two year period.
The MS Society, which lobbied Mr McGimpsey to speed up the drug's availability here, said the development could potentially be prescribed to several hundred people in Northern Ireland. It has been available in the rest of the UK since last year.
Patricia Gordon, the charity's director, said the development marked the real benefit for people with MS since the return of power to Stormont last May.
"This new drug could make a real difference to hundreds of people with MS across Northern Ireland and the minister is to be congratulated in his efforts to help deliver its availability.
"Now that we've achieved the first real benefit for people with MS under devolution, we will be lobbying the minister to look at other areas which need to be addressed, such as the provision of physiotherapy," she said.
"We will be making a presentation to the minister about the fact that Northern Ireland has the second highest instance of MS per capita in the world, looking at ways of identifying the reasons for this terrible statistic and how the MS Society can work more closely with the department to address to help improve the lives of people with MS."
Mr McGimpsey made the announcement during a visit to the MS Society's headquarters.
"Tysabri has the potential to help people suffering from a particularly severe type of MS and I am therefore pleased to announce that the Department has endorsed the NICE guidance on the use of this drug," he said.
"Health and Social Services Boards and Trusts are preparing for the managed introduction of Tysabri so that patients can be assured of safe delivery and administration of this treatment."
"I know that... waiting times for these drugs had been unacceptably long. To address this, an additional £2m was invested over the last two years to ensure that by March this year, no patient eligible for disease modifying therapy should wait more than 13 weeks for their treatment to commence. I am confident that this target will be met."
http://www.belfasttelegraph.co.uk/health/article3423895.ece
Antwort auf Beitrag Nr.: 33.339.446 von bernie55 am 12.02.08 11:44:28

Tests close in on cause of Alzheimer's
By KEN FOSKETT
The Atlanta Journal-Constitution
Published on: 02/18/08
http://www.ajc.com/metro/content/health/stories/2008/02/17/a…
By KEN FOSKETT
The Atlanta Journal-Constitution
Published on: 02/18/08
http://www.ajc.com/metro/content/health/stories/2008/02/17/a…
Elan, Wyeth Buck Standard in Alzheimer's Trial
by ADAM FEUERSTEIN
02/27/08 - 06:50 AM EST
http://www.thestreet.com/_yahoo/newsanalysis/business-news-u…
by ADAM FEUERSTEIN
02/27/08 - 06:50 AM EST
http://www.thestreet.com/_yahoo/newsanalysis/business-news-u…
Antwort auf Beitrag Nr.: 33.490.275 von bernie55 am 27.02.08 13:27:58Was denkst du über den Feuerstein Artikel, Cyberhexe ????
...also es scheint wohl so zu sein , dass der NTB Test ( von ELAN und WYETH ) eine andere Aussagekraft/ bzw. andere Kriterien hinsichtlich der Messung kognitiver Veränderung bei Alzheimer Patienten zu haben scheint als der üblich durchgeführte ADAS-cog Test.
Two Alzheimer's researchers familiar with the study protocols for the bapineuzumab phase III studies have confirmed that NTB was chosen as the primary endpoint, with the ADAS-cog test listed as a secondary endpoint.
.....eine anderes Testverfahren.....andere Ergebnisse ?????
What we need are better Alzheimer's disease drugs, not better tests," says Davies. "If a drug cannot show improvement in cognitive function using the ADAS-cog test, then the drug is not worth bothering with."
...scheint aber alles mit der FDA abgesprochen zu sein...
This is all being done in close cooperation with the FDA and other regulators who are fine with the approach," Birt adds
...vielleicht scheint der NTB Test doch genauer zu sein ????
that the companies believe the NTB may be more sensitive than the ADAS-cog in measuring cognitive function in patients with milder forms of Alzheimer's.
....Skepsis bei Adam Feuerstein hinsichtlich des NBT Tests...
Most investors expect bapineuzumab to demonstrate any profound effect on cognitive function via an improvement in ADAS-cog scores. But if bapineuzumab follows the path of Elan's past Alzheimer's drug candidates, it may be the NTB, and not ADAS-cog, that picks up any improvement in mental status.
Given the unproven and uncertain nature of the NTB, investors may be in for an unpleasant surprise.
Was denkst du über den Feuerstein Artikel, Cyberhexe ????
...also es scheint wohl so zu sein , dass der NTB Test ( von ELAN und WYETH ) eine andere Aussagekraft/ bzw. andere Kriterien hinsichtlich der Messung kognitiver Veränderung bei Alzheimer Patienten zu haben scheint als der üblich durchgeführte ADAS-cog Test.
Two Alzheimer's researchers familiar with the study protocols for the bapineuzumab phase III studies have confirmed that NTB was chosen as the primary endpoint, with the ADAS-cog test listed as a secondary endpoint.
.....eine anderes Testverfahren.....andere Ergebnisse ?????
What we need are better Alzheimer's disease drugs, not better tests," says Davies. "If a drug cannot show improvement in cognitive function using the ADAS-cog test, then the drug is not worth bothering with."
...scheint aber alles mit der FDA abgesprochen zu sein...
This is all being done in close cooperation with the FDA and other regulators who are fine with the approach," Birt adds
...vielleicht scheint der NTB Test doch genauer zu sein ????
that the companies believe the NTB may be more sensitive than the ADAS-cog in measuring cognitive function in patients with milder forms of Alzheimer's.
....Skepsis bei Adam Feuerstein hinsichtlich des NBT Tests...
Most investors expect bapineuzumab to demonstrate any profound effect on cognitive function via an improvement in ADAS-cog scores. But if bapineuzumab follows the path of Elan's past Alzheimer's drug candidates, it may be the NTB, and not ADAS-cog, that picks up any improvement in mental status.
Given the unproven and uncertain nature of the NTB, investors may be in for an unpleasant surprise.
Was denkst du über den Feuerstein Artikel, Cyberhexe ????
und plop 22,80, is ja nicht so nett. 

COOLE Zusammenfassung von ELANs zukünftigem Projekten und ELANs AD PROGRAMM !!!!!!
Developmental potential disease-modifying drugs for mild-moderate Alzheimer’s disease
...u.a...
Clearly the need is great for therapies that halt the natural degenerative course of Alzheimer’s disease. Out of the select potential disease-modifying drugs in development for Mild to Moderate AD, only Elan’s AAB-001 (phase III) and ELND-005 (phase II) have received “fast track” status from the FDA—a process allowing a faster path to approval for “drugs and biologics intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs”.
This has left the future of Elan heavily exposed to the Amyloid Beta as a target. We believe the principal findings from the past decades of scientific research point heavily to a central role of Amyloid Beta in Alzheimer’s Disease pathogenesis—if evolving clinical data refutes a central role for Amyloid Beta in AD, then the future of Elan’s pipeline will likely be put in question. If Amyloid Beta is centrally involved in AD pathogenesis—then,in our view, Elan potentially offers the most exciting Alzheimer’s pipeline in the industry.
http://www.investorvillage.com/smbd.asp?mb=160&mn=206202&pt=…
Developmental potential disease-modifying drugs for mild-moderate Alzheimer’s disease
...u.a...
Clearly the need is great for therapies that halt the natural degenerative course of Alzheimer’s disease. Out of the select potential disease-modifying drugs in development for Mild to Moderate AD, only Elan’s AAB-001 (phase III) and ELND-005 (phase II) have received “fast track” status from the FDA—a process allowing a faster path to approval for “drugs and biologics intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs”.
This has left the future of Elan heavily exposed to the Amyloid Beta as a target. We believe the principal findings from the past decades of scientific research point heavily to a central role of Amyloid Beta in Alzheimer’s Disease pathogenesis—if evolving clinical data refutes a central role for Amyloid Beta in AD, then the future of Elan’s pipeline will likely be put in question. If Amyloid Beta is centrally involved in AD pathogenesis—then,in our view, Elan potentially offers the most exciting Alzheimer’s pipeline in the industry.
http://www.investorvillage.com/smbd.asp?mb=160&mn=206202&pt=…
Executive Health
Attacking Alzheimer's
Robert Langreth 04.21.08, 12:00 AM ET
Ein Artikel u.a über ELAN / WYETH und
Dennis Selkoe , dem " Champion der Amyloid Theorie" !!!!

http://www.forbes.com/free_forbes/2008/0421/094.html
Attacking Alzheimer's
Robert Langreth 04.21.08, 12:00 AM ET
Ein Artikel u.a über ELAN / WYETH und
Dennis Selkoe , dem " Champion der Amyloid Theorie" !!!!

http://www.forbes.com/free_forbes/2008/0421/094.html
Der "Elan Company Day" war überaus beeindruckend. Anbei sehr anschaulich die prognostizierte Entwicklung der Pipeline:
http://img379.imageshack.us/my.php?image=pipeline2008hf4.jpg
http://img174.imageshack.us/my.php?image=pipeline2010gp2.png
Besonders hervorzuheben ist die Option mit Tysabri direkt in eine Ph3-Studie zur Behandlung von "Ulcerative Colitis" einzusteigen sowie Phase2-Studien in der Krebstherapie (Multiple myeloma) aufzunehmen. Ergenisse von Bapineuzumab werden Ende Juli erwartet.
http://img379.imageshack.us/my.php?image=pipeline2008hf4.jpg
http://img174.imageshack.us/my.php?image=pipeline2010gp2.png
Besonders hervorzuheben ist die Option mit Tysabri direkt in eine Ph3-Studie zur Behandlung von "Ulcerative Colitis" einzusteigen sowie Phase2-Studien in der Krebstherapie (Multiple myeloma) aufzunehmen. Ergenisse von Bapineuzumab werden Ende Juli erwartet.
Antwort auf Beitrag Nr.: 34.062.765 von Cyberhexe am 09.05.08 10:41:42http://www1.investorvillage.com/smbd.asp?mb=160&mn=231472&pt…
...Elans Presse wird besser!
...Elans Presse wird besser!
Acorda, Elan drug improves walking for MS patients
Mon Jun 2, 2008 6:01am EDT
By Ransdell Pierson
NEW YORK, June 2 (Reuters) - Acorda Therapeutics Inc. (ACOR.O: Quote, Profile, Research) said on Monday it will seek U.S. marketing approval early next year for the first medicine to improve walking among patients with multiple sclerosis, following highly favorable results of a second late-stage study.
The latest Phase 3 trial involved 240 MS patients in the United States and Canada with some degree of walking disability, divided into groups that either took the Acorda medicine or placebos twice daily.
Almost 43 percent of patients taking the medicine, called fampridine, showed consistent improvement in walking speed during the two-month study, compared with 9.3 percent of patients taking placebos, Acorda said.
The results were highly statistically significant and similar to those seen in an earlier 14-week study of the medicine. Patients who responded to the medicine in both Phase 3 studies, on average, required about 25 percent less time to to walk a distance of 25 feet.
Moreover, leg strength improved by a statistically significant degree among the fampridine group in the latest trial, meeting a secondary goal of the study.
"There is no approved treatment today that addresses walking disability for patients with multiple sclerosis," Ron Cohen, Acorda's chief executive, said in an interview late on Sunday.
"Walking disability is a fundamental feature of multiple sclerosis and one of the most worrisome features because it can imply loss of independence," said Cohen, whose tiny biotechnology company is based in Hawthorne, New York.
Cohen cautioned that although fampridine has been shown to improve the mobility of patients, it does not slow the progression of multiple sclerosis. Therefore, he said it will likely be used alongside standard interferon treatments that do slow progression, which include Biogen Idec Inc's (BIIB.O: Quote, Profile, Research) Avonex and the newer treatment Tysabri developed by Biogen Idec and Elan Corp (ELN.L: Quote, Profile, Research)
He noted, however, that the interferon treatments have not been shown to improve walking abilities, as fampridine has done.
Multiple sclerosis is a progressive disease in which the body's immune system attacks, and thereby wears away, the protective layer of a protein called myelin that insulates nerve fibers in the brain and spinal cord. More than 400,000 Americans are estimated to have the disease, a high percentage of whom eventually develop walking difficulties or related motor problems.
The Acorda drug, which is being developed in partnership with Elan and will guarantee the Irish drugmaker about 20 percent of its net sales, works by preventing potassium from leaking from nerve fibers whose myelin sheath has been denuded.
Cohen said Acorda, based on the favorable results of the two late-stage studies, aims to seek U.S. approval for fampridine in the first quarter of 2009. It could reach the market later that year or in 2010, he said, and might cost between $5,000 to $10,000 per year.
Although industry analysts have estimated the medicine could generate U.S. sales of up to $500 million, and considerable sales overseas, Cohen declined to comment on its commercial potential. (Editing by Lincoln Feast)
http://www.reuters.com/article/marketsNews/idINN014094622008…
Mon Jun 2, 2008 6:01am EDT
By Ransdell Pierson
NEW YORK, June 2 (Reuters) - Acorda Therapeutics Inc. (ACOR.O: Quote, Profile, Research) said on Monday it will seek U.S. marketing approval early next year for the first medicine to improve walking among patients with multiple sclerosis, following highly favorable results of a second late-stage study.
The latest Phase 3 trial involved 240 MS patients in the United States and Canada with some degree of walking disability, divided into groups that either took the Acorda medicine or placebos twice daily.
Almost 43 percent of patients taking the medicine, called fampridine, showed consistent improvement in walking speed during the two-month study, compared with 9.3 percent of patients taking placebos, Acorda said.
The results were highly statistically significant and similar to those seen in an earlier 14-week study of the medicine. Patients who responded to the medicine in both Phase 3 studies, on average, required about 25 percent less time to to walk a distance of 25 feet.
Moreover, leg strength improved by a statistically significant degree among the fampridine group in the latest trial, meeting a secondary goal of the study.
"There is no approved treatment today that addresses walking disability for patients with multiple sclerosis," Ron Cohen, Acorda's chief executive, said in an interview late on Sunday.
"Walking disability is a fundamental feature of multiple sclerosis and one of the most worrisome features because it can imply loss of independence," said Cohen, whose tiny biotechnology company is based in Hawthorne, New York.
Cohen cautioned that although fampridine has been shown to improve the mobility of patients, it does not slow the progression of multiple sclerosis. Therefore, he said it will likely be used alongside standard interferon treatments that do slow progression, which include Biogen Idec Inc's (BIIB.O: Quote, Profile, Research) Avonex and the newer treatment Tysabri developed by Biogen Idec and Elan Corp (ELN.L: Quote, Profile, Research)
He noted, however, that the interferon treatments have not been shown to improve walking abilities, as fampridine has done.
Multiple sclerosis is a progressive disease in which the body's immune system attacks, and thereby wears away, the protective layer of a protein called myelin that insulates nerve fibers in the brain and spinal cord. More than 400,000 Americans are estimated to have the disease, a high percentage of whom eventually develop walking difficulties or related motor problems.
The Acorda drug, which is being developed in partnership with Elan and will guarantee the Irish drugmaker about 20 percent of its net sales, works by preventing potassium from leaking from nerve fibers whose myelin sheath has been denuded.
Cohen said Acorda, based on the favorable results of the two late-stage studies, aims to seek U.S. approval for fampridine in the first quarter of 2009. It could reach the market later that year or in 2010, he said, and might cost between $5,000 to $10,000 per year.
Although industry analysts have estimated the medicine could generate U.S. sales of up to $500 million, and considerable sales overseas, Cohen declined to comment on its commercial potential. (Editing by Lincoln Feast)
http://www.reuters.com/article/marketsNews/idINN014094622008…
Antwort auf Beitrag Nr.: 34.216.868 von bernie55 am 02.06.08 12:32:57interessante Zusamnmenfassung über EDT:
http://www.elan.com/Images/EDT%20general%20present%20aug%202…
http://www.elan.com/Images/EDT%20general%20present%20aug%202…
Bapineuzumab in Patients With Mild to Moderate Alzheimer's Disease
This study is currently recruiting participants.
Verified by Elan Pharmaceuticals, June 2008
Sponsored by: Elan Pharmaceuticals
Information provided by: Elan Pharmaceuticals
ClinicalTrials.gov Identifier: NCT00575055
ClinicalTrials.gov Identifier: NCT00574132
http://clinicaltrials.gov/ct2/show/NCT00575055?term=aab-001&…
http://clinicaltrials.gov/ct2/show/NCT00574132?term=aab-001&…
This study is currently recruiting participants.
Verified by Elan Pharmaceuticals, June 2008
Sponsored by: Elan Pharmaceuticals
Information provided by: Elan Pharmaceuticals
ClinicalTrials.gov Identifier: NCT00575055
ClinicalTrials.gov Identifier: NCT00574132
http://clinicaltrials.gov/ct2/show/NCT00575055?term=aab-001&…
http://clinicaltrials.gov/ct2/show/NCT00574132?term=aab-001&…
Zur Vorfreude auf Bapineuzumab:
Villes Thema des Monats Juni: Antikörper gegen Amyloid-beta
http://www.wallstreet-online.de/diskussion/1134050-neustebei…
Villes Thema des Monats Juni: Antikörper gegen Amyloid-beta
http://www.wallstreet-online.de/diskussion/1134050-neustebei…
 > 26,51 USD <
> 26,51 USD < 
...voll der " home run "...

Antwort auf Beitrag Nr.: 34.275.660 von bernie55 am 10.06.08 17:24:16903801
Geld
16,89
Brief
17,43
Zeit
10.06. 17:25
871331
Geld
16,93
Brief
17,19
Zeit
10.06. 17:26
Geld
16,89
Brief
17,43
Zeit
10.06. 17:25
871331
Geld
16,93
Brief
17,19
Zeit
10.06. 17:26
Antwort auf Beitrag Nr.: 34.275.755 von Birgit.Tersteegen am 10.06.08 17:33:52Kurs kommt wieder runter, aber das kennen wir ja bereits...
25,74 USD

25,74 USD
Antwort auf Beitrag Nr.: 34.275.804 von bernie55 am 10.06.08 17:39:28..auf jeden Fall zur Zeit gutes Volumen...
...Spekulation im IV.....eventuelle Übernahme für 45 USD ???...was ich mir in der aktuellen Situation nicht vorstellen kann.........Vorfeude auf die morgige GS Konferenz ???......bald werden wir alle schlauer sein...
...Spekulation im IV.....eventuelle Übernahme für 45 USD ???...was ich mir in der aktuellen Situation nicht vorstellen kann.........Vorfeude auf die morgige GS Konferenz ???......bald werden wir alle schlauer sein...
Antwort auf Beitrag Nr.: 34.275.836 von bernie55 am 10.06.08 17:42:33Elan and Wyeth Announce Encouraging Top-line Results from Phase 2 Clinical Trial of Bapineuzumab for Alzheimer's Disease
Tuesday June 17, 2:00 am ET
-- Safety And Efficacy Findings Support Design Of Phase 3 Program
-- Primary Efficacy Endpoints In Overall Study Population Not Statistically Significant
-- Statistically Significant And Clinically Meaningful Benefits Seen In ApoE4 Non-Carriers
-- Overall Results Support Prior Decision To Initiate Phase 3
-- Detailed Data Presentation At ICAD July 29, 2008
http://biz.yahoo.com/bw/080617/20080616006659.html?.v=1
statistisch Signifikanz bei ApoE4 Non-Carriers - und dies bei einer derart kleinen Teilnehmerzahl...das ist der Hammer!
Tuesday June 17, 2:00 am ET
-- Safety And Efficacy Findings Support Design Of Phase 3 Program
-- Primary Efficacy Endpoints In Overall Study Population Not Statistically Significant
-- Statistically Significant And Clinically Meaningful Benefits Seen In ApoE4 Non-Carriers
-- Overall Results Support Prior Decision To Initiate Phase 3
-- Detailed Data Presentation At ICAD July 29, 2008
http://biz.yahoo.com/bw/080617/20080616006659.html?.v=1
statistisch Signifikanz bei ApoE4 Non-Carriers - und dies bei einer derart kleinen Teilnehmerzahl...das ist der Hammer!
Antwort auf Beitrag Nr.: 34.313.453 von Cyberhexe am 17.06.08 08:11:49...statistische Signifianz in einer derat kleinen Studie bedeutet: the drug works - man darf gespannt sein, in wie fern die Arzneimittelbehörden die Verfügbarkeit für die betroffenen Patienten beschleunigen.
Elan and Wyeth Announce Encouraging Top-line Results from Phase 2 Clinical Trial of Bapineuzumab for Alzheimer's Disease
-- Safety And Efficacy Findings Support Design Of Phase 3 Program
-- Primary Efficacy Endpoints In Overall Study Population Not
Statistically Significant
-- Statistically Significant And Clinically Meaningful Benefits
Seen In ApoE4 Non-Carriers
-- Overall Results Support Prior Decision To Initiate Phase 3
-- Detailed Data Presentation At ICAD July 29, 2008
DUBLIN, Ireland & MADISON, N.J.--(BUSINESS WIRE)--June 17, 2008--Elan Corporation, plc (NYSE: ELN) and Wyeth (NYSE: WYE) today announced encouraging preliminary findings from a Phase 2 study of bapineuzumab (AAB-001) in patients with mild to moderate Alzheimer's disease. In the 18-month trial, bapineuzumab appeared to have clinical activity in treating Alzheimer's disease.
Efficacy Findings
The study did not attain statistical significance on the primary efficacy endpoints in the overall study population. Post-hoc analyses did show statistically significant and clinically meaningful benefits in important subgroups.
In non-carriers of the Apolipoprotein E4 (ApoE4) allele, estimated in the literature to be from 40 to 70 percent of the Alzheimer's disease population, post-hoc analyses showed statistically significant and clinically meaningful benefits associated with bapineuzumab treatment on several key efficacy endpoints, including the Alzheimer's Disease Assessment Scale (ADAS-cog), the Neuropsychological Test Battery (NTB), the Mini Mental State Examination (MMSE) and the Clinical Dementia Rating - Sum of Boxes (CDR-SB). A favorable directional change was seen on the Disability Assessment Scale for Dementia (DAD), although this was not statistically significant.
Additionally in non-carriers, preliminary evaluation of MRI results showed less loss of brain volume among treated patients versus placebo patients, a finding that was statistically significant. Smaller increases in ventricular volume were seen in treated patients compared to placebo patients, although this finding was not statistically significant. Progression of Alzheimer's disease is generally associated with loss in brain volume and increases in ventricular volume. Further, treatment-related benefits seen on MRI were correlated to the favorable clinical changes observed in non-carriers.
In similar post-hoc analyses of carriers of the ApoE4 allele, no clinical benefits or statistically significant effects were observed on efficacy endpoints or the brain volume endpoint. However, favorable directional changes were observed on a number of endpoints. Preliminary analyses suggest possible increase of ventricular volume in treated patients versus placebo patients. The clinical significance of this finding is currently unclear and analyses are ongoing.
Safety Findings
As expected given the nature of the population studied, adverse events were very common in both placebo and bapineuzumab-treated patients. In non-carriers, the number of patients experiencing serious adverse events was similar between placebo and bapineuzumab-treated patients. In carriers, serious adverse events were more frequently observed in bapineuzumab-treated patients than in placebo patients. In addition, vasogenic edema was reported in the treated population with an increased frequency in carriers and at higher doses. No cases were reported in placebo patients. In the ongoing Phase 3 studies, carriers of the ApoE4 allele are being treated with a lower dose to minimize the risk of vasogenic edema. The Companies believe that the overall safety findings from this Phase 2 trial support their prior decision to move to Phase 3 studies.
CEO Comments
"The preliminary analyses of the Phase 2 study are a continued validation of the amyloid approach to Alzheimer's disease and an important milestone in our companies' ongoing commitment to bring new treatment options to patients," said Kelly Martin, President and CEO of Elan. "These results clinically support our decision to move into Phase 3 last year."
"We are encouraged by these findings. We remain driven by science and focused on patients as we work to bring this treatment to those who desperately need new options," said Bernard Poussot, President and CEO, Wyeth. "We recognize there is a great deal of hard work left as we move from this phase of learning towards confirming the potential of bapineuzumab."
Elan and Wyeth plan to continue all four studies in the previously disclosed bapineuzumab Phase 3 clinical program and will review and discuss these data with regulatory authorities and leading medical experts.
These findings reflect preliminary analyses of the Phase 2 data and its implications for ongoing clinical development of bapineuzumab. In this trial, there were imbalances in patient numbers and characteristics at baseline between subgroups studied that may or may not have affected these results. Further analysis will continue in advance of a planned scientific presentation of detailed results of this study at the International Conference on Alzheimer's Disease (ICAD) in Chicago, July 29, 2008.
http://www.elan.com/news/full.asp?ID=1166655
Elan and Wyeth Announce Encouraging Top-line Results from Phase 2 Clinical Trial of Bapineuzumab for Alzheimer's Disease
-- Safety And Efficacy Findings Support Design Of Phase 3 Program
-- Primary Efficacy Endpoints In Overall Study Population Not
Statistically Significant
-- Statistically Significant And Clinically Meaningful Benefits
Seen In ApoE4 Non-Carriers
-- Overall Results Support Prior Decision To Initiate Phase 3
-- Detailed Data Presentation At ICAD July 29, 2008
DUBLIN, Ireland & MADISON, N.J.--(BUSINESS WIRE)--June 17, 2008--Elan Corporation, plc (NYSE: ELN) and Wyeth (NYSE: WYE) today announced encouraging preliminary findings from a Phase 2 study of bapineuzumab (AAB-001) in patients with mild to moderate Alzheimer's disease. In the 18-month trial, bapineuzumab appeared to have clinical activity in treating Alzheimer's disease.
Efficacy Findings
The study did not attain statistical significance on the primary efficacy endpoints in the overall study population. Post-hoc analyses did show statistically significant and clinically meaningful benefits in important subgroups.
In non-carriers of the Apolipoprotein E4 (ApoE4) allele, estimated in the literature to be from 40 to 70 percent of the Alzheimer's disease population, post-hoc analyses showed statistically significant and clinically meaningful benefits associated with bapineuzumab treatment on several key efficacy endpoints, including the Alzheimer's Disease Assessment Scale (ADAS-cog), the Neuropsychological Test Battery (NTB), the Mini Mental State Examination (MMSE) and the Clinical Dementia Rating - Sum of Boxes (CDR-SB). A favorable directional change was seen on the Disability Assessment Scale for Dementia (DAD), although this was not statistically significant.
Additionally in non-carriers, preliminary evaluation of MRI results showed less loss of brain volume among treated patients versus placebo patients, a finding that was statistically significant. Smaller increases in ventricular volume were seen in treated patients compared to placebo patients, although this finding was not statistically significant. Progression of Alzheimer's disease is generally associated with loss in brain volume and increases in ventricular volume. Further, treatment-related benefits seen on MRI were correlated to the favorable clinical changes observed in non-carriers.
In similar post-hoc analyses of carriers of the ApoE4 allele, no clinical benefits or statistically significant effects were observed on efficacy endpoints or the brain volume endpoint. However, favorable directional changes were observed on a number of endpoints. Preliminary analyses suggest possible increase of ventricular volume in treated patients versus placebo patients. The clinical significance of this finding is currently unclear and analyses are ongoing.
Safety Findings
As expected given the nature of the population studied, adverse events were very common in both placebo and bapineuzumab-treated patients. In non-carriers, the number of patients experiencing serious adverse events was similar between placebo and bapineuzumab-treated patients. In carriers, serious adverse events were more frequently observed in bapineuzumab-treated patients than in placebo patients. In addition, vasogenic edema was reported in the treated population with an increased frequency in carriers and at higher doses. No cases were reported in placebo patients. In the ongoing Phase 3 studies, carriers of the ApoE4 allele are being treated with a lower dose to minimize the risk of vasogenic edema. The Companies believe that the overall safety findings from this Phase 2 trial support their prior decision to move to Phase 3 studies.
CEO Comments
"The preliminary analyses of the Phase 2 study are a continued validation of the amyloid approach to Alzheimer's disease and an important milestone in our companies' ongoing commitment to bring new treatment options to patients," said Kelly Martin, President and CEO of Elan. "These results clinically support our decision to move into Phase 3 last year."
"We are encouraged by these findings. We remain driven by science and focused on patients as we work to bring this treatment to those who desperately need new options," said Bernard Poussot, President and CEO, Wyeth. "We recognize there is a great deal of hard work left as we move from this phase of learning towards confirming the potential of bapineuzumab."
Elan and Wyeth plan to continue all four studies in the previously disclosed bapineuzumab Phase 3 clinical program and will review and discuss these data with regulatory authorities and leading medical experts.
These findings reflect preliminary analyses of the Phase 2 data and its implications for ongoing clinical development of bapineuzumab. In this trial, there were imbalances in patient numbers and characteristics at baseline between subgroups studied that may or may not have affected these results. Further analysis will continue in advance of a planned scientific presentation of detailed results of this study at the International Conference on Alzheimer's Disease (ICAD) in Chicago, July 29, 2008.
http://www.elan.com/news/full.asp?ID=1166655
...und es ist davon auszugehen, dass in den umfangreicheren p3-Studien auch die Vorteile für die Apoe4-Träger statistisch signifikant nachweisbar sind.
Abgesehen davon besitzen etwa 60% der AD-Patienten dieses Allel nicht, so dass alleine diese Population ein unglaubliches Potential darstellt.
Abgesehen davon besitzen etwa 60% der AD-Patienten dieses Allel nicht, so dass alleine diese Population ein unglaubliches Potential darstellt.
Antwort auf Beitrag Nr.: 34.314.309 von Cyberhexe am 17.06.08 10:22:11Danke Hexchen---Du warst die erste mit den News--und mit der fachlichen Einschätzung sowieso!!!!!!!!!!!!!!!!!!!!!!!!!

Antwort auf Beitrag Nr.: 34.314.309 von Cyberhexe am 17.06.08 10:22:11.....erst mal danke für die schnellen infos......
Elan, Wyeth drug helps some Alzheimer's patients
6/17/2008 3:07:00 AM (Reuters)
By Ben Hirschler LONDON (Reuters) -
Elan and Wyeth's key new drug bapineuzumab helped a proportion of patients with Alzheimer's disease in an intermediate clinical trial, supporting a prior decision to start final Phase III tests. The two companies said on Tuesday the drug did not achieve overall statistically significant results in the main goals of the Phase II clinical trial, but did achieve significance results in important subgroups in the study. Shares in Irish drugmaker Elan rose 5.1 percent to 18.08 euros on the news by 8:55 a.m.
The update on the antibody medicine, also known as AAB-001, is perhaps the year's most keenly awaited biotech trial result. If successful in final-stage trials, the medicine could be the world's first drug to modify the course of Alzheimer's, the most common cause of dementia, rather than just relieving its symptoms. Some analysts have forecast eventual annual sales of $13 billion (6.6 billion pounds), which would make it the biggest drug ever. But the project remains high risk for Elan and its U.S. partner Wyeth, given past failures in the Alzheimer's treatment field and looming competition from others companies, such as Eli Lilly and Myriad Genetics. Because of the nature of the clinical study and the limited number of patients involved, the Phase II trial had not been expected to show overall statistical significance. But Elan and Wyeth said statistically significant and clinically meaningful benefits were seen in a genetic sub-group of patients known as ApoE4 non-carriers, who make up between 40 and 70 percent of the Alzheimer's disease population.
'BETTER THAN EXPECTED' "Overall, these sets of data are much better than we expected, given the strong response observed in non-ApoE4 carriers," said Ian Hunter, an analyst at Goodbody Stockbrokers in Dublin. He increased his fair-value estimate for Elan's U.S. shares to around $30 from $28.50 previously on the back of the results. The stock closed in New York on Monday at $27.11. People who carry a gene that causes their bodies to produce a substance called apolipoprotein (Apo) E4 are known to be at increased risk of developing Alzheimer's, but many patients still get the condition without this genetic variation. Bapineuzumab aims to fight deposits called beta amyloid plaques, which are linked to the degenerative brain condition. "The preliminary analyses of the Phase II study are a continued validation of the amyloid approach to Alzheimer's disease," Elan Chief Executive Kelly Martin said. "These results clinically support our decision to move into Phase III last year." Detailed results will be presented at the International Conference on Alzheimer's Disease in Chicago on July 29. In the Phase II trial, ApoE4 non-carriers showed benefit from bapineuzumab treatment based on a number of different scoring systems that measure Alzheimer's disease. Loss of brain volume, which is associated with Alzheimer's, was also significantly less in this set of patients. Given the world's ageing population and the unmet need for an effective treatment, new medicines for Alzheimer's are seen as one of the big untapped opportunities for the pharmaceuticals industry. Existing acetylcholinesterase inhibitor drugs, like Eisai and Pfizer's market-leader Aricept, can reduce symptoms but do not modify the course of the disease. For a background story on bapineuzumab, please click on (Additional reporting by Mark Potter; Editing by David Holmes and Quentin Bryar)
http://www.investorvillage.com/mbnews.asp?mb=160&pt=qn&xid=4…
Elan, Wyeth drug helps some Alzheimer's patients
6/17/2008 3:07:00 AM (Reuters)
By Ben Hirschler LONDON (Reuters) -
Elan and Wyeth's key new drug bapineuzumab helped a proportion of patients with Alzheimer's disease in an intermediate clinical trial, supporting a prior decision to start final Phase III tests. The two companies said on Tuesday the drug did not achieve overall statistically significant results in the main goals of the Phase II clinical trial, but did achieve significance results in important subgroups in the study. Shares in Irish drugmaker Elan rose 5.1 percent to 18.08 euros on the news by 8:55 a.m.
The update on the antibody medicine, also known as AAB-001, is perhaps the year's most keenly awaited biotech trial result. If successful in final-stage trials, the medicine could be the world's first drug to modify the course of Alzheimer's, the most common cause of dementia, rather than just relieving its symptoms. Some analysts have forecast eventual annual sales of $13 billion (6.6 billion pounds), which would make it the biggest drug ever. But the project remains high risk for Elan and its U.S. partner Wyeth, given past failures in the Alzheimer's treatment field and looming competition from others companies, such as Eli Lilly and Myriad Genetics. Because of the nature of the clinical study and the limited number of patients involved, the Phase II trial had not been expected to show overall statistical significance. But Elan and Wyeth said statistically significant and clinically meaningful benefits were seen in a genetic sub-group of patients known as ApoE4 non-carriers, who make up between 40 and 70 percent of the Alzheimer's disease population.
'BETTER THAN EXPECTED' "Overall, these sets of data are much better than we expected, given the strong response observed in non-ApoE4 carriers," said Ian Hunter, an analyst at Goodbody Stockbrokers in Dublin. He increased his fair-value estimate for Elan's U.S. shares to around $30 from $28.50 previously on the back of the results. The stock closed in New York on Monday at $27.11. People who carry a gene that causes their bodies to produce a substance called apolipoprotein (Apo) E4 are known to be at increased risk of developing Alzheimer's, but many patients still get the condition without this genetic variation. Bapineuzumab aims to fight deposits called beta amyloid plaques, which are linked to the degenerative brain condition. "The preliminary analyses of the Phase II study are a continued validation of the amyloid approach to Alzheimer's disease," Elan Chief Executive Kelly Martin said. "These results clinically support our decision to move into Phase III last year." Detailed results will be presented at the International Conference on Alzheimer's Disease in Chicago on July 29. In the Phase II trial, ApoE4 non-carriers showed benefit from bapineuzumab treatment based on a number of different scoring systems that measure Alzheimer's disease. Loss of brain volume, which is associated with Alzheimer's, was also significantly less in this set of patients. Given the world's ageing population and the unmet need for an effective treatment, new medicines for Alzheimer's are seen as one of the big untapped opportunities for the pharmaceuticals industry. Existing acetylcholinesterase inhibitor drugs, like Eisai and Pfizer's market-leader Aricept, can reduce symptoms but do not modify the course of the disease. For a background story on bapineuzumab, please click on (Additional reporting by Mark Potter; Editing by David Holmes and Quentin Bryar)
http://www.investorvillage.com/mbnews.asp?mb=160&pt=qn&xid=4…
.....erst mal danke für die schnellen infos
....das war purer Zufall, wobei es auf ein paar Minuten, Stunden, Tage auch nicht ankommt. Das Ergebnis ist jedoch spektakulär und KM hat, wie meistens, nicht zu viel versprochen, denn statistische Signifikanz, wenn auch nur in einer Kohorte, konnte man beio dieser Studiengrösse nun wirklich nicht erwarten.
Bin mal gespannt, on nun die Studie mit den Nichtträgern des AD-Allels für alle Teilnehmer geöffnet wird, also auch die Placebo-Empfänger das Medikament erhalten. Wäre eigentlich die logische Folge!
http://www.clinicaltrials.gov/ct2/show/NCT00667810?term=bapi…
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Non-Carriers
....das war purer Zufall, wobei es auf ein paar Minuten, Stunden, Tage auch nicht ankommt. Das Ergebnis ist jedoch spektakulär und KM hat, wie meistens, nicht zu viel versprochen, denn statistische Signifikanz, wenn auch nur in einer Kohorte, konnte man beio dieser Studiengrösse nun wirklich nicht erwarten.
Bin mal gespannt, on nun die Studie mit den Nichtträgern des AD-Allels für alle Teilnehmer geöffnet wird, also auch die Placebo-Empfänger das Medikament erhalten. Wäre eigentlich die logische Folge!
http://www.clinicaltrials.gov/ct2/show/NCT00667810?term=bapi…
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Non-Carriers
Antwort auf Beitrag Nr.: 34.314.667 von Cyberhexe am 17.06.08 11:02:27denn statistische Signifikanz, wenn auch nur in einer Kohorte, konnte man beio dieser Studiengrösse nun wirklich nicht erwarten.
Bin mal gespannt, on nun die Studie mit den Nichtträgern des AD-Allels für alle Teilnehmer geöffnet wird, also auch die Placebo-Empfänger das Medikament erhalten. Wäre eigentlich die logische Folge!
Wenn nun die Safety Daten des PIII Trials nach 6 Monaten ??? erbracht werden können , kann dann eigentlich schon ein " final approval" für die " Nichtträger des APOE4 " ausgesprochen werden ?????
Bin mal gespannt, on nun die Studie mit den Nichtträgern des AD-Allels für alle Teilnehmer geöffnet wird, also auch die Placebo-Empfänger das Medikament erhalten. Wäre eigentlich die logische Folge!
Wenn nun die Safety Daten des PIII Trials nach 6 Monaten ??? erbracht werden können , kann dann eigentlich schon ein " final approval" für die " Nichtträger des APOE4 " ausgesprochen werden ?????
Antwort auf Beitrag Nr.: 34.314.855 von bernie55 am 17.06.08 11:27:16Die ursprünglich doppelt verblindete p2-Studie wurde nun für alle Studienteilnehmer geöffnet (open label):
http://www.clinicaltrials.gov/ct2/show/NCT00606476?term=bapi…
...gemäss Protokoll wird diese im Dez 2008 beendet - da es sich um eine Pivotal-Studie handelt, könnte dise Studie m.E. in Verbindung mit Daten zur Arzneimittelsicherheit der initialisierten p3-Studien eine Zulassung begründen (für Nichtträger des Allels). In wie weit dies jedoch wahrscheinlich ist, kann ich nicht beurteilen. Möglicherweise wird hierüber im IV-Forum etwas ausführlicher diskutiert.
http://www.clinicaltrials.gov/ct2/show/NCT00606476?term=bapi…
...gemäss Protokoll wird diese im Dez 2008 beendet - da es sich um eine Pivotal-Studie handelt, könnte dise Studie m.E. in Verbindung mit Daten zur Arzneimittelsicherheit der initialisierten p3-Studien eine Zulassung begründen (für Nichtträger des Allels). In wie weit dies jedoch wahrscheinlich ist, kann ich nicht beurteilen. Möglicherweise wird hierüber im IV-Forum etwas ausführlicher diskutiert.
na, ob da nicht ein Torcetrapib lauert? 
was vielleicht gerne übersehen wird... der primäre Endpunkt wurde verfehlt... der Rest ist schönes Data Mining... Provenge lässt grüßen
Hope Mixes With Doubt For Alzheimer's
http://www.forbes.com/business/2008/06/17/alzheimers-pharmac…
Matthew Herper and Robert Langreth 06.17.08, 5:35 PM ET
A promising new treatment for Alzheimer's disease helped some patients but may have harmed others, according to a preliminary study of 240 patients released today by the drug's makers.
Still, Elan (nyse: ELN - news - people ) of Dublin, Ireland, and Wyeth (nyse: WYE - news - people ) of Madison, N.J., said they were encouraged by the results, and the stock prices of both companies' shares rose in early trading. The companies have already started studies in thousands of people in order to get the drug approved, but those will take at least two years.
Bapineuzumab is one of the most-watched experimental treatments among drug and biotech investors. It was designed to attack and remove what some researchers believe is a root cause of Alzheimer's, the tell-tale amyloid plaques that build up in patients' brains. If it works, sales could reach $5 billion a year, according to Wall Street forecasts. But some scientists say bapineuzumab is based on a flawed conception of what causes Alzheimer's, making its chances of working far from assured.
The new results shed only a little light on the debate. According to a press release, the study failed to meet its pre-stated goal of helping patients do better on any of several questionnaires and tests designed to measure Alzheimer's severity.
But a subset of patients who did not have a genetic variant called APOE4, showed statistically significant improvements across four different tests for Alzheimer's symptoms, Wyeth and Elan said, when compared to a control group of patients who did not get the drug. They also appear to have lost less brain volume, according to MRI scans.
Meanwhile, in patients who had the APOE4 gene variant, which increases the risk of developing Alzheimer's by as much as tenfold, the drug had little benefit and increased risk of brain swelling due to leaky blood vessels, called vasogenic edema. Roughly half of Alzheimer's patients have the APOE4 gene variant.
"In my view, these results are very, very slightly hopeful, but not more than this," says John Hardy, a University College, London, neuroscientist and geneticist who was among the first to point to a link between amyloid, the protein bapineuzumab attacks, and Alzheimer's. He says because the companies didn't decide to divide patients into those with and without APOE until after the study finished, there is a meaningful risk that the positive results are due solely to chance. (kommt mir bekannt vor...)
(kommt mir bekannt vor...)
Other researchers are more optimistic. "This is exactly what you would want to see to justify going to a big phase III program," says Steven Ferris, director of the NYU Langone Alzheimer's Disease Center and a paid adviser to Elan. "These are pretty good results." He noted that those without APOE4 also showed less brain shrinkage on MRI scans as well as on cognitive tests. That, he says, is exactly what would be needed for the drug to be approved by the Food and Drug Administration as a disease-modifying agent, not just a treatment of symptoms.
Lon S. Schneider, a psychiatrist and Alzheimer's disease expert at the University of Southern California Keck School of Medicine, adds: "I don't think this is particularly earth shaking. The basic thing they are saying is we looked at our data and it continues to give us faith to go ahead."
The news release is also notable for what it does not include . It doesn't say how many patients were in the subset that appeared to benefit or what the magnitude of the benefit was. The news release also does not reveal whether higher doses of the drug showed more or less of an effect than lower doses. The news release also does not say how many different subsets of patients were analyzed, which is particularly important because the more subsets you analyze, the more you are likely to have a positive result merely by chance.
. It doesn't say how many patients were in the subset that appeared to benefit or what the magnitude of the benefit was. The news release also does not reveal whether higher doses of the drug showed more or less of an effect than lower doses. The news release also does not say how many different subsets of patients were analyzed, which is particularly important because the more subsets you analyze, the more you are likely to have a positive result merely by chance.
The big question behind bapineuzumab is whether it is targeting a cause of Alzheimer's or a symptom. The dominant explanation of Alzheimer's disease contends that the massive brain cell death is due to the buildup of plaques containing a protein called beta amyloid built up in the brain. Bapineuzumab is an antibody that attacks beta amyloid. Eli Lilly (nyse: LLY - news - people ) and Pfizer (nyse: PFE - news - people ) are among numerous other companies testing similar approaches.
One big mystery is why hitting amyloid with a drug would benefit people without the APOE4 gene but not those with it. There are few clinical differences, experts say, between those with APOE4 and those without it once they develop the disease.
hitting amyloid with a drug would benefit people without the APOE4 gene but not those with it. There are few clinical differences, experts say, between those with APOE4 and those without it once they develop the disease.
That difference is "unexpected but not inexplicable," says Zaven Khachaturian, president of Keep Memory Alive in Las Vegas and former head of Alzheimer's research at the National Institutes of Health. Alzheimer's is probably caused by a complex interaction of many genes, and it is possible those with the APOE4 gene are less able to repair their injured brains. These patients tend to have more amyloid built up in their blood vessels, not just in their brains.
but not inexplicable," says Zaven Khachaturian, president of Keep Memory Alive in Las Vegas and former head of Alzheimer's research at the National Institutes of Health. Alzheimer's is probably caused by a complex interaction of many genes, and it is possible those with the APOE4 gene are less able to repair their injured brains. These patients tend to have more amyloid built up in their blood vessels, not just in their brains.
For investors, the huge potential of bapineuzumab and the very real risks create a very big betting opportunity. Wall Street analysts differ on the chances that the drug eventually proves effective and is approved. Timothy Anderson at Sanford C. Bernstein wrote in a note to investors that he models only a 30% chance of approval but still sees Wyeth as worth buying because of the valuation of the stock.
James Kelly, the pharmaceuticals analyst at Goldman Sachs, wrote in a note that the results should slightly increase Wall Street's view of bapineuzumab's chances. However, he said, the new data should cool hopes that the drug could be approved in this mid-stage. Barbara Ryan at Deutsche Bank wrote that the results were "as good as could be expected at this time," and that shares will trade up for now. But she advises investors to be careful because of other disappointments that could hit the company.
Barbara Ryan at Deutsche Bank wrote that the results were "as good as could be expected at this time," and that shares will trade up for now. But she advises investors to be careful because of other disappointments that could hit the company.
David Amsellem and Jacob Rasmussen of FBR Capital Markets called the data "underwhelming." They write that even in the subgroup of patients without the APOE4 gene, the results were statistically significant only for one of two primary endpoints, and note that unspecified imbalances mentioned in the news release could skew the results.
They write that even in the subgroup of patients without the APOE4 gene, the results were statistically significant only for one of two primary endpoints, and note that unspecified imbalances mentioned in the news release could skew the results.
The last experimental drug to generate sales forecasts as big as the ones analysts are ballparking for bapineuzumab was torcetrapib, a Pfizer pill designed to reduce heart attacks. Torcetrapib actually increased the death rate and was canned.
James Nicoll, a neuropathologist at the University of Southampton, performed autopsies on nine patients who received a plaque-removing vaccine Elan and Wyeth tried in an earlier trial. (Side effects torpedoed that approach.) He found that the vaccine patients had dramatic reductions in plaque compared to a control group. Yet eight of nine still developed end-stage dementia.
Nicoll called the news release this morning "tantalizing." However, he added: "There is quite a lot of information you would like to see that isn't there. If there is efficacy, it is not of a very large magnitude."
mfg ipollit

was vielleicht gerne übersehen wird... der primäre Endpunkt wurde verfehlt... der Rest ist schönes Data Mining... Provenge lässt grüßen

Hope Mixes With Doubt For Alzheimer's
http://www.forbes.com/business/2008/06/17/alzheimers-pharmac…
Matthew Herper and Robert Langreth 06.17.08, 5:35 PM ET
A promising new treatment for Alzheimer's disease helped some patients but may have harmed others, according to a preliminary study of 240 patients released today by the drug's makers.
Still, Elan (nyse: ELN - news - people ) of Dublin, Ireland, and Wyeth (nyse: WYE - news - people ) of Madison, N.J., said they were encouraged by the results, and the stock prices of both companies' shares rose in early trading. The companies have already started studies in thousands of people in order to get the drug approved, but those will take at least two years.
Bapineuzumab is one of the most-watched experimental treatments among drug and biotech investors. It was designed to attack and remove what some researchers believe is a root cause of Alzheimer's, the tell-tale amyloid plaques that build up in patients' brains. If it works, sales could reach $5 billion a year, according to Wall Street forecasts. But some scientists say bapineuzumab is based on a flawed conception of what causes Alzheimer's, making its chances of working far from assured.
The new results shed only a little light on the debate. According to a press release, the study failed to meet its pre-stated goal of helping patients do better on any of several questionnaires and tests designed to measure Alzheimer's severity.
But a subset of patients who did not have a genetic variant called APOE4, showed statistically significant improvements across four different tests for Alzheimer's symptoms, Wyeth and Elan said, when compared to a control group of patients who did not get the drug. They also appear to have lost less brain volume, according to MRI scans.
Meanwhile, in patients who had the APOE4 gene variant, which increases the risk of developing Alzheimer's by as much as tenfold, the drug had little benefit and increased risk of brain swelling due to leaky blood vessels, called vasogenic edema. Roughly half of Alzheimer's patients have the APOE4 gene variant.
"In my view, these results are very, very slightly hopeful, but not more than this," says John Hardy, a University College, London, neuroscientist and geneticist who was among the first to point to a link between amyloid, the protein bapineuzumab attacks, and Alzheimer's. He says because the companies didn't decide to divide patients into those with and without APOE until after the study finished, there is a meaningful risk that the positive results are due solely to chance.
 (kommt mir bekannt vor...)
(kommt mir bekannt vor...) Other researchers are more optimistic. "This is exactly what you would want to see to justify going to a big phase III program," says Steven Ferris, director of the NYU Langone Alzheimer's Disease Center and a paid adviser to Elan. "These are pretty good results." He noted that those without APOE4 also showed less brain shrinkage on MRI scans as well as on cognitive tests. That, he says, is exactly what would be needed for the drug to be approved by the Food and Drug Administration as a disease-modifying agent, not just a treatment of symptoms.
Lon S. Schneider, a psychiatrist and Alzheimer's disease expert at the University of Southern California Keck School of Medicine, adds: "I don't think this is particularly earth shaking. The basic thing they are saying is we looked at our data and it continues to give us faith to go ahead."
The news release is also notable for what it does not include
 . It doesn't say how many patients were in the subset that appeared to benefit or what the magnitude of the benefit was. The news release also does not reveal whether higher doses of the drug showed more or less of an effect than lower doses. The news release also does not say how many different subsets of patients were analyzed, which is particularly important because the more subsets you analyze, the more you are likely to have a positive result merely by chance.
. It doesn't say how many patients were in the subset that appeared to benefit or what the magnitude of the benefit was. The news release also does not reveal whether higher doses of the drug showed more or less of an effect than lower doses. The news release also does not say how many different subsets of patients were analyzed, which is particularly important because the more subsets you analyze, the more you are likely to have a positive result merely by chance. The big question behind bapineuzumab is whether it is targeting a cause of Alzheimer's or a symptom. The dominant explanation of Alzheimer's disease contends that the massive brain cell death is due to the buildup of plaques containing a protein called beta amyloid built up in the brain. Bapineuzumab is an antibody that attacks beta amyloid. Eli Lilly (nyse: LLY - news - people ) and Pfizer (nyse: PFE - news - people ) are among numerous other companies testing similar approaches.
One big mystery is why
 hitting amyloid with a drug would benefit people without the APOE4 gene but not those with it. There are few clinical differences, experts say, between those with APOE4 and those without it once they develop the disease.
hitting amyloid with a drug would benefit people without the APOE4 gene but not those with it. There are few clinical differences, experts say, between those with APOE4 and those without it once they develop the disease. That difference is "unexpected
 but not inexplicable," says Zaven Khachaturian, president of Keep Memory Alive in Las Vegas and former head of Alzheimer's research at the National Institutes of Health. Alzheimer's is probably caused by a complex interaction of many genes, and it is possible those with the APOE4 gene are less able to repair their injured brains. These patients tend to have more amyloid built up in their blood vessels, not just in their brains.
but not inexplicable," says Zaven Khachaturian, president of Keep Memory Alive in Las Vegas and former head of Alzheimer's research at the National Institutes of Health. Alzheimer's is probably caused by a complex interaction of many genes, and it is possible those with the APOE4 gene are less able to repair their injured brains. These patients tend to have more amyloid built up in their blood vessels, not just in their brains. For investors, the huge potential of bapineuzumab and the very real risks create a very big betting opportunity. Wall Street analysts differ on the chances that the drug eventually proves effective and is approved. Timothy Anderson at Sanford C. Bernstein wrote in a note to investors that he models only a 30% chance of approval but still sees Wyeth as worth buying because of the valuation of the stock.
James Kelly, the pharmaceuticals analyst at Goldman Sachs, wrote in a note that the results should slightly increase Wall Street's view of bapineuzumab's chances. However, he said, the new data should cool hopes that the drug could be approved in this mid-stage.
 Barbara Ryan at Deutsche Bank wrote that the results were "as good as could be expected at this time," and that shares will trade up for now. But she advises investors to be careful because of other disappointments that could hit the company.
Barbara Ryan at Deutsche Bank wrote that the results were "as good as could be expected at this time," and that shares will trade up for now. But she advises investors to be careful because of other disappointments that could hit the company. David Amsellem and Jacob Rasmussen of FBR Capital Markets called the data "underwhelming."
 They write that even in the subgroup of patients without the APOE4 gene, the results were statistically significant only for one of two primary endpoints, and note that unspecified imbalances mentioned in the news release could skew the results.
They write that even in the subgroup of patients without the APOE4 gene, the results were statistically significant only for one of two primary endpoints, and note that unspecified imbalances mentioned in the news release could skew the results. The last experimental drug to generate sales forecasts as big as the ones analysts are ballparking for bapineuzumab was torcetrapib, a Pfizer pill designed to reduce heart attacks. Torcetrapib actually increased the death rate and was canned.
James Nicoll, a neuropathologist at the University of Southampton, performed autopsies on nine patients who received a plaque-removing vaccine Elan and Wyeth tried in an earlier trial. (Side effects torpedoed that approach.) He found that the vaccine patients had dramatic reductions in plaque compared to a control group. Yet eight of nine still developed end-stage dementia.

Nicoll called the news release this morning "tantalizing." However, he added: "There is quite a lot of information you would like to see that isn't there. If there is efficacy, it is not of a very large magnitude."

mfg ipollit
http://www.rte.ie/business/2008/morningrep/download/0618davy…
Elan climbed to an almost four-year high in late US trading, ending the day at $30 even (+10.7%), which we feel is a fair reaction to the top-line Phase II data on
Bapineuzumab. On balance, the data were better than our expectations, especially with what look to be robust efficacy results in the ApoE4 non-carrier group. Given the newsflow vacuum between now and the presentation of full Phase II data at ICAD, the stock is likely to remain quite volatile. Beyond that, we expect a busy H2 for newsflow at Elan, including more information on its AD filing and manufacturing strategies, updates on Tysabri revenues and several R&D milestones for its early and mid-stage development pipeline.
Increased confidence in the development prospects for Bapineuzumab and its ultimate commercial potential lead us to upgrade our risked valuation to $31 (range of $27-32). Our models suggest unrisked upside to approximately $42, based on what may be a conservative $4.5bn peak revenue potential in AD.
#19525 von Cyberhexe 17.06.08 20:14:24 Beitrag Nr.: 34.319.434
Dieses Posting: versenden | melden
Folgende Antwort bezieht sich auf Beitrag Nr.: 34.317.924 von Holgus am 17.06.08 17:09:50
--------------------------------------------------------------------------------
...die Ergebnisse sind besser als erwartet, statistische Signifikanz, wenn auch nur in der Untergruppe der Nichtträger des Allels Apoe4, konnte man nämlich in Anbetracht der Stufdiengrösse nicht erwarten. Dass diese dennoch erreicht wurde, ist m.E. überwältigend und der letzte Beweis dafür, dass Bapineuzumab wirkt.
Jedoch konnte man trotzdem nicht erwarten, dass sich der Kurs an einem Tag verdoppelt, sprich die Marktkapitalisierung um weitere 10 Milliarden USD zulegt. Dies ist einfach nicht realistisch, da immer noch einiges schief gehen kann bis der Wirkstoff dann wirklich am Markt platziert ist. Die Wahrscheinlichkeit einer Marktzulassung ist mit dem heutigen Ergebnis allerdings um einiges gestiegen, und verspricht auf die kommenden Monate/Jahre eine überaus attraktive Kursphantasie. Kurzfristige Verdoppelungen sind jedoch bei Unternehmen mit einer Marktkapitalisierung jenseits von 10 Milliarden USD die ganz ganz grosse Ausnahme. Dies ist eher bei kleineren Unternehmen möglich, wenn diese einen Wirkstoff durch die Klinik an den Markt bringen (vielleicht Dendreon mit Provenge in 2009). Allerdings ist bei diesen kleinen Unternehmen das Risiko nun ungleich grösser. All jene, die einer Kursverdoppelung/-verdreifachung etwas Zeit geben, dürften jedoch nun bei Elan sehr gut aufgehoben sein.
ch
Elan climbed to an almost four-year high in late US trading, ending the day at $30 even (+10.7%), which we feel is a fair reaction to the top-line Phase II data on
Bapineuzumab. On balance, the data were better than our expectations, especially with what look to be robust efficacy results in the ApoE4 non-carrier group. Given the newsflow vacuum between now and the presentation of full Phase II data at ICAD, the stock is likely to remain quite volatile. Beyond that, we expect a busy H2 for newsflow at Elan, including more information on its AD filing and manufacturing strategies, updates on Tysabri revenues and several R&D milestones for its early and mid-stage development pipeline.
Increased confidence in the development prospects for Bapineuzumab and its ultimate commercial potential lead us to upgrade our risked valuation to $31 (range of $27-32). Our models suggest unrisked upside to approximately $42, based on what may be a conservative $4.5bn peak revenue potential in AD.
#19525 von Cyberhexe 17.06.08 20:14:24 Beitrag Nr.: 34.319.434
Dieses Posting: versenden | melden
Folgende Antwort bezieht sich auf Beitrag Nr.: 34.317.924 von Holgus am 17.06.08 17:09:50
--------------------------------------------------------------------------------
...die Ergebnisse sind besser als erwartet, statistische Signifikanz, wenn auch nur in der Untergruppe der Nichtträger des Allels Apoe4, konnte man nämlich in Anbetracht der Stufdiengrösse nicht erwarten. Dass diese dennoch erreicht wurde, ist m.E. überwältigend und der letzte Beweis dafür, dass Bapineuzumab wirkt.
Jedoch konnte man trotzdem nicht erwarten, dass sich der Kurs an einem Tag verdoppelt, sprich die Marktkapitalisierung um weitere 10 Milliarden USD zulegt. Dies ist einfach nicht realistisch, da immer noch einiges schief gehen kann bis der Wirkstoff dann wirklich am Markt platziert ist. Die Wahrscheinlichkeit einer Marktzulassung ist mit dem heutigen Ergebnis allerdings um einiges gestiegen, und verspricht auf die kommenden Monate/Jahre eine überaus attraktive Kursphantasie. Kurzfristige Verdoppelungen sind jedoch bei Unternehmen mit einer Marktkapitalisierung jenseits von 10 Milliarden USD die ganz ganz grosse Ausnahme. Dies ist eher bei kleineren Unternehmen möglich, wenn diese einen Wirkstoff durch die Klinik an den Markt bringen (vielleicht Dendreon mit Provenge in 2009). Allerdings ist bei diesen kleinen Unternehmen das Risiko nun ungleich grösser. All jene, die einer Kursverdoppelung/-verdreifachung etwas Zeit geben, dürften jedoch nun bei Elan sehr gut aufgehoben sein.
ch
wenn die Optimisten hier sogar nur eine 30% Zulassungswahrscheinlichkeit sehen, ist es wahrscheinlich lohnender Elan auf diesem Niveau zu shorten. Die Theorie hinter Bapineuzumab steht auf sehr wackeligen Beinen... in der Summe mit anderen Anti Beta Amyolid Kandidaten könnte die ganze Geschichte ein weiteres Milliarden Grab für die Pharmas werden... 
zu der gestrigen "News"...
1.) Für die 240 Patienten hat diese Studie den primären Endpunkt laut der gestrigen Meldung verfehlt! Es konnte keine statistisch signifikante Verbesserung festgestellt werden.
2.) angeblich signifikante Verbesserungen gibt es nur bei den Patienten, denen die Genvariante APOE4 fehlt. Diese Aussage halte ich nach dem aktuell bekannten Daten für zweifelhaft, da
a) die Größe dieser Subgruppe in der Studie nicht bekannt gegeben wurde. Je kleiner diese Gruppe in den 240 Patienten ist, umso zufälliger sind solche Ergebnisse, selbst dann, wenn sie angeblich statistisch signifikant sind. Die Ergebnisse sind wahrscheinlich nicht reproduzierbar in einer PIII.
b) die Subgruppe wurde nachträglich definiert. Dies ist sehr kritisch, da man überlicherweise einfach beliebige Gruppen im Nachhinein bei bekannten Ergebnissen durchgehen kann und immer irgendeine findet, bei der zufällig das Ergebnis positiv ausfällt. Subgruppen müssen vor Beginn der Studie definiert sein, um überhaupt einen Wert zu haben.
c) Es gibt angeblich keine theoretische Erklärung, warum die Wirkung von Bapineuzumab von APOE4 abhängen soll. Dies weisst auch eher auf zufällig positive Ergebnisse hin.
d) Die Verbesserung bei dieser speziellen Subgruppe soll 2 bis 2,5 sein... die Grenze der klinischen Relevanz soll bei >2 liegen. Also u.U. zufällige Ergebnisse, die auch nur minimal an der Grenze zu einer wirklich relevanten Verbesserung liegen.
3.) Die Subgruppe "ohne APOE4" ist zwar groß mit 40-70%. Bei den anderen 30-60% "mit APOE4" konnte allerdings keine Wirkung nachgewiesen werden, obwohl diese Gruppe ein 10fach höheres Risiko besitzt an Alzheimer zu erkranken. Ich würde auch eher vermuten, dass man besser eine Wirkung an Hoch-Risiko-Patienten nachweisen kann, weil da ein Nichtwirken klar auffällt. Bei Patienten "ohne APOE4" mit weniger Risiko kann das positive Ergebnis wie jetzt bei der PII auch bloßes statistisches Rauschen sein!
4.) Es wurden keine Aussagen über Nebenwirkungen gemacht. Diese können sehr kritisch sein, da das Medikament auf das Gehirn einwirkt. Nebenwirkungen wie Gehirnschwellungen können den potentiellen positiven Effekt aufheben und ins Gegenteil drehen. Die Vakzine von Elan als Vorgänger von Bapineuzumab wurde wegen Nebenwirkungen gestoppt.
5.) Es fehlen Angaben zu den unterschiedlichen Dosierungen und deren Effektivität... wenn sich die Effektivität nicht in den Dosierungen wiederspiegelt, dann weißt dies ebenfalls auf einen Fehler von der Beta Amyloid Theorie und damit der Unwirksamkeit von Bapineuzumab hin.
Ich denke, das Risiko sollte offensichtlich sein. Interessanter für Elan sind wohl noch die anstehenden PIII Daten von Flurizan, die parallel zu den vollständigen Bapineuzumab PII Daten nächsten Monat veröffentlich werden sollen.
mfg ipollit

zu der gestrigen "News"...
1.) Für die 240 Patienten hat diese Studie den primären Endpunkt laut der gestrigen Meldung verfehlt! Es konnte keine statistisch signifikante Verbesserung festgestellt werden.
2.) angeblich signifikante Verbesserungen gibt es nur bei den Patienten, denen die Genvariante APOE4 fehlt. Diese Aussage halte ich nach dem aktuell bekannten Daten für zweifelhaft, da
a) die Größe dieser Subgruppe in der Studie nicht bekannt gegeben wurde. Je kleiner diese Gruppe in den 240 Patienten ist, umso zufälliger sind solche Ergebnisse, selbst dann, wenn sie angeblich statistisch signifikant sind. Die Ergebnisse sind wahrscheinlich nicht reproduzierbar in einer PIII.
b) die Subgruppe wurde nachträglich definiert. Dies ist sehr kritisch, da man überlicherweise einfach beliebige Gruppen im Nachhinein bei bekannten Ergebnissen durchgehen kann und immer irgendeine findet, bei der zufällig das Ergebnis positiv ausfällt. Subgruppen müssen vor Beginn der Studie definiert sein, um überhaupt einen Wert zu haben.
c) Es gibt angeblich keine theoretische Erklärung, warum die Wirkung von Bapineuzumab von APOE4 abhängen soll. Dies weisst auch eher auf zufällig positive Ergebnisse hin.
d) Die Verbesserung bei dieser speziellen Subgruppe soll 2 bis 2,5 sein... die Grenze der klinischen Relevanz soll bei >2 liegen. Also u.U. zufällige Ergebnisse, die auch nur minimal an der Grenze zu einer wirklich relevanten Verbesserung liegen.
3.) Die Subgruppe "ohne APOE4" ist zwar groß mit 40-70%. Bei den anderen 30-60% "mit APOE4" konnte allerdings keine Wirkung nachgewiesen werden, obwohl diese Gruppe ein 10fach höheres Risiko besitzt an Alzheimer zu erkranken. Ich würde auch eher vermuten, dass man besser eine Wirkung an Hoch-Risiko-Patienten nachweisen kann, weil da ein Nichtwirken klar auffällt. Bei Patienten "ohne APOE4" mit weniger Risiko kann das positive Ergebnis wie jetzt bei der PII auch bloßes statistisches Rauschen sein!
4.) Es wurden keine Aussagen über Nebenwirkungen gemacht. Diese können sehr kritisch sein, da das Medikament auf das Gehirn einwirkt. Nebenwirkungen wie Gehirnschwellungen können den potentiellen positiven Effekt aufheben und ins Gegenteil drehen. Die Vakzine von Elan als Vorgänger von Bapineuzumab wurde wegen Nebenwirkungen gestoppt.
5.) Es fehlen Angaben zu den unterschiedlichen Dosierungen und deren Effektivität... wenn sich die Effektivität nicht in den Dosierungen wiederspiegelt, dann weißt dies ebenfalls auf einen Fehler von der Beta Amyloid Theorie und damit der Unwirksamkeit von Bapineuzumab hin.
Ich denke, das Risiko sollte offensichtlich sein. Interessanter für Elan sind wohl noch die anstehenden PIII Daten von Flurizan, die parallel zu den vollständigen Bapineuzumab PII Daten nächsten Monat veröffentlich werden sollen.
mfg ipollit
Antwort auf Beitrag Nr.: 34.323.573 von ipollit am 18.06.08 12:50:39und das Spiel geht weiter...
Alzheimer's Trial Still a Gamble Despite Early Optimism
By SHIRLEY S. WANG
June 18, 2008
Promising results from a midstage Alzheimer's drug study released Tuesday offer encouraging news to pharmaceutical companies that are gambling billions of dollars on a popular but unproven theory of what causes the devastating illness.
Elan Corp. and Wyeth said a small clinical trial of a treatment designed to slow the advance of dementia in Alzheimer's patients showed signs of effectiveness.
Like most Alzheimer's drugs now in development, bapineuzumab is designed to remove or reduce a sticky substance in the brain known as amyloid, which many scientists believe is a primary cause of Alzheimer's symptoms.
The drug failed on overall measures of success but showed enough promise to offer hope to Wall Street and those looking for any positive sign in the treatment of this so far incurable disease. Still, bapineuzumab must prove more effective in later-stage clinical trials if it has any hope of reaching the market.
In the bigger picture, too, laboratory tests have provided little evidence that reducing amyloid improves cognitive symptoms in mice, let alone in humans. Yet drug makers have been willing to invest heavily in this approach.
"The biggest concern is that we might have too many eggs in one basket," says P. Murali Doraiswamy, a Duke University professor and an Alzheimer's and clinical-trials expert who has consulted with various pharmaceutical companies, including those that make Alzheimer's drugs. "If the theory backfires or if amyloid turns out not to be the essential culprit, then a lot of research effort may not bear fruit."
Wyeth's shares were up $2.08, or 4.8%, to $45.16, and Elan's American depositary shares jumped $2.89, or 11%, to $30 as of 4 p.m. composite trading Tuesday on the New York Stock Exchange.
Leerink Swann, an investment bank specializing in health care, upgraded Wyeth to "outperform" on learning results of the 240-patient bapineuzumab study.
There are more drugs in the neurological-disease pipeline for Alzheimer's than for any other malady except pain, according to Pharmaceutical Research and Manufacturers of America, the industry trade group. Drug makers likely will spend more than $1 billion a year in 2009 and beyond on Alzheimer's research and development, estimates Seamus Fernandez, a Leerink Swann analyst.
The investor and scientific communities are awaiting several other study outcomes as well, to gauge whether amyloid is the proper focus for drug makers. Data from Myriad Genetics Inc.'s key Phase III clinical study on Flurizan, the largest and longest human trial for an Alzheimer's treatment to date, will be unveiled in the next week or two, according to the Salt Lake City company. The full data from both the Elan/Wyeth and Myriad studies will be presented next month at the International Conference on Alzheimer's Disease.
Myriad has spent $200 million so far on Flurizan, which aims to inhibit enzymes that create amyloid. The results of its 1,684-patient trial will offer the best evidence to date whether amyloid reduction improves cognitive ability. Elan, based in Dublin, and Wyeth, of Madison, N.J., began a 4,100-patient, $300 million Phase III trial in December, even before seeing Tuesday's complete Phase II results.
Alzheimer's medicines are a market valued at more than $3 billion a year world-wide -- and analysts estimate that the figure could nearly triple in the next 10 years with the arrival of any treatment that slows progression of the disease. More than five million Americans are thought to have Alzheimer's, and direct and indirect costs for care associated with Alzheimer's and other dementias are more than $148 billion a year, according to Tony Butler, an analyst at Lehman Brothers.
Drug companies have focused on the amyloid hypothesis because a considerable body of research suggests that the brains of Alzheimer's patients contain large amounts of certain types of amyloid. But in the relatively few studies in which amyloid has been removed from mice's brains, cognitive symptoms haven't necessarily improved. And human autopsies show that the mere presence of large amounts of amyloid doesn't always correlate with the presence of Alzheimer's, according to Bruce Miller, clinical director of the University of California at San Francisco Memory and Aging Center, who has consulted with Myriad.
Bapineuzumab uses antibodies to attack and remove amyloid on the assumption that doing so will retard the disease's progress. It is expected by many analysts to be the first disease-modifying Alzheimer's drug to hit the market.
Available Alzheimer's drugs, such as Eisai Inc. and Pfizer Inc.'s Aricept and H. Lundbeck AS and Forest Laboratories Inc.'s Namenda, ameliorate symptoms but don't actually slow progression of the disease.
An Eisai spokeswoman said the company is increasing efforts to educate caregivers about its Alzheimer's treatment, and started a new direct-to-consumer campaign focusing on the signs and symptoms of Alzheimer's.
Bapineuzumab mostly helped people who aren't genetically predisposed to developing Alzheimer's. They showed significant improvement on a number of cognitive tests and less loss of brain volume. People carrying the gene associated with higher Alzheimer's risk demonstrated some benefit on the tests, but not at a statistically significant level.
mfg ipollit
Alzheimer's Trial Still a Gamble Despite Early Optimism
By SHIRLEY S. WANG
June 18, 2008
Promising results from a midstage Alzheimer's drug study released Tuesday offer encouraging news to pharmaceutical companies that are gambling billions of dollars on a popular but unproven theory of what causes the devastating illness.
Elan Corp. and Wyeth said a small clinical trial of a treatment designed to slow the advance of dementia in Alzheimer's patients showed signs of effectiveness.
Like most Alzheimer's drugs now in development, bapineuzumab is designed to remove or reduce a sticky substance in the brain known as amyloid, which many scientists believe is a primary cause of Alzheimer's symptoms.
The drug failed on overall measures of success but showed enough promise to offer hope to Wall Street and those looking for any positive sign in the treatment of this so far incurable disease. Still, bapineuzumab must prove more effective in later-stage clinical trials if it has any hope of reaching the market.
In the bigger picture, too, laboratory tests have provided little evidence that reducing amyloid improves cognitive symptoms in mice, let alone in humans. Yet drug makers have been willing to invest heavily in this approach.
"The biggest concern is that we might have too many eggs in one basket," says P. Murali Doraiswamy, a Duke University professor and an Alzheimer's and clinical-trials expert who has consulted with various pharmaceutical companies, including those that make Alzheimer's drugs. "If the theory backfires or if amyloid turns out not to be the essential culprit, then a lot of research effort may not bear fruit."
Wyeth's shares were up $2.08, or 4.8%, to $45.16, and Elan's American depositary shares jumped $2.89, or 11%, to $30 as of 4 p.m. composite trading Tuesday on the New York Stock Exchange.
Leerink Swann, an investment bank specializing in health care, upgraded Wyeth to "outperform" on learning results of the 240-patient bapineuzumab study.
There are more drugs in the neurological-disease pipeline for Alzheimer's than for any other malady except pain, according to Pharmaceutical Research and Manufacturers of America, the industry trade group. Drug makers likely will spend more than $1 billion a year in 2009 and beyond on Alzheimer's research and development, estimates Seamus Fernandez, a Leerink Swann analyst.
The investor and scientific communities are awaiting several other study outcomes as well, to gauge whether amyloid is the proper focus for drug makers. Data from Myriad Genetics Inc.'s key Phase III clinical study on Flurizan, the largest and longest human trial for an Alzheimer's treatment to date, will be unveiled in the next week or two, according to the Salt Lake City company. The full data from both the Elan/Wyeth and Myriad studies will be presented next month at the International Conference on Alzheimer's Disease.
Myriad has spent $200 million so far on Flurizan, which aims to inhibit enzymes that create amyloid. The results of its 1,684-patient trial will offer the best evidence to date whether amyloid reduction improves cognitive ability. Elan, based in Dublin, and Wyeth, of Madison, N.J., began a 4,100-patient, $300 million Phase III trial in December, even before seeing Tuesday's complete Phase II results.
Alzheimer's medicines are a market valued at more than $3 billion a year world-wide -- and analysts estimate that the figure could nearly triple in the next 10 years with the arrival of any treatment that slows progression of the disease. More than five million Americans are thought to have Alzheimer's, and direct and indirect costs for care associated with Alzheimer's and other dementias are more than $148 billion a year, according to Tony Butler, an analyst at Lehman Brothers.
Drug companies have focused on the amyloid hypothesis because a considerable body of research suggests that the brains of Alzheimer's patients contain large amounts of certain types of amyloid. But in the relatively few studies in which amyloid has been removed from mice's brains, cognitive symptoms haven't necessarily improved. And human autopsies show that the mere presence of large amounts of amyloid doesn't always correlate with the presence of Alzheimer's, according to Bruce Miller, clinical director of the University of California at San Francisco Memory and Aging Center, who has consulted with Myriad.
Bapineuzumab uses antibodies to attack and remove amyloid on the assumption that doing so will retard the disease's progress. It is expected by many analysts to be the first disease-modifying Alzheimer's drug to hit the market.
Available Alzheimer's drugs, such as Eisai Inc. and Pfizer Inc.'s Aricept and H. Lundbeck AS and Forest Laboratories Inc.'s Namenda, ameliorate symptoms but don't actually slow progression of the disease.
An Eisai spokeswoman said the company is increasing efforts to educate caregivers about its Alzheimer's treatment, and started a new direct-to-consumer campaign focusing on the signs and symptoms of Alzheimer's.
Bapineuzumab mostly helped people who aren't genetically predisposed to developing Alzheimer's. They showed significant improvement on a number of cognitive tests and less loss of brain volume. People carrying the gene associated with higher Alzheimer's risk demonstrated some benefit on the tests, but not at a statistically significant level.
mfg ipollit
Antwort auf Beitrag Nr.: 34.323.573 von ipollit am 18.06.08 12:50:39...deine Kritikpunkte sind klar dargestellt.....THX...
..deshalb warten nicht nur wir, sondern auch viele andere Investoren auf den 29.07.08.......dann wird hoffentlich vieles klarer zu beurteilen sein....
In keeping with Elan/Wyeth's original target, the full Phase II data will be presented at the ICAD conference on July 29, 2008. This will be of great interest to the scientific and investor communities alike.
Key points of interest will include
(i) the magnitude of movements in efficacy endpoints,
(ii) patient responses within each dose cohort,
(iii) the split of carriers/non-carriers in the study,
(iv) whether non-carriers demonstrated stabilisation or improvement and
(v) the potential of the treatment for carriers.
..deshalb warten nicht nur wir, sondern auch viele andere Investoren auf den 29.07.08.......dann wird hoffentlich vieles klarer zu beurteilen sein....
In keeping with Elan/Wyeth's original target, the full Phase II data will be presented at the ICAD conference on July 29, 2008. This will be of great interest to the scientific and investor communities alike.
Key points of interest will include
(i) the magnitude of movements in efficacy endpoints,
(ii) patient responses within each dose cohort,
(iii) the split of carriers/non-carriers in the study,
(iv) whether non-carriers demonstrated stabilisation or improvement and
(v) the potential of the treatment for carriers.
..hier eine Grafik über die Zusammenhänge von dem APOE Gen und dem Alzheimer Risiko.
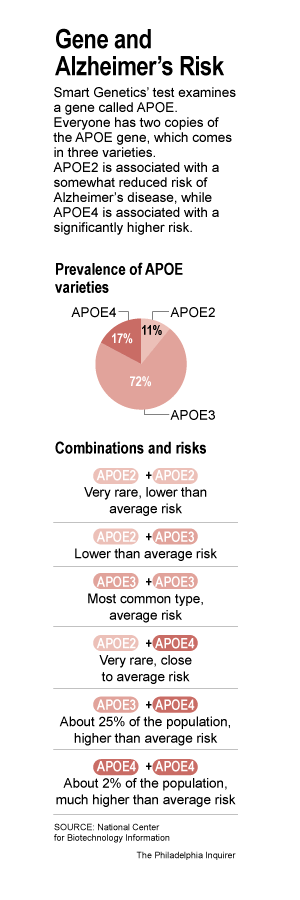

Antwort auf Beitrag Nr.: 34.323.573 von ipollit am 18.06.08 12:50:391.) Für die 240 Patienten hat diese Studie den primären Endpunkt laut der gestrigen Meldung verfehlt! Es konnte keine statistisch signifikante Verbesserung festgestellt werden.
Wenn man den Studienentwurf betrachtet und dabei nicht übersieht, dass in 4 unterschiedliche Kohorte zu je 60 Teilnehmern aufgeteilt wurde, welche im Verhältnis 8:7 (Verum zu Placebo) doppelt verblindet randomisiert wurden, dann konnte man in Anbetracht der kleinen Teilnehmerzahl keine statistische Signifikanz erwarten. Denn statistische Signifikanz nachzuweisen bedeutet auszuschliessen, dass das erzielte Resultat zufällig zustandegekommen ist.
Die Tatsache, dass bei den Nichtträgern des Allels bei einer derat kleinen Studie dennoch statistisch signifikant Verbesserungen gegenüber der Plazebogruppe beobachtet wurden, ist das erstaunliche an der Meldung. Und wer dies nicht wahrhaben will, der soll halt diese grosse Chance verpassen und bei GPC dem Konkurs entgegenfiebern!
Wenn man den Studienentwurf betrachtet und dabei nicht übersieht, dass in 4 unterschiedliche Kohorte zu je 60 Teilnehmern aufgeteilt wurde, welche im Verhältnis 8:7 (Verum zu Placebo) doppelt verblindet randomisiert wurden, dann konnte man in Anbetracht der kleinen Teilnehmerzahl keine statistische Signifikanz erwarten. Denn statistische Signifikanz nachzuweisen bedeutet auszuschliessen, dass das erzielte Resultat zufällig zustandegekommen ist.
Die Tatsache, dass bei den Nichtträgern des Allels bei einer derat kleinen Studie dennoch statistisch signifikant Verbesserungen gegenüber der Plazebogruppe beobachtet wurden, ist das erstaunliche an der Meldung. Und wer dies nicht wahrhaben will, der soll halt diese grosse Chance verpassen und bei GPC dem Konkurs entgegenfiebern!
Antwort auf Beitrag Nr.: 34.324.176 von Cyberhexe am 18.06.08 13:53:40...Du spachst mir aus der Seele....



Antwort auf Beitrag Nr.: 34.324.394 von Birgit.Tersteegen am 18.06.08 14:15:09..ach ja....unsere " Seelensprache "....



.im übrigen finde ich es interessant, dass User, die sich bisher in diesem Board überhaupt nicht zu Wort gemeldet haben, plötzlich auftauchen, wenn akuelle " HammerNews " herauskommen und dann Kritiken vom Stapel lassen....
> ( was natürlich auch gewünscht ist !!! ) <
..jedoch habe auch ich manchmal das Gefühl, dass ein gewisser " emotionaler " Unterton mitschwingt......
..siehe DNDN....jetzt ELAN....mmmhhhh....
> ( was natürlich auch gewünscht ist !!! ) <
..jedoch habe auch ich manchmal das Gefühl, dass ein gewisser " emotionaler " Unterton mitschwingt......
..siehe DNDN....jetzt ELAN....mmmhhhh....
...also, jetzt wieder zum Alltag...
..und den 29.07.08 vormerken !!!!!

..und den 29.07.08 vormerken !!!!!

Ken Kam's Comments
Many in the financial industry want to believe that access to real-time data services like Bloomberg, Reuters, and Thompson give them an advantage over individual investors who lack access.
Perhaps this was true in the past, but today when the internet disseminates information quickly and virtually for free, access to information is no longer the competitive advantage it once was.
The real value no longer comes from having the news first, but in knowing how to interpret that news and therefore what actions to take. This takes good judgment and in this regard individual investors often have an advantage over Wall Street.
Today we saw a great example of this in action. When the market opened, both Elan and Wyeth traded up as much as 7% as the results of their Phase II trial for AAB-001 hit the newswires. Then, Bloomberg ran a story with the headline, “Elan Wyeth Alzheimer's drug didn't meet study goal,” and both stocks trimmed their gains.
While Bloomberg’s headline is accurate, it conveys a strong sense that AAB-001 was a failure, and nothing could be farther from the truth.
The drug showed a statistically significant beneficial effect on patients who don’t carry the ApoE4 gene. Somewhere between 40% and 60% of Alzheimer’s patients don’t have the ApoE4 gene, so an equally accurate headline could have been “Elan Wyeth Alzheimer’s drug found effective for many patients.”
There were 240 patients in the Phase II trial, so in order for the beneficial effect to be statistically significant, it had to be a big effect. It is now clear why the Phase III trial was designed so patients who carry ApoE4 are tracked separately from those who do not.
If the Phase III trial confirms the results, AAB-001 will offer hope for millions of patients who don’t carry ApoE4.
AAB-001 may be the first drug that can really alter the course of Alzheimer’s disease for many patients.
Wall Street may think these results were mediocre. But those who read more than the headline came to a much different conclusion.
Elan closed at $30, up 10% for the day.
When Wall Street disagrees with the judgment of the investors with the best track records at Marketocracy its easy for me to decide what to do. I am still not selling.
http://www.investorvillage.com/smbd.asp?mb=160&mn=243091&pt=…
Many in the financial industry want to believe that access to real-time data services like Bloomberg, Reuters, and Thompson give them an advantage over individual investors who lack access.
Perhaps this was true in the past, but today when the internet disseminates information quickly and virtually for free, access to information is no longer the competitive advantage it once was.
The real value no longer comes from having the news first, but in knowing how to interpret that news and therefore what actions to take. This takes good judgment and in this regard individual investors often have an advantage over Wall Street.
Today we saw a great example of this in action. When the market opened, both Elan and Wyeth traded up as much as 7% as the results of their Phase II trial for AAB-001 hit the newswires. Then, Bloomberg ran a story with the headline, “Elan Wyeth Alzheimer's drug didn't meet study goal,” and both stocks trimmed their gains.
While Bloomberg’s headline is accurate, it conveys a strong sense that AAB-001 was a failure, and nothing could be farther from the truth.
The drug showed a statistically significant beneficial effect on patients who don’t carry the ApoE4 gene. Somewhere between 40% and 60% of Alzheimer’s patients don’t have the ApoE4 gene, so an equally accurate headline could have been “Elan Wyeth Alzheimer’s drug found effective for many patients.”
There were 240 patients in the Phase II trial, so in order for the beneficial effect to be statistically significant, it had to be a big effect. It is now clear why the Phase III trial was designed so patients who carry ApoE4 are tracked separately from those who do not.
If the Phase III trial confirms the results, AAB-001 will offer hope for millions of patients who don’t carry ApoE4.
AAB-001 may be the first drug that can really alter the course of Alzheimer’s disease for many patients.
Wall Street may think these results were mediocre. But those who read more than the headline came to a much different conclusion.
Elan closed at $30, up 10% for the day.
When Wall Street disagrees with the judgment of the investors with the best track records at Marketocracy its easy for me to decide what to do. I am still not selling.
http://www.investorvillage.com/smbd.asp?mb=160&mn=243091&pt=…
Antwort auf Beitrag Nr.: 34.323.599 von ipollit am 18.06.08 12:52:44und das Spiel geht weiter...
..wie wahr..
Alzheimer's Drug Shows Progress
Wednesday June 18, 8:08 am ET
By Catherine Arnst
The much anticipated results of a clinical trial of one of the more promising Alzheimer's drugs, released June 17, were mixed at best. But in the Alzheimer's world that qualifies as a success -- and on Wall Street, it was a winner as well.
Wyeth Pharmaceuticals (NYSE:WYE - News) and Elan Corp. (NYSE:ELN - News) announced that the Phase II trial of their jointly developed drug, bapineuzumab, did not attain statistically significant results in the overall group of 240 patients. But in a subset of patients that lacked a high-risk gene variant for the disease, the drug significantly improved symptoms in five different tests. Patients in the gene-free group also lost less brain volume.
Investors chose to read the trial as a positive, boosting Elan's share price by 2.89, or 10.7%, to 30. The stock hit a six-year high of 30.30 during the day's trading. Wyeth's shares rose by 2.08, or 4.8%, to 45.16.
Targeting the Amyloid Protein
The gene in question, APOE4, is carried by about half of all Alzheimer's patients and usually signals earlier onset of the disease. A drug that might help only those patients without the gene may not sound all that meaningful, but the 18-month trial marks one of the first hints of success in the search for a drug meant to change the course of the disease for which there is currently no treatment.
The four drugs approved so far for Alzheimer's treat only symptoms, and work only for a few months. The bapineuzumab trial also represents one of the first successes for a number of drugs in the pipeline that are trying to modify the disease by clearing away a brain-destroying protein called amyloid that is a hallmark of Alzheimer's.
If the results play out in a much larger Phase III trial already under way -- and that is still a big if -- then bapineuzumab would represent a major advance in one of the greatest unmet needs in medicine.
A Potential Multibillion-Dollar Market
"It's fair to say that both myself and the other investigators on this trial are quite excited," says Dr. Anton Porsteinsson, director of the Alzheimer's disease care, research, and education program at the University of Rochester, who is treating patients in both the Phase II and III trials of the drug. "It is the first time a study of meaningful size and scope has shown that beta amyloid modulation makes a difference."
Wall Street's enthusiasm reflects the multibillion-dollar market that any successful Alzheimer's drug could tap into. The Centers for Disease Control and Prevention announced last week that in 2006, the last year for which data were compiled, Alzheimer's moved up from the seventh to the sixth leading cause of death in the U.S. There are currently 5.2 million Americans living with the disease, and the Alzheimer's Association expects that number to increase to 9 million by 2020. One in 10 people over 65 develop the brain-destroying disease; over age 85, the odds rise to one in two.
Most pharmaceutical analysts had not expected statistically significant results from the Wyeth-Elan trial because of the relatively small number of patients. Brian McCarthy, a biotech analyst with Merriman Curhan Ford, said the results were encouraging, however, because the patients in the non-APOE4 group showed improvement across a broad spectrum of tests.
Out of Failure, Some Success
This could still end up being a statistical fluke however, if the overall number of patients in the non-APOE4 group turns out to be very small. "We want to take a look at the full test results before reaching any conclusions, but this subset analysis is certainly very interesting," McCarthy said. Wyeth and Elan will present the full results at the International Conference on Alzheimer's Disease in Chicago at the end of July.
Bapineuzumab is an antibody, given by injection, that grew out of one of the more spectacular drug failures of recent years -- a therapeutic vaccine meant to alter the course of Alzheimer's. The vaccine was also developed by Elan and Wyeth, and was once considered the most promising drug in the pipeline against the dreaded disease. But in 2002 the trial had to be stopped because several patients developed a deadly swelling of the brain.
A year later, after some trial participants had died of Alzheimer's, autoposies revealed that those patients who received the vaccine had much lower levels of amyloid in their brains, a sticky plaque that destroys neurons. Although there is no definitive proof that amyloid is the cause of Alzheimer's, the evidence that the vaccine did what it was supposed to gave the two companies reason to pursue a drug that would attack amyloid without the vaccine's side effects.
Elan and Wyeth said that bapineuzumab, also known as AAB-001, did cause some brain swelling in the Phase II trial. To minimize this danger, the drug is being administered at a lower dose in the Phase III trial, which started last year.
http://www.investorvillage.com/smbd.asp?mb=160&mn=243091&pt=…
..wie wahr..
Alzheimer's Drug Shows Progress
Wednesday June 18, 8:08 am ET
By Catherine Arnst
The much anticipated results of a clinical trial of one of the more promising Alzheimer's drugs, released June 17, were mixed at best. But in the Alzheimer's world that qualifies as a success -- and on Wall Street, it was a winner as well.
Wyeth Pharmaceuticals (NYSE:WYE - News) and Elan Corp. (NYSE:ELN - News) announced that the Phase II trial of their jointly developed drug, bapineuzumab, did not attain statistically significant results in the overall group of 240 patients. But in a subset of patients that lacked a high-risk gene variant for the disease, the drug significantly improved symptoms in five different tests. Patients in the gene-free group also lost less brain volume.
Investors chose to read the trial as a positive, boosting Elan's share price by 2.89, or 10.7%, to 30. The stock hit a six-year high of 30.30 during the day's trading. Wyeth's shares rose by 2.08, or 4.8%, to 45.16.
Targeting the Amyloid Protein
The gene in question, APOE4, is carried by about half of all Alzheimer's patients and usually signals earlier onset of the disease. A drug that might help only those patients without the gene may not sound all that meaningful, but the 18-month trial marks one of the first hints of success in the search for a drug meant to change the course of the disease for which there is currently no treatment.
The four drugs approved so far for Alzheimer's treat only symptoms, and work only for a few months. The bapineuzumab trial also represents one of the first successes for a number of drugs in the pipeline that are trying to modify the disease by clearing away a brain-destroying protein called amyloid that is a hallmark of Alzheimer's.
If the results play out in a much larger Phase III trial already under way -- and that is still a big if -- then bapineuzumab would represent a major advance in one of the greatest unmet needs in medicine.
A Potential Multibillion-Dollar Market
"It's fair to say that both myself and the other investigators on this trial are quite excited," says Dr. Anton Porsteinsson, director of the Alzheimer's disease care, research, and education program at the University of Rochester, who is treating patients in both the Phase II and III trials of the drug. "It is the first time a study of meaningful size and scope has shown that beta amyloid modulation makes a difference."
Wall Street's enthusiasm reflects the multibillion-dollar market that any successful Alzheimer's drug could tap into. The Centers for Disease Control and Prevention announced last week that in 2006, the last year for which data were compiled, Alzheimer's moved up from the seventh to the sixth leading cause of death in the U.S. There are currently 5.2 million Americans living with the disease, and the Alzheimer's Association expects that number to increase to 9 million by 2020. One in 10 people over 65 develop the brain-destroying disease; over age 85, the odds rise to one in two.
Most pharmaceutical analysts had not expected statistically significant results from the Wyeth-Elan trial because of the relatively small number of patients. Brian McCarthy, a biotech analyst with Merriman Curhan Ford, said the results were encouraging, however, because the patients in the non-APOE4 group showed improvement across a broad spectrum of tests.
Out of Failure, Some Success
This could still end up being a statistical fluke however, if the overall number of patients in the non-APOE4 group turns out to be very small. "We want to take a look at the full test results before reaching any conclusions, but this subset analysis is certainly very interesting," McCarthy said. Wyeth and Elan will present the full results at the International Conference on Alzheimer's Disease in Chicago at the end of July.
Bapineuzumab is an antibody, given by injection, that grew out of one of the more spectacular drug failures of recent years -- a therapeutic vaccine meant to alter the course of Alzheimer's. The vaccine was also developed by Elan and Wyeth, and was once considered the most promising drug in the pipeline against the dreaded disease. But in 2002 the trial had to be stopped because several patients developed a deadly swelling of the brain.
A year later, after some trial participants had died of Alzheimer's, autoposies revealed that those patients who received the vaccine had much lower levels of amyloid in their brains, a sticky plaque that destroys neurons. Although there is no definitive proof that amyloid is the cause of Alzheimer's, the evidence that the vaccine did what it was supposed to gave the two companies reason to pursue a drug that would attack amyloid without the vaccine's side effects.
Elan and Wyeth said that bapineuzumab, also known as AAB-001, did cause some brain swelling in the Phase II trial. To minimize this danger, the drug is being administered at a lower dose in the Phase III trial, which started last year.
http://www.investorvillage.com/smbd.asp?mb=160&mn=243091&pt=…
Elan – anticipated pipeline newsflow in 6-12 months
Products Newsflow > Anticipated timeline:
- Bapineuzumab Full Phase II data ICAD 26th-31st July
- Phase II imaging data End 2008/early 2009
- Filing/manufacturing decisions H2 2008
- Interim analyses (if included) Possibly H1 2009
- Bapineuzumab SubQ Initiate Phase II H2 2008
- ACC-001 Final study report on Phase I H2 2008
- ELND005 Initiate Phase II in early AD By end-2008
- ELND006 Initiate Phase I H2 2008
- ELND007 IND filing H2 2009
- Tysabri Revenue, patient and safety updates Quarterly
- Pre-IND meeting on UC indication H2 2008
- ELND002 Phase I data H2 2008
- ELND004 Initiate Phase II By end-2008
Source: Elan
Products Newsflow > Anticipated timeline:
- Bapineuzumab Full Phase II data ICAD 26th-31st July
- Phase II imaging data End 2008/early 2009
- Filing/manufacturing decisions H2 2008
- Interim analyses (if included) Possibly H1 2009
- Bapineuzumab SubQ Initiate Phase II H2 2008
- ACC-001 Final study report on Phase I H2 2008
- ELND005 Initiate Phase II in early AD By end-2008
- ELND006 Initiate Phase I H2 2008
- ELND007 IND filing H2 2009
- Tysabri Revenue, patient and safety updates Quarterly
- Pre-IND meeting on UC indication H2 2008
- ELND002 Phase I data H2 2008
- ELND004 Initiate Phase II By end-2008
Source: Elan
Antwort auf Beitrag Nr.: 34.325.877 von bernie55 am 18.06.08 16:41:23hallo Bernie,
anbei der richtige Link zu dem Artikel.
http://www.businessweek.com/bwdaily/dnflash/content/jun2008/…
anbei der richtige Link zu dem Artikel.
http://www.businessweek.com/bwdaily/dnflash/content/jun2008/…
Antwort auf Beitrag Nr.: 34.325.877 von bernie55 am 18.06.08 16:41:23ein guter Artikel! Allerdings...
"A year later, after some trial participants had died of Alzheimer's, autoposies revealed that those patients who received the vaccine had much lower levels of amyloid in their brains, a sticky plaque that destroys neurons."
das mag soweit richtig sein... allerdings gibt es zu dieser PII noch weitere Daten, die mich damals skeptisch gemacht haben, ob der Ansatz mit Beta Amyloid nicht falsch ist. Ich habe vorher jedenfalls Bapineuzumab auch positiv gesehen und es wird sicherlich ein Multi-Mrd Blockbuster, wenn es denn wirklich einen Effekt bei Alzheimer hat. Allerdings zeigten die weiteren Langzeit-Daten zu der Vakzine PII, dass zwar eindeutig wie oben geschrieben, das Beta Amyloid Plaque in den behandelten Personen reduziert war, was ja das Ziel ist. Allerdings haben die Patienten trotzdem die Demenz entwickelt, unabhängig davon, ob Beta Amyloid vorhanden war oder nicht. Entsprechendes hat sich auch aus Tierversuchen ergeben. Das ganze ist schon eine wahnsinnige Sache, die nur durch die hohe mögliche Gewinnchance erklärlich ist: Es gibt bis heute keinen Beleg dafür, das die Beta-Amyloid-Theorie richtig ist! Sie ist sogar unter Wissenschaftlern durchaus umstritten. Und trotzdem pumpen aktuell die Pharmas Milliarden in diese Theorie. Wenn es sich zeigen sollte, dass sie falsch ist, dann wäre das ganze Geld und er ganze Zeitaufwand umsonst gewesen!
Und in der Tat... es ist ja witzig, aber es besteht eindeutig ein Zusammenhang zu Dendreon und Provenge. Auch dort wurden nachträglich entsprechend angepasste Analysen durchgeführt und ebenfalls fehlt die Theorie des Wirkmechanismus hinter Provenge. Wenn man sich mal andere kleinere Biotechs anschaut, dann ist dies das typische Vorgehen, wenn etwas nicht funktioniert hat. Ein Paradebeispiel ist natürlich auch GPC: Satraplatin hat den primären Endpunkt verfehlt und nun werden diese Subgruppen Analysen durchgeführt... und irgendetwas wird schon (zufällig) (statistisch signifikant) positiv sein (wobei diese Analysen anders als z.B. jetzt bei Bapineuzumab wenigstens bereits im Studienprotokoll definiert waren). Naja... aber ich gebe zu, auch GPC ist da kein Stück besser und macht ebenfalls diesen Mist!
Ich finde eure Argumentation etwas paradox, ehrlich gesagt: Auf der einen Seite sagt ihr, dass 240 Patienten zu wenig wären, um statistisch signifikante Ergebnisse zu produzieren, auf der anderen Seite soll ein Subgruppe der 240 Patienten, die deutlich weniger Patienten enthält, klar signifikante Ergebnisse produziert haben???? Ist das nicht erstmal sehr seltsam? Da argumentiert ihr dann, dass gerade das das geniale wäre, dass eine so kleine Menge statistisch signifikante Ergebnisse liefert. Wenn man sich allerdings mit Statistik ein klein wenig auskennt, dann weiß man auch, dass je kleiner die Gruppe ist, umso schneller es passieren kann, dass die Ergebnisse zufällig (ja, es hört sich komisch an, ist aber so) statistisch signifikant sind. (steht ja da auch: "This could still end up being a statistical fluke however, if the overall number of patients in the non-APOE4 group turns out to be very small." )
Ist das nicht erstmal sehr seltsam? Da argumentiert ihr dann, dass gerade das das geniale wäre, dass eine so kleine Menge statistisch signifikante Ergebnisse liefert. Wenn man sich allerdings mit Statistik ein klein wenig auskennt, dann weiß man auch, dass je kleiner die Gruppe ist, umso schneller es passieren kann, dass die Ergebnisse zufällig (ja, es hört sich komisch an, ist aber so) statistisch signifikant sind. (steht ja da auch: "This could still end up being a statistical fluke however, if the overall number of patients in the non-APOE4 group turns out to be very small." )
Es heißt zwar schön "randomisierte doppeltverblindete" Studie... das gilt aber nur für die komplette Studie, nicht für diese Subgruppen. So ist die angeblich positive Subgruppe nicht randomisiert, also mit Ungleichgewichten im Zustand der Patienten, Alter usw! Dies gibt Elan sogar selbst zu: "Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results." Zudem ist eine nachträgliche Analyse auch nicht mehr verblindet, da man ja die Analyse sich erst überlegt, wenn man die Ergebnisse der Studie auf dem Tisch hat. Das ist dann natürlich nicht mehr blind, sondern man gestaltet die Analyse so, dass deren Ergebnis wie gewünscht positiv ist, während genauso logisch negative Analysen und Ergebnisse unter den Tisch fallen.
Vielleicht solltet ihr euch auch mal Gedanken machen, was passieren kann, wenn die vollständigen Ergebnisse der PII im Juli weniger positiv ausfallen... ich weiß nicht, ob dann nicht Elan deutlich überbewertet sind. Ich schätze das Abwärtsrisiko ist >50%... und ihr solltet Myriad's Flurizan im Auge behalten. Flurizan basiert auf der gleichen Beta-Amyloid Theorie. Myriad wird zur gleichen Zeit im Juli die PIII Daten (>1000 Patienten) veröffentlichen. Wenn diese negativ ausfallen, wird dass auch deutliche Zweifel an der Theorie wecken... und damit indirekt an Bapineuzumab.
hier noch ein paar Kommentare in Englisch...
http://www.fool.com/investing/high-growth/2008/06/18/elans-a…
Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results. When you combine this with a small study size, murky trial endpoints, and unexpected post-hoc subgroup data, investors should feel just as ambiguous about bapineuzumab's prospects as they were before the phase 2 study data came out. Therefore, I think the risk of bapineuzumab failing in the clinic has not gone down.
http://seekingalpha.com/article/81715-alzheimer-drug-fails-t…" target="_blank" rel="nofollow ugc noopener">http://seekingalpha.com/article/81715-alzheimer-drug-fails-t…
Alzheimer Drug Fails Trial
For a drug expected to top $7B in annual sales, even a partial success looks like a failure to me. I’ve talked about the efficacy in modulating APP for the treatment of Alzheimer’s a few times and Derek Lowe @ has some great articles, so if you’re not familiar, I‘d check them out to catch up.
On to the news. I’m going to try and summarize the press release from Elan and Wyeth without all the spin. Bapineuzumab failed to reach significance on the primary endpoint in a phase 2 trial.
Simple, right? Maybe not. The trial did show efficacy, though magnitude wasn’t released, in a small subgroup of patients (in non-carriers of the ApoE4 allele, estimated to be ~40% of Alzheimer’s patients).
So, right off the bat, this should cut analyst expectations by 60% right?
Interestingly, in trying to explain away not hitting the primary endpoint for the general population, the release mentions that the study wasn’t powered to detect changes in ADAS-cog.
Fantastic. Let me just break down this flow and see if we can make more sense of it.
1.) Run a trial that is not powered to detect primary endpoint changes.
2.) Fail primary, ADAS-cog endpoint.
3.) Release that you succeeded in a subgroup of patients on many endpoints (that you admittedly aren’t powered to detect)
4.) Conclude with statements like, “bapineuzumab appeared to have clinical activity in treating Alzheimer’s disease“, and “…the Phase 2 study are a continued validation of the amyloid approach to Alzheimer’s disease”.
Fundamentally, I’m happy that there are companies with deep-pockets attempting to treat debilitating diseases in novel ways but doing things over and over, expecting different results is foolish.
Naja... ich werde vorerst nicht in Elan investieren, das ist mir zu riskant... ist aber ein sehr interessantes Thema und die Chancen und Risiken sind entsprechend hoch. Schön wäre es für die zig Millionen Alzheimer Patienten, wenn Bapineuzumab helfen würde.
Ansonsten viel Glück den Investierten!
mfg ipollit
"A year later, after some trial participants had died of Alzheimer's, autoposies revealed that those patients who received the vaccine had much lower levels of amyloid in their brains, a sticky plaque that destroys neurons."
das mag soweit richtig sein... allerdings gibt es zu dieser PII noch weitere Daten, die mich damals skeptisch gemacht haben, ob der Ansatz mit Beta Amyloid nicht falsch ist. Ich habe vorher jedenfalls Bapineuzumab auch positiv gesehen und es wird sicherlich ein Multi-Mrd Blockbuster, wenn es denn wirklich einen Effekt bei Alzheimer hat. Allerdings zeigten die weiteren Langzeit-Daten zu der Vakzine PII, dass zwar eindeutig wie oben geschrieben, das Beta Amyloid Plaque in den behandelten Personen reduziert war, was ja das Ziel ist. Allerdings haben die Patienten trotzdem die Demenz entwickelt, unabhängig davon, ob Beta Amyloid vorhanden war oder nicht. Entsprechendes hat sich auch aus Tierversuchen ergeben. Das ganze ist schon eine wahnsinnige Sache, die nur durch die hohe mögliche Gewinnchance erklärlich ist: Es gibt bis heute keinen Beleg dafür, das die Beta-Amyloid-Theorie richtig ist! Sie ist sogar unter Wissenschaftlern durchaus umstritten. Und trotzdem pumpen aktuell die Pharmas Milliarden in diese Theorie. Wenn es sich zeigen sollte, dass sie falsch ist, dann wäre das ganze Geld und er ganze Zeitaufwand umsonst gewesen!
Und in der Tat... es ist ja witzig, aber es besteht eindeutig ein Zusammenhang zu Dendreon und Provenge. Auch dort wurden nachträglich entsprechend angepasste Analysen durchgeführt und ebenfalls fehlt die Theorie des Wirkmechanismus hinter Provenge. Wenn man sich mal andere kleinere Biotechs anschaut, dann ist dies das typische Vorgehen, wenn etwas nicht funktioniert hat. Ein Paradebeispiel ist natürlich auch GPC: Satraplatin hat den primären Endpunkt verfehlt und nun werden diese Subgruppen Analysen durchgeführt... und irgendetwas wird schon (zufällig) (statistisch signifikant) positiv sein (wobei diese Analysen anders als z.B. jetzt bei Bapineuzumab wenigstens bereits im Studienprotokoll definiert waren). Naja... aber ich gebe zu, auch GPC ist da kein Stück besser und macht ebenfalls diesen Mist!
Ich finde eure Argumentation etwas paradox, ehrlich gesagt: Auf der einen Seite sagt ihr, dass 240 Patienten zu wenig wären, um statistisch signifikante Ergebnisse zu produzieren, auf der anderen Seite soll ein Subgruppe der 240 Patienten, die deutlich weniger Patienten enthält, klar signifikante Ergebnisse produziert haben????
 Ist das nicht erstmal sehr seltsam? Da argumentiert ihr dann, dass gerade das das geniale wäre, dass eine so kleine Menge statistisch signifikante Ergebnisse liefert. Wenn man sich allerdings mit Statistik ein klein wenig auskennt, dann weiß man auch, dass je kleiner die Gruppe ist, umso schneller es passieren kann, dass die Ergebnisse zufällig (ja, es hört sich komisch an, ist aber so) statistisch signifikant sind. (steht ja da auch: "This could still end up being a statistical fluke however, if the overall number of patients in the non-APOE4 group turns out to be very small." )
Ist das nicht erstmal sehr seltsam? Da argumentiert ihr dann, dass gerade das das geniale wäre, dass eine so kleine Menge statistisch signifikante Ergebnisse liefert. Wenn man sich allerdings mit Statistik ein klein wenig auskennt, dann weiß man auch, dass je kleiner die Gruppe ist, umso schneller es passieren kann, dass die Ergebnisse zufällig (ja, es hört sich komisch an, ist aber so) statistisch signifikant sind. (steht ja da auch: "This could still end up being a statistical fluke however, if the overall number of patients in the non-APOE4 group turns out to be very small." )Es heißt zwar schön "randomisierte doppeltverblindete" Studie... das gilt aber nur für die komplette Studie, nicht für diese Subgruppen. So ist die angeblich positive Subgruppe nicht randomisiert, also mit Ungleichgewichten im Zustand der Patienten, Alter usw! Dies gibt Elan sogar selbst zu: "Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results." Zudem ist eine nachträgliche Analyse auch nicht mehr verblindet, da man ja die Analyse sich erst überlegt, wenn man die Ergebnisse der Studie auf dem Tisch hat. Das ist dann natürlich nicht mehr blind, sondern man gestaltet die Analyse so, dass deren Ergebnis wie gewünscht positiv ist, während genauso logisch negative Analysen und Ergebnisse unter den Tisch fallen.
Vielleicht solltet ihr euch auch mal Gedanken machen, was passieren kann, wenn die vollständigen Ergebnisse der PII im Juli weniger positiv ausfallen... ich weiß nicht, ob dann nicht Elan deutlich überbewertet sind. Ich schätze das Abwärtsrisiko ist >50%... und ihr solltet Myriad's Flurizan im Auge behalten. Flurizan basiert auf der gleichen Beta-Amyloid Theorie. Myriad wird zur gleichen Zeit im Juli die PIII Daten (>1000 Patienten) veröffentlichen. Wenn diese negativ ausfallen, wird dass auch deutliche Zweifel an der Theorie wecken... und damit indirekt an Bapineuzumab.
hier noch ein paar Kommentare in Englisch...
http://www.fool.com/investing/high-growth/2008/06/18/elans-a…
Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results. When you combine this with a small study size, murky trial endpoints, and unexpected post-hoc subgroup data, investors should feel just as ambiguous about bapineuzumab's prospects as they were before the phase 2 study data came out. Therefore, I think the risk of bapineuzumab failing in the clinic has not gone down.
http://seekingalpha.com/article/81715-alzheimer-drug-fails-t…" target="_blank" rel="nofollow ugc noopener">http://seekingalpha.com/article/81715-alzheimer-drug-fails-t…
Alzheimer Drug Fails Trial
For a drug expected to top $7B in annual sales, even a partial success looks like a failure to me. I’ve talked about the efficacy in modulating APP for the treatment of Alzheimer’s a few times and Derek Lowe @ has some great articles, so if you’re not familiar, I‘d check them out to catch up.
On to the news. I’m going to try and summarize the press release from Elan and Wyeth without all the spin. Bapineuzumab failed to reach significance on the primary endpoint in a phase 2 trial.
Simple, right? Maybe not. The trial did show efficacy, though magnitude wasn’t released, in a small subgroup of patients (in non-carriers of the ApoE4 allele, estimated to be ~40% of Alzheimer’s patients).
So, right off the bat, this should cut analyst expectations by 60% right?
Interestingly, in trying to explain away not hitting the primary endpoint for the general population, the release mentions that the study wasn’t powered to detect changes in ADAS-cog.
Fantastic. Let me just break down this flow and see if we can make more sense of it.
1.) Run a trial that is not powered to detect primary endpoint changes.
2.) Fail primary, ADAS-cog endpoint.
3.) Release that you succeeded in a subgroup of patients on many endpoints (that you admittedly aren’t powered to detect)
4.) Conclude with statements like, “bapineuzumab appeared to have clinical activity in treating Alzheimer’s disease“, and “…the Phase 2 study are a continued validation of the amyloid approach to Alzheimer’s disease”.
Fundamentally, I’m happy that there are companies with deep-pockets attempting to treat debilitating diseases in novel ways but doing things over and over, expecting different results is foolish.
Naja... ich werde vorerst nicht in Elan investieren, das ist mir zu riskant... ist aber ein sehr interessantes Thema und die Chancen und Risiken sind entsprechend hoch. Schön wäre es für die zig Millionen Alzheimer Patienten, wenn Bapineuzumab helfen würde.
Ansonsten viel Glück den Investierten!
mfg ipollit
Antwort auf Beitrag Nr.: 34.325.877 von bernie55 am 18.06.08 16:41:23"Elan and Wyeth said that bapineuzumab, also known as AAB-001, did cause some brain swelling in the Phase II trial. To minimize this danger, the drug is being administered at a lower dose in the Phase III trial, which started last year."
auch etwas kritisch... wegen Hirnschwellungen wurde die Dosierung der PIII gesenkt. Wenn ich Cyberhexe richtig verstanden habe, gibt es 4 Kohorten mit jeweils einer anderen Dosierung und etwas weniger als die Hälfte erhalten Placebos. D.h. 4 x 60 Patienten gesamt... in einer Dosierung etwas mehr als 30 Patienten, die Bapineuzumab erhalten. Im Schnitt sind grob geschätzt 50% ohne ApoE4, was aber in dieser Studie deutlich abweichen kann, da die Patienten nicht danach ausgewählt wurden. Aber im Schnitt befinden sich etwa 15 "ohne ApoE4"-Patienten in einer Dosierung.
Insgesamt werden es vielleicht nur 4 x 15 also 60 Patienten sein, die diese angeblich statistisch signifikant positive Subgruppe bilden.
Wenn man auch noch höhere Dosierungen aufgrund der Nebenwirkungen streicht, fällt davon nochmal ein Teil heraus.
Am Ende basiert die PIII letztlich vielleicht auf den Ergebnissen von 30 nicht randomisierten Patienten, die mit Bapineuzumab behandelt wurden. Da finde ich die Formulierung von Elan "glasklare Ergebnisse" schon ziemlich verzerrt, wenn das alles sein soll!
mfg ipollit
auch etwas kritisch... wegen Hirnschwellungen wurde die Dosierung der PIII gesenkt. Wenn ich Cyberhexe richtig verstanden habe, gibt es 4 Kohorten mit jeweils einer anderen Dosierung und etwas weniger als die Hälfte erhalten Placebos. D.h. 4 x 60 Patienten gesamt... in einer Dosierung etwas mehr als 30 Patienten, die Bapineuzumab erhalten. Im Schnitt sind grob geschätzt 50% ohne ApoE4, was aber in dieser Studie deutlich abweichen kann, da die Patienten nicht danach ausgewählt wurden. Aber im Schnitt befinden sich etwa 15 "ohne ApoE4"-Patienten in einer Dosierung.
Insgesamt werden es vielleicht nur 4 x 15 also 60 Patienten sein, die diese angeblich statistisch signifikant positive Subgruppe bilden.
Wenn man auch noch höhere Dosierungen aufgrund der Nebenwirkungen streicht, fällt davon nochmal ein Teil heraus.
Am Ende basiert die PIII letztlich vielleicht auf den Ergebnissen von 30 nicht randomisierten Patienten, die mit Bapineuzumab behandelt wurden. Da finde ich die Formulierung von Elan "glasklare Ergebnisse" schon ziemlich verzerrt, wenn das alles sein soll!
mfg ipollit
>>NEW RUMOR! Exciting Breakthrough in Alzheimers!
I'm hearing a rumor that another subgroup has been identified RETROSPECTIVELY from the Phase 2 data showing statistical significance! The rumor is that those patients wearing light colored shoes showed statistical significance while those who were wearing dark colored shoes actually got worse! I think a lower dose for the dark colored shoes group will be probably be implemented in the Phase 3 trials. Let's hope so. No word yet on what would happen if the dark colored shoe group switched to a lighter colored shoe. Lot of research still needs to be done.<<

I'm hearing a rumor that another subgroup has been identified RETROSPECTIVELY from the Phase 2 data showing statistical significance! The rumor is that those patients wearing light colored shoes showed statistical significance while those who were wearing dark colored shoes actually got worse! I think a lower dose for the dark colored shoes group will be probably be implemented in the Phase 3 trials. Let's hope so. No word yet on what would happen if the dark colored shoe group switched to a lighter colored shoe. Lot of research still needs to be done.<<

Antwort auf Beitrag Nr.: 34.316.351 von Cyberhexe am 17.06.08 14:27:41da es sich um eine Pivotal-Studie handelt, könnte dise Studie m.E. in Verbindung mit Daten zur Arzneimittelsicherheit der initialisierten p3-Studien eine Zulassung begründen (für Nichtträger des Allels). In wie weit dies jedoch wahrscheinlich ist, kann ich nicht beurteilen. Möglicherweise wird hierüber im IV-Forum etwas ausführlicher diskutiert.
..habe was dazu im IV Bord gefunden
I’ve just one point to make in relation to the comments pasted below from you msg.
“Lars and Selkoe BOTH have said "It will take one year of safety data AND a positive adas-cog results to permit approval." ADAS-Cog positive was part of top line announcement on aab-001.
One year of a less than full enrollment safety data HITS in Dec 08. Full enrollment safety for one year SHOULD hit Oct 09. A Subpart e filing broadly hinted at by Allison Hume ( remember there were NO questions asked) at company day, would be a rolling filing and approval on aab-001 could happen before Thanksgiving 09 or Christmas 09. “
Although some speculated here, including myself, that because the p2 trials were of 18-mth duration over three years, and were indeed designated as pivotal, that a filing on p2 data was possible. This view however didn’t take until Lars made a related comment during 1q ’07. This was a few months after the first p2 cohort had completed and he may well have seen some data at that point. Remember, there were four separate p2 cohorts, the first of which started during 1q05 and completed in Nov/Dec ‘06.
Lars stated, without prompt or question, that there was no way the FDA would approve Bap on p2 data without at least 6 months of safety data for 1000 patients. That’s when this issue got legs. He said six months, not 12. Very recently Kelly Martin made a similar comment. He stated that the FDA would require 6-12 months of safety data from p3 before final approval.
For me, the bcs is 6 months, and 12 months is a shoe in for the lower dose to non-carriers. There has been no indication that the data needs to be available at the time of filing, and could well be supplemented via sub-e. This would allow for a filing soon after the first p3 trial has been fully enrolled, or final approval no longer than eight or nine months from the day the p3 trial has been fully enrolled.
I’m looking for approval based on p2 efficacy with 12 months p3 safety data for the lower dose to non-carriers. I believe sub-e will come in to play and allow for a filing b4 the safety data is available. I see a distinct possibility that it could all happen six months earlier.
I also believe that all patients will start on the lower dose anyway, and that the lower dose to non-carriers could be on the market b4 mid ’09 at best, or six months later than that at most. It depends on whether we require 6 or 12 months safety data.
In my view, they are going to push ahead with the lower dose to non-carriers.
Kind regards,
Peadar ‘Og
http://www.investorvillage.com/smbd.asp?mb=160&mn=243502&pt=…
..habe was dazu im IV Bord gefunden
I’ve just one point to make in relation to the comments pasted below from you msg.
“Lars and Selkoe BOTH have said "It will take one year of safety data AND a positive adas-cog results to permit approval." ADAS-Cog positive was part of top line announcement on aab-001.
One year of a less than full enrollment safety data HITS in Dec 08. Full enrollment safety for one year SHOULD hit Oct 09. A Subpart e filing broadly hinted at by Allison Hume ( remember there were NO questions asked) at company day, would be a rolling filing and approval on aab-001 could happen before Thanksgiving 09 or Christmas 09. “
Although some speculated here, including myself, that because the p2 trials were of 18-mth duration over three years, and were indeed designated as pivotal, that a filing on p2 data was possible. This view however didn’t take until Lars made a related comment during 1q ’07. This was a few months after the first p2 cohort had completed and he may well have seen some data at that point. Remember, there were four separate p2 cohorts, the first of which started during 1q05 and completed in Nov/Dec ‘06.
Lars stated, without prompt or question, that there was no way the FDA would approve Bap on p2 data without at least 6 months of safety data for 1000 patients. That’s when this issue got legs. He said six months, not 12. Very recently Kelly Martin made a similar comment. He stated that the FDA would require 6-12 months of safety data from p3 before final approval.
For me, the bcs is 6 months, and 12 months is a shoe in for the lower dose to non-carriers. There has been no indication that the data needs to be available at the time of filing, and could well be supplemented via sub-e. This would allow for a filing soon after the first p3 trial has been fully enrolled, or final approval no longer than eight or nine months from the day the p3 trial has been fully enrolled.
I’m looking for approval based on p2 efficacy with 12 months p3 safety data for the lower dose to non-carriers. I believe sub-e will come in to play and allow for a filing b4 the safety data is available. I see a distinct possibility that it could all happen six months earlier.
I also believe that all patients will start on the lower dose anyway, and that the lower dose to non-carriers could be on the market b4 mid ’09 at best, or six months later than that at most. It depends on whether we require 6 or 12 months safety data.
In my view, they are going to push ahead with the lower dose to non-carriers.
Kind regards,
Peadar ‘Og
http://www.investorvillage.com/smbd.asp?mb=160&mn=243502&pt=…
...übrigens werden in den p3-Studien Träger des Apoe4-Allels und Nichtträger unterschieden:
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Non-Carriers
http://www.clinicaltrials.gov/ct2/show/NCT00667810?term=bapi…
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Carriers
http://www.clinicaltrials.gov/ct2/show/NCT00676143?term=bapi…
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Non-Carriers
http://www.clinicaltrials.gov/ct2/show/NCT00667810?term=bapi…
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Carriers
http://www.clinicaltrials.gov/ct2/show/NCT00676143?term=bapi…
Elan, Wyeth: Response to Skeptics on Alzheimer's Data
Elan (ELN) and Wyeth (WYE) presented their phase II data on June 17th with bapineuzumab in Alzheimer’s Disease.
In brief, the data showed that out of the 240 patients in the trial, those who had no apoE4 had statistical improvement compared to those who did. The skeptics made their case, to which I reply:
1. The prospective analysis failed.
The success was with retrospective analysis. My response to that is that in phase II studies where there are sometimes too many variables, often variables that are not known or identified when the study originated, it is not unusual to undertake retrospective analysis to identify positive trends. And in this case, it was more than a trend – it was statistically significant. Keep in mind that this was a phase II study – not a pivotal phase III study, where the analysis would have to be prospectively defined. I hope Elan/Wyeth made appropriate changes to their Phase III study and statistical analysis plan [SAP] to reflect this.
2. There was no mention of dose response.
Fair enough - but that does not mean there was no dose response. Absence of affirmation does not mean affirmation of absence. While a dose response is scientifically appealing, take a look at it practically. If the drug worked at a dose, and there was no effect whatever with smaller doses, would you refuse to take the effective dose if you had AD?
3. The sample size was small.
Of course it was – it was a phase II study. On the flip side – maybe it is impressive that there was statistical significance even with such a small sample size?
4. If you data-mine enough, you will find something statistically significant.
Not entirely true – having data mined through many studies, I can state with confidence that there are some truly negative studies. More importantly, if the data mining finds something significant that is scientifically reasonable, should it not be given some importance and explored in a prospective, controlled, larger study? Many drugs, including Viagra, came out of such exercises.
5. It only worked on patients with ‘milder’ disease.
Even if true, does that imply those with mild disease should not be treated? Should they be allowed to progress to severe disease?
6. It worked in only a small subset of patients (approximately 40%). I would not call 40% small, and 40% of tens of millions (just in the US) is still a large number.
The details of the study will be presented on Tuesday, July 29, during an afternoon session at the ICAD meeting in Chicago. Let the data speak for itself.
http://seekingalpha.com/article/81940-elan-wyeth-response-to…
Elan (ELN) and Wyeth (WYE) presented their phase II data on June 17th with bapineuzumab in Alzheimer’s Disease.
In brief, the data showed that out of the 240 patients in the trial, those who had no apoE4 had statistical improvement compared to those who did. The skeptics made their case, to which I reply:
1. The prospective analysis failed.
The success was with retrospective analysis. My response to that is that in phase II studies where there are sometimes too many variables, often variables that are not known or identified when the study originated, it is not unusual to undertake retrospective analysis to identify positive trends. And in this case, it was more than a trend – it was statistically significant. Keep in mind that this was a phase II study – not a pivotal phase III study, where the analysis would have to be prospectively defined. I hope Elan/Wyeth made appropriate changes to their Phase III study and statistical analysis plan [SAP] to reflect this.
2. There was no mention of dose response.
Fair enough - but that does not mean there was no dose response. Absence of affirmation does not mean affirmation of absence. While a dose response is scientifically appealing, take a look at it practically. If the drug worked at a dose, and there was no effect whatever with smaller doses, would you refuse to take the effective dose if you had AD?
3. The sample size was small.
Of course it was – it was a phase II study. On the flip side – maybe it is impressive that there was statistical significance even with such a small sample size?
4. If you data-mine enough, you will find something statistically significant.
Not entirely true – having data mined through many studies, I can state with confidence that there are some truly negative studies. More importantly, if the data mining finds something significant that is scientifically reasonable, should it not be given some importance and explored in a prospective, controlled, larger study? Many drugs, including Viagra, came out of such exercises.
5. It only worked on patients with ‘milder’ disease.
Even if true, does that imply those with mild disease should not be treated? Should they be allowed to progress to severe disease?
6. It worked in only a small subset of patients (approximately 40%). I would not call 40% small, and 40% of tens of millions (just in the US) is still a large number.
The details of the study will be presented on Tuesday, July 29, during an afternoon session at the ICAD meeting in Chicago. Let the data speak for itself.
http://seekingalpha.com/article/81940-elan-wyeth-response-to…
New clue to Alzheimer's found in form of protein
By RANDOLPH E. SCHMID (AP Science Writer)
From Associated Press
June 22, 2008 2:16 PM EDT
WASHINGTON - Researchers have uncovered a new clue to the cause of Alzheimer's disease. The brains of people with the memory-robbing form of dementia are cluttered with a plaque made up of beta-amyloid, a sticky protein. But there long has been a question whether this is a cause of the disease or a side effect. Also involved are tangles of a protein called tau; some scientists suspect this is the cause.
Now, researchers have caused Alzheimer's symptoms in rats by injecting them with one particular form of beta-amyloid. Injections with other forms of beta-amyloid did not cause illness, which may explain why some people have beta-amyloid plaque in their brains but do not show disease symptoms.
The findings by a team led by Dr. Ganesh M. Shankar and "Dr. Dennis J. Selkoe" of Harvard Medical School were reported in Sunday's online edition of the journal Nature Medicine.
The researchers used extracts from the brains of people who donated their bodies to medicine.
Forms of soluble beta-amyloid containing different numbers of molecules, as well as insoluble cores of the brain plaque, were injected into the brains of mice. There was no detectable effect from the insoluble plaque or the soluble one-molecule or three-molecule forms, the researchers found.
But the two-molecule form of soluble beta-amyloid produced characteristics of Alzheimer's in the rats, they reported.
Those rats had impaired memory function, especially for newly learned behaviors. When the mouse brains were inspected, the density brain cells was reduced by 47 percent with the beta-amyloid seeming to affect synapses, the connections between cells that are essential for communication between them.
The research, for the first time, showed the effect of a particular type of beta-amyloid in the brain, said Dr. Marcelle Morrison-Bogorad, director of the division of neuroscience at the National Institute on Aging, which helped fund the research.
It was surprising that only one of the three types had an effect, she said in a telephone interview.
Morrison-Bogorad said the findings may help explain the discovery of plaque in the brains of people who do not develop dementia. For some time, doctors have wondered why they find some brains in autopsy that are heavily coated with beta-amyloid, but the person did not have Alzheimer's.
The answer may lie in the two types of beta-amyloid that did not cause symptoms.
Now, the question is why one has the damaging effect and not others.
"A lot of work needs to be done," Morrison-Bogorad said. "Nature keeps sending us down paths that look straight at the beginning, but there are a lot of curves before we get to the end."
Dr. Richard J. Hodes, director of the National Institute on Aging, said that "while more research is needed to replicate and extend these findings, this study has put yet one more piece into place in the puzzle that is Alzheimer's."
In addition to the Institute on Aging, the research was funded by Science Foundation Ireland, Wellcome Trust, the McKnight and Ellison foundations and the Lefler Small Grant Fund
http://www.investorvillage.com/smbd.asp?mb=160&mn=245028&pt=…
By RANDOLPH E. SCHMID (AP Science Writer)
From Associated Press
June 22, 2008 2:16 PM EDT
WASHINGTON - Researchers have uncovered a new clue to the cause of Alzheimer's disease. The brains of people with the memory-robbing form of dementia are cluttered with a plaque made up of beta-amyloid, a sticky protein. But there long has been a question whether this is a cause of the disease or a side effect. Also involved are tangles of a protein called tau; some scientists suspect this is the cause.
Now, researchers have caused Alzheimer's symptoms in rats by injecting them with one particular form of beta-amyloid. Injections with other forms of beta-amyloid did not cause illness, which may explain why some people have beta-amyloid plaque in their brains but do not show disease symptoms.
The findings by a team led by Dr. Ganesh M. Shankar and "Dr. Dennis J. Selkoe" of Harvard Medical School were reported in Sunday's online edition of the journal Nature Medicine.
The researchers used extracts from the brains of people who donated their bodies to medicine.
Forms of soluble beta-amyloid containing different numbers of molecules, as well as insoluble cores of the brain plaque, were injected into the brains of mice. There was no detectable effect from the insoluble plaque or the soluble one-molecule or three-molecule forms, the researchers found.
But the two-molecule form of soluble beta-amyloid produced characteristics of Alzheimer's in the rats, they reported.
Those rats had impaired memory function, especially for newly learned behaviors. When the mouse brains were inspected, the density brain cells was reduced by 47 percent with the beta-amyloid seeming to affect synapses, the connections between cells that are essential for communication between them.
The research, for the first time, showed the effect of a particular type of beta-amyloid in the brain, said Dr. Marcelle Morrison-Bogorad, director of the division of neuroscience at the National Institute on Aging, which helped fund the research.
It was surprising that only one of the three types had an effect, she said in a telephone interview.
Morrison-Bogorad said the findings may help explain the discovery of plaque in the brains of people who do not develop dementia. For some time, doctors have wondered why they find some brains in autopsy that are heavily coated with beta-amyloid, but the person did not have Alzheimer's.
The answer may lie in the two types of beta-amyloid that did not cause symptoms.
Now, the question is why one has the damaging effect and not others.
"A lot of work needs to be done," Morrison-Bogorad said. "Nature keeps sending us down paths that look straight at the beginning, but there are a lot of curves before we get to the end."
Dr. Richard J. Hodes, director of the National Institute on Aging, said that "while more research is needed to replicate and extend these findings, this study has put yet one more piece into place in the puzzle that is Alzheimer's."
In addition to the Institute on Aging, the research was funded by Science Foundation Ireland, Wellcome Trust, the McKnight and Ellison foundations and the Lefler Small Grant Fund
http://www.investorvillage.com/smbd.asp?mb=160&mn=245028&pt=…
Davy Starting Points
Elan Corp
Next AD newsflow to come from Myriad Genetics
Price $34.56 Target: $31.00 Issued: 18/06/08 Previous: $25.50 Issued: 23/01/08
As we continue to digest Bapineuzumab’s promising top-line Phase II data, Myriad Genetics is expected to announce Phase III data on its own AD prospect, Flurizan.These data are expected this week.
Flurizan is a gamma secretase modulator – that is, it attempts to partly inhibit the function of this particular enzyme so that beta amyloid production can be reduced.
Full inhibition of this enzyme has had implications for other systemic functions that have proved very difficult to overcome. Myriad is conducting two Phase III studies, and is reporting on the 1,684-patient US trial imminently.
In Phase II, Flurizan did not meet statistical significance in mild-to-moderate AD,but did show positive trends in post-hoc analyses of the mild AD patient subset.Further analysis found statistical significance for two functional endpoints, in mild AD patients who also had high blood concentrations of the drug. The cognitive improvement over placebo, though favourable, was modest and not statistically significant.
On balance, we believe Bapineuzumab offers more promising and clinically significant efficacy signals, based on the top-line data released last week. Indeed, both products may have complementary mechanisms of action:Flurizan to inhibit production, and Bapineuzumab to clear existing amyloid.
The stakes are obviously very high for Myriad given that this will be a Phase III result. Expectations, as reflected in Myriad’s share price performance, have been set reasonably low: the stock is only up 6.7% year-to-date — though that conceals a near-45% rebound since early March. But in a nascent commercial AD market,any reasonable efficacy signals, combined with a benign safety profile, could offer approval potential for this and other products.
http://www.rte.ie/business/2008/morningrep/download/0624davy…
Elan Corp
Next AD newsflow to come from Myriad Genetics
Price $34.56 Target: $31.00 Issued: 18/06/08 Previous: $25.50 Issued: 23/01/08
As we continue to digest Bapineuzumab’s promising top-line Phase II data, Myriad Genetics is expected to announce Phase III data on its own AD prospect, Flurizan.These data are expected this week.
Flurizan is a gamma secretase modulator – that is, it attempts to partly inhibit the function of this particular enzyme so that beta amyloid production can be reduced.
Full inhibition of this enzyme has had implications for other systemic functions that have proved very difficult to overcome. Myriad is conducting two Phase III studies, and is reporting on the 1,684-patient US trial imminently.
In Phase II, Flurizan did not meet statistical significance in mild-to-moderate AD,but did show positive trends in post-hoc analyses of the mild AD patient subset.Further analysis found statistical significance for two functional endpoints, in mild AD patients who also had high blood concentrations of the drug. The cognitive improvement over placebo, though favourable, was modest and not statistically significant.
On balance, we believe Bapineuzumab offers more promising and clinically significant efficacy signals, based on the top-line data released last week. Indeed, both products may have complementary mechanisms of action:Flurizan to inhibit production, and Bapineuzumab to clear existing amyloid.
The stakes are obviously very high for Myriad given that this will be a Phase III result. Expectations, as reflected in Myriad’s share price performance, have been set reasonably low: the stock is only up 6.7% year-to-date — though that conceals a near-45% rebound since early March. But in a nascent commercial AD market,any reasonable efficacy signals, combined with a benign safety profile, could offer approval potential for this and other products.
http://www.rte.ie/business/2008/morningrep/download/0624davy…
Study Evaluating the Efficacy and Safety of Bapineuzumab in Alzheimer Disease Patients
This study is currently recruiting participants.
Verified by Wyeth, June 2008
> study details as provided by Wyeth:
Primary Outcome Measures:
Alzheimer's Disease Assessment Scale-Cognitive Subscale; Disability Assessment for Dementia [ Time Frame: 78 weeks ] [ Designated as safety issue: No ]
Secondary Outcome Measures:
Neuropsychological Test Battery; Clinical Dementia Rating Scale [ Time Frame: 78 Weeks ] [ Designated as safety issue: No ]
Estimated Enrollment: 1250
Study Start Date: May 2008
Estimated Study Completion Date: April 2011
Estimated Primary Completion Date: April 2011 (Final data collection date for primary outcome measure)
>Arms >Assigned Interventions
A: Experimental Drug: bapineuzumab
B: Experimental Drug: bapineuzumab
C: Experimental Drug: bapineuzumab
D: Placebo Comparator Drug: placebo
http://clinicaltrials.gov/ct2/show/NCT00667810?term=AAB-001&…
This study is currently recruiting participants.
Verified by Wyeth, June 2008
> study details as provided by Wyeth:
Primary Outcome Measures:
Alzheimer's Disease Assessment Scale-Cognitive Subscale; Disability Assessment for Dementia [ Time Frame: 78 weeks ] [ Designated as safety issue: No ]
Secondary Outcome Measures:
Neuropsychological Test Battery; Clinical Dementia Rating Scale [ Time Frame: 78 Weeks ] [ Designated as safety issue: No ]
Estimated Enrollment: 1250
Study Start Date: May 2008
Estimated Study Completion Date: April 2011
Estimated Primary Completion Date: April 2011 (Final data collection date for primary outcome measure)
>Arms >Assigned Interventions
A: Experimental Drug: bapineuzumab
B: Experimental Drug: bapineuzumab
C: Experimental Drug: bapineuzumab
D: Placebo Comparator Drug: placebo
http://clinicaltrials.gov/ct2/show/NCT00667810?term=AAB-001&…
ein weiterer Hinweis, dass die Beta-Amyloid-Theorie auf wackligen Beinen steht... Flurizan ist gescheitert!
kommt das jemanden bekannt vor?
"Myriad Genetics Inc. on Monday said its drug Flurizan failed to effect a significant improvement in patients with mild to moderate Alzheimer's disease, though some benefit was seen in certain patients with mild forms of the disease.
The Salt Lake City-based company said that while the drug, Flurizan, did not reach the main goal of a mid-stage, or Phase II clinical trial, enough positive trends were seen in patients with mild Alzheimer's disease to warrant continuation of a Phase III, or late-stage trial.
The main goals of the trial included showing statistically significant improvements in the ability to carry out the activities of daily living such as eating and dressing and an slowing in the rate of cognitive decline.
Patients with mild forms of the disease who were taking 800 milligrams of the drug twice daily did show significant improvements in these measures, the company said. The company is hopeful the drug may have some benefit in patients with mild Alzheimer's disease, tells Reuters. Myriad said the 12-month study of 207 patients showed that some test goals achieved statistical significance for patients with a mild version of Alzheimer's disease. The clinical study measured three indicators of daily living and cognitive function."
also...
- keine signifikante Verbesserung in der PII für alle 207 Patienten
- aber unglaublicherweise wurde bei einer Untergruppe (mit leichteren Krankheitsverlauf) bereits jetzt eine statistisch signifikante Verbesserung erzielt!!!! Wenn das mal kein gutes Zeichen ist!
Wenn das mal kein gutes Zeichen ist! 
- die Phase III wurde bereits vor dem Vorliegen der Phase II Ergebnisse begonnen. Mensch, dann müssen die doch sich sehr sicher sein, dass Flurizan ein Blockbuster wird!
- das Unternehmen ist sehr hoffnungsvoll und passt die PIII nun entsprechend dieser Subgruppe an!
**************
Und wie geht diese Geschichte nun aus? Was ist 3 Jahre später das Ergebnis? Haben wir den erhofften Blockbuster? Der Kurs ist immerhin nur gestiegen und gestiegen...
die Adhoc fällt nicht allzu lange aus...
Myriad Genetics Reports Results of U.S. Phase 3 Trial of Flurizan(TM) in Alzheimer's Disease
Flurizan Fails to Achieve Significance on Either Co-Primary Endpoint; Company Has Decided to Discontinue Its Development of Flurizan
June 30, 2008: 02:30 AM EST
Myriad Genetics, Inc. (NASDAQ: MYGN) today announced results of the Act-Earli-AD trial, an 18-month Phase 3 study of Flurizan (tarenflurbil) in patients with mild Alzheimer's disease. The study did not achieve statistical significance on either of its primary endpoints -- cognition and activities of daily living.
"We are disappointed that Flurizan failed to achieve significance in this study, and we will now discontinue development of this compound ," said Peter Meldrum, President and Chief Executive Officer of Myriad Genetics, Inc. "The discontinuation of Flurizan will reduce our pharmaceutical development spend substantially and should enable Myriad to achieve profitability next year, ending June 30, 2009."
," said Peter Meldrum, President and Chief Executive Officer of Myriad Genetics, Inc. "The discontinuation of Flurizan will reduce our pharmaceutical development spend substantially and should enable Myriad to achieve profitability next year, ending June 30, 2009."
During fiscal 2008, Myriad spent approximately $60 million on development of Flurizan. The remaining expenses to wrap up its Flurizan program are projected to be approximately $8 million in total, spread primarily over the next two fiscal quarters.
Ups... waren die Ergebnisse der PII nicht noch signifikant positiv? Was ist passiert? Warum kommt etwas anderes dabei heraus, wenn man nun fast 1700 Patienten einbezieht???
Fakt ist...
- die PIII ist definitiv fehlgeschlagen!
- Flurizan wird eingestampft!
- positiv ist zu anzumerken, dass Myriad hier keine Spielchen mehr anfängt mit Subgruppen und Subanalysen, um noch irgendwelche zu suchen, bei denen Flurizan zufällig doch positiv aussieht... so wie es andere gerne machen.
*****************
Und nun kommt die Elan-Geschichte...
nochmal zu den Punkten oben: wir haben eine PII mit...
- keine signifikante Verbesserung in der PII für alle 240 Patienten
- aber unglaublicherweise wurde bei einer Untergruppe (bei denen das Gen ApoE4 fehlt, d.h. die ein geringeres Risiko haben, Alzheimer zu entwickeln) bereits jetzt eine statistisch signifikante Verbesserung erzielt!!!! Wenn das mal kein gutes Zeichen ist!
Wenn das mal kein gutes Zeichen ist! 
- die Phase III wurde bereits vor dem Vorliegen der Phase II Ergebnisse begonnen. Mensch, dann müssen die doch sich sehr sicher sein, dass Bapineuzumab ein Blockbuster wird!
- das Unternehmen ist sehr hoffnungsvoll und passt die PIII nun entsprechend dieser Subgruppe (ohne ApoE4) an!
na... sieht jemand einen Zusammenhang? Reiner Zufall?
mfg ipollit
kommt das jemanden bekannt vor?
"Myriad Genetics Inc. on Monday said its drug Flurizan failed to effect a significant improvement in patients with mild to moderate Alzheimer's disease, though some benefit was seen in certain patients with mild forms of the disease.
The Salt Lake City-based company said that while the drug, Flurizan, did not reach the main goal of a mid-stage, or Phase II clinical trial, enough positive trends were seen in patients with mild Alzheimer's disease to warrant continuation of a Phase III, or late-stage trial.
The main goals of the trial included showing statistically significant improvements in the ability to carry out the activities of daily living such as eating and dressing and an slowing in the rate of cognitive decline.
Patients with mild forms of the disease who were taking 800 milligrams of the drug twice daily did show significant improvements in these measures, the company said. The company is hopeful the drug may have some benefit in patients with mild Alzheimer's disease, tells Reuters. Myriad said the 12-month study of 207 patients showed that some test goals achieved statistical significance for patients with a mild version of Alzheimer's disease. The clinical study measured three indicators of daily living and cognitive function."
also...
- keine signifikante Verbesserung in der PII für alle 207 Patienten
- aber unglaublicherweise wurde bei einer Untergruppe (mit leichteren Krankheitsverlauf) bereits jetzt eine statistisch signifikante Verbesserung erzielt!!!!
 Wenn das mal kein gutes Zeichen ist!
Wenn das mal kein gutes Zeichen ist! 
- die Phase III wurde bereits vor dem Vorliegen der Phase II Ergebnisse begonnen. Mensch, dann müssen die doch sich sehr sicher sein, dass Flurizan ein Blockbuster wird!

- das Unternehmen ist sehr hoffnungsvoll und passt die PIII nun entsprechend dieser Subgruppe an!
**************
Und wie geht diese Geschichte nun aus? Was ist 3 Jahre später das Ergebnis? Haben wir den erhofften Blockbuster? Der Kurs ist immerhin nur gestiegen und gestiegen...
die Adhoc fällt nicht allzu lange aus...
Myriad Genetics Reports Results of U.S. Phase 3 Trial of Flurizan(TM) in Alzheimer's Disease
Flurizan Fails to Achieve Significance on Either Co-Primary Endpoint; Company Has Decided to Discontinue Its Development of Flurizan
June 30, 2008: 02:30 AM EST
Myriad Genetics, Inc. (NASDAQ: MYGN) today announced results of the Act-Earli-AD trial, an 18-month Phase 3 study of Flurizan (tarenflurbil) in patients with mild Alzheimer's disease. The study did not achieve statistical significance on either of its primary endpoints -- cognition and activities of daily living.
"We are disappointed that Flurizan failed to achieve significance in this study, and we will now discontinue development of this compound
 ," said Peter Meldrum, President and Chief Executive Officer of Myriad Genetics, Inc. "The discontinuation of Flurizan will reduce our pharmaceutical development spend substantially and should enable Myriad to achieve profitability next year, ending June 30, 2009."
," said Peter Meldrum, President and Chief Executive Officer of Myriad Genetics, Inc. "The discontinuation of Flurizan will reduce our pharmaceutical development spend substantially and should enable Myriad to achieve profitability next year, ending June 30, 2009." During fiscal 2008, Myriad spent approximately $60 million on development of Flurizan. The remaining expenses to wrap up its Flurizan program are projected to be approximately $8 million in total, spread primarily over the next two fiscal quarters.
Ups... waren die Ergebnisse der PII nicht noch signifikant positiv? Was ist passiert? Warum kommt etwas anderes dabei heraus, wenn man nun fast 1700 Patienten einbezieht???
Fakt ist...
- die PIII ist definitiv fehlgeschlagen!
- Flurizan wird eingestampft!
- positiv ist zu anzumerken, dass Myriad hier keine Spielchen mehr anfängt mit Subgruppen und Subanalysen, um noch irgendwelche zu suchen, bei denen Flurizan zufällig doch positiv aussieht... so wie es andere gerne machen.
*****************
Und nun kommt die Elan-Geschichte...
nochmal zu den Punkten oben: wir haben eine PII mit...
- keine signifikante Verbesserung in der PII für alle 240 Patienten
- aber unglaublicherweise wurde bei einer Untergruppe (bei denen das Gen ApoE4 fehlt, d.h. die ein geringeres Risiko haben, Alzheimer zu entwickeln) bereits jetzt eine statistisch signifikante Verbesserung erzielt!!!!
 Wenn das mal kein gutes Zeichen ist!
Wenn das mal kein gutes Zeichen ist! 
- die Phase III wurde bereits vor dem Vorliegen der Phase II Ergebnisse begonnen. Mensch, dann müssen die doch sich sehr sicher sein, dass Bapineuzumab ein Blockbuster wird!

- das Unternehmen ist sehr hoffnungsvoll und passt die PIII nun entsprechend dieser Subgruppe (ohne ApoE4) an!
na... sieht jemand einen Zusammenhang? Reiner Zufall?

mfg ipollit
Antwort auf Beitrag Nr.: 34.403.484 von ipollit am 30.06.08 13:10:58@ipolit
..unabhängig von deiner Fachkompetenz, vor der ich wirklich Respekt habe , verstehe ich deine hämisch-ironische Art absolut nicht mehr.......
Du präsentierst dich als Fachmann...OK !!!
...aber als " Besserwisser" mit der " ätschebätsche Mentalität " disqualifizierst du dich leider selbst....schade !!!!
.....glaub mir, es würde dir besser stehen, , wenn du dein Wissen anders präsentieren würdest.....
....vielleicht erinnnerst du dich noch an den Juli 2007......keiner hat sich in irgendeiner Form hämisch geäußert, als die von dir so hochgelobte GPC einen Crashflug in Richtung Süden gemacht hat......da hat Dir dein Fachwissen im übrigen leider auch nicht geholfen....
...du warst sogar so frustriert, dass du dich am 25.07.07 in deinem Thread Biotechdepot 2007 mit folgenden Worten verabschiedet hast...
so... ich glaube diesen Thread kann ich schließen!
bye bye w : o...
....nochmals , ich finde deine " kritschen " Anmerkungen wirklich gut, da wir als Biotech-Laien einen kritischeren Einblick in die Thematik bekommen, jedoch würde ich es mir wünschen, mal einen ipolit Fachmann zu lesen, der sein Fachwissen zeigt und weniger seinen " ätschebätsche " Touch heraushängen lässt.
Grüße bernie55
..unabhängig von deiner Fachkompetenz, vor der ich wirklich Respekt habe , verstehe ich deine hämisch-ironische Art absolut nicht mehr.......
Du präsentierst dich als Fachmann...OK !!!
...aber als " Besserwisser" mit der " ätschebätsche Mentalität " disqualifizierst du dich leider selbst....schade !!!!
.....glaub mir, es würde dir besser stehen, , wenn du dein Wissen anders präsentieren würdest.....
....vielleicht erinnnerst du dich noch an den Juli 2007......keiner hat sich in irgendeiner Form hämisch geäußert, als die von dir so hochgelobte GPC einen Crashflug in Richtung Süden gemacht hat......da hat Dir dein Fachwissen im übrigen leider auch nicht geholfen....
...du warst sogar so frustriert, dass du dich am 25.07.07 in deinem Thread Biotechdepot 2007 mit folgenden Worten verabschiedet hast...
so... ich glaube diesen Thread kann ich schließen!
bye bye w : o...

....nochmals , ich finde deine " kritschen " Anmerkungen wirklich gut, da wir als Biotech-Laien einen kritischeren Einblick in die Thematik bekommen, jedoch würde ich es mir wünschen, mal einen ipolit Fachmann zu lesen, der sein Fachwissen zeigt und weniger seinen " ätschebätsche " Touch heraushängen lässt.
Grüße bernie55
Antwort auf Beitrag Nr.: 34.403.916 von bernie55 am 30.06.08 14:13:14....nochmals , ich finde deine " kritschen " Anmerkungen wirklich gut, da wir als Biotech-Laien einen kritischeren Einblick in die Thematik bekommen
..da wäre es angebracht, mal deine Meinung zu den genauen PII Ergebnissen zu hören.....
..da wäre es angebracht, mal deine Meinung zu den genauen PII Ergebnissen zu hören.....
Antwort auf Beitrag Nr.: 34.329.190 von ipollit am 19.06.08 00:30:21Naja... ich werde vorerst nicht in Elan investieren, das ist mir zu riskant... ist aber ein sehr interessantes Thema und die Chancen und Risiken sind entsprechend hoch. Schön wäre es für die zig Millionen Alzheimer Patienten, wenn Bapineuzumab helfen würde.
Ansonsten viel Glück den Investierten!
mfg ipollit
...du kannst ja auch anders, ipolit..
..schade, vielleicht hättest du doch einige €s vor der ersten Ankündigung zu den PII Daten investieren sollen....
...es hätte sich zumindest bis heute schon ein bisschen gelohnt.....
Ansonsten viel Glück den Investierten!
mfg ipollit
...du kannst ja auch anders, ipolit..

..schade, vielleicht hättest du doch einige €s vor der ersten Ankündigung zu den PII Daten investieren sollen....
...es hätte sich zumindest bis heute schon ein bisschen gelohnt.....
@Cyberhexe
@ipolit
..so das habe ich gerade im Internet gefunden:
Upcoming Alzheimer's drugs seek to slow the course of the disease by intervening in the beta-amyloid pathway, focusing on a toxic soluble form of amyloid that later congeals into amyloid plaque, according to Jeffrey Cummings, MD, director of the Alzheimer's Disease Center at UCLA. The plaque itself, largely benign, is an after-effect of the damaging process.
Was ist bzw. was soll Flurizan bewirken ??
Flurizan, a gamma secretase modulator, interferes with the manufacture of "amyloid precursor protein," the parent protein of amyloid. With Flurizan, gamma secretase produces less of the most-toxic variety of soluble amyloid and more of a less-toxic sort, Cummings explains.
Was ist bzw. was soll BAP bewirken ???
Bapineuzumab, a humanized monoclonal antibody, is infused intravenously to remove plaque—as well as the toxic intermediate—from the brain, Cummings said. PBT2, a chelator or metal-protein attenuating compound, is currently in a Phase II trial in Sweden. According to Cummings, the drug is designed to inhibit the aggregation of amyloid before it becomes toxic. Researchers theorize that accumulation of metals in the brain converts beta-amyloid into an enzyme that catalyzes production of hydrogen peroxide and damages brain cells.
entnommen aus:
http://pharmexec.findpharma.com/pharmexec/Articles/The-Pharm…
@Cyberhexe
@ipolit
Flurizan und BAP basieren beide auf der Beta-Amyloid-Theorie -
beide Mittel, so wie ich es verstanden habe, gehen doch auf verschiedene Art und Weise an das Problem heran ???? ..siehe oben..
Kann man beide Mittel dann über " einen Kamm scheren ???
Es wäre schön, wenn ich eure Meinung dazu hören könnte !!!!
Grüße bernie55
@ipolit
..so das habe ich gerade im Internet gefunden:
Upcoming Alzheimer's drugs seek to slow the course of the disease by intervening in the beta-amyloid pathway, focusing on a toxic soluble form of amyloid that later congeals into amyloid plaque, according to Jeffrey Cummings, MD, director of the Alzheimer's Disease Center at UCLA. The plaque itself, largely benign, is an after-effect of the damaging process.
Was ist bzw. was soll Flurizan bewirken ??
Flurizan, a gamma secretase modulator, interferes with the manufacture of "amyloid precursor protein," the parent protein of amyloid. With Flurizan, gamma secretase produces less of the most-toxic variety of soluble amyloid and more of a less-toxic sort, Cummings explains.
Was ist bzw. was soll BAP bewirken ???
Bapineuzumab, a humanized monoclonal antibody, is infused intravenously to remove plaque—as well as the toxic intermediate—from the brain, Cummings said. PBT2, a chelator or metal-protein attenuating compound, is currently in a Phase II trial in Sweden. According to Cummings, the drug is designed to inhibit the aggregation of amyloid before it becomes toxic. Researchers theorize that accumulation of metals in the brain converts beta-amyloid into an enzyme that catalyzes production of hydrogen peroxide and damages brain cells.
entnommen aus:
http://pharmexec.findpharma.com/pharmexec/Articles/The-Pharm…
@Cyberhexe
@ipolit
Flurizan und BAP basieren beide auf der Beta-Amyloid-Theorie -
beide Mittel, so wie ich es verstanden habe, gehen doch auf verschiedene Art und Weise an das Problem heran ???? ..siehe oben..
Kann man beide Mittel dann über " einen Kamm scheren ???
Es wäre schön, wenn ich eure Meinung dazu hören könnte !!!!
Grüße bernie55

Antwort auf Beitrag Nr.: 34.404.661 von bernie55 am 30.06.08 15:49:02...wird gerade auch im IV Board diskutiert...
Flurizan vs monoclonal
Both bind Abeta but the kinetics and subsequent processes diverge after that. Positive results with flurizan would have provided further support for the argument that Abeta is a valiid target in the treatment of AD. Because of the signifigant differences between the approaches flurizan's failure should not affect the outllook for ELN's passive immunity trial. That's the way I look at it but who can predict.
------------------------------------------------------------------
flurizan is a gamma-secretase modulator, it binds to gamma-secretase and changes its 3 dimentional structure and it would still cleave APP, it may not be producing Abeta42, but it will still make other soluable amyloid peptides, using DS's words at the company day, "it just shifted them around", it is predictable it won't work.
------------------------------------------------------------------
The epidemiological data showed reduced AD risk for chronic ( multi-year) NSAID takers. Flurizan is similar
but was recently shown to bind to APP and reduce the amount of APP making it through
gamma secretase. This would be great as if could avoid side effects of gamma secretase inhibitors but it isn't clear how much inhibition you get or if there is any benefit against existing deposits ( which may or may not slowly dissolve and create the picomolar dimer concentrations that seem to be toxic).
Depending on details, it may or may not bind to abeta in any forms.
Comparing this to the antibody approach, let me relate a few observations in a stream of consciosness approach. Just looking at the pictures, it looks like the antibodies tend to bind on lower residues while
the gxxxg motifs tend to be at the other end ( which is where you suspect dimerization). So, ok,
it wouldn't be unreasonable for antibodies to lack a lot of specificity to a particular n-mer. Someone made a comment, IIRC, that the other end of the antibody "stick" matters. That is, just binding at the Fab side is only 1/2 the story you need someone to see the Fc side and actually remove the abeta. Presumably the NSAID's don't have this " remove me " structure
Flurizan vs monoclonal
Both bind Abeta but the kinetics and subsequent processes diverge after that. Positive results with flurizan would have provided further support for the argument that Abeta is a valiid target in the treatment of AD. Because of the signifigant differences between the approaches flurizan's failure should not affect the outllook for ELN's passive immunity trial. That's the way I look at it but who can predict.
------------------------------------------------------------------
flurizan is a gamma-secretase modulator, it binds to gamma-secretase and changes its 3 dimentional structure and it would still cleave APP, it may not be producing Abeta42, but it will still make other soluable amyloid peptides, using DS's words at the company day, "it just shifted them around", it is predictable it won't work.
------------------------------------------------------------------
The epidemiological data showed reduced AD risk for chronic ( multi-year) NSAID takers. Flurizan is similar
but was recently shown to bind to APP and reduce the amount of APP making it through
gamma secretase. This would be great as if could avoid side effects of gamma secretase inhibitors but it isn't clear how much inhibition you get or if there is any benefit against existing deposits ( which may or may not slowly dissolve and create the picomolar dimer concentrations that seem to be toxic).
Depending on details, it may or may not bind to abeta in any forms.
Comparing this to the antibody approach, let me relate a few observations in a stream of consciosness approach. Just looking at the pictures, it looks like the antibodies tend to bind on lower residues while
the gxxxg motifs tend to be at the other end ( which is where you suspect dimerization). So, ok,
it wouldn't be unreasonable for antibodies to lack a lot of specificity to a particular n-mer. Someone made a comment, IIRC, that the other end of the antibody "stick" matters. That is, just binding at the Fab side is only 1/2 the story you need someone to see the Fc side and actually remove the abeta. Presumably the NSAID's don't have this " remove me " structure
Antwort auf Beitrag Nr.: 34.404.661 von bernie55 am 30.06.08 15:49:02naja... Fachkompetenz habe ich höchstens sehr eingeschränkt. Ich gebe dir ja Recht, dass mich manches ein wenig aufregt, so dass ich vielleicht die Dinge etwas unpassend schreibe. Manchmal liegt es aber auch ein wenig an den Reaktionen, dass ich dann nicht so sachlich bleibe, wie es eigentlich angemessen wäre.
und es sind noch zwei Dinge, ob die Idee bzw. fundamentalen Gründe hinter einer Investition richtig oder falsch sind oder ob man gut investiert und sich bezügl der Richtung des Aktienkurs richtig positioniert hat. Ich interessiere mich schon eher für die fundamentalen Daten... was die Kursbewegungen betrifft, so liege ich doch meistens daneben, das gebe ich gerne zu.
Ich habe doch schonmal gesagt, dass GPC für mich eine gravierende Fehlinvestition war, die eigentlich so nicht passieren sollte. Gerade wenn man sich zu sicher ist, dann geht es schnell schief. Vielleicht schreibe ich auch deshalb hier, um einfach eine andere Sichtweise darzustellen, so dass man vielleicht mal über seine Investition nachdenkt. Aber im wesentlichen ist es auch ein wenig Ärger darüber, dass die Kurse selten den Fundamentaldaten folgen: wie auch heute wieder ELN im Plus und MYGN's Abschlag ist so minimal, dass er kaum relevant ist (wobei MYGN ja nicht nur Flurizan ist und anscheinend das Scheitern weitgehend erwartet wurde)
zu deiner Frage: Flurizan und BAP sind natürlich unterschiedlich vom Ansatz. Beide zielen zwar darauf ab, Beta-Amyloid-Plaques im Gehirn zu reduzieren... dies ist die Beta-Amyloid-Theorie, bei der man davon ausgeht, dass die Verklumpungen von Beta-Amyloid Ursache für die Zerstörung der Nervenzellen und damit Alzheimer sind. BAP macht dies aktiv, indem es als Antikörper die Verklumpungen markiert, so dass die Immunabwehr darauf reagiert und die Plaques auflöst. Flurizan geht einen anderen Weg, indem es die Bildung von Beta-Amyloid-Plaques verhindern soll, also dass sich überhaupt Verklumpungen bilden. Flurizan kann von diesem Ansatz aus schonmal gar nicht gegen bereits vorhandene Plaques wirken, BAP dagegen schon. Bei Alzheimer im Anfangsstadium sollte aber auch Flurizan die Bildung von Beta-Amyloid-Plaques verhindern und somit die Entstehung von Alzheimer zumindest bremsen, wenn die Theorie richtig ist.
Ein Risiko bei BAP könnte darin bestehen, dass das Gehirn anschwillt, was fatal wäre. Dies liegt aber u.U. bereits am Wirkmechanismus selbst, denn BAP aktiviert ja die Körperabwehr, der Körper attakiert dabei die Plaques im Gehirn so wie irgendwelche Fremdkörper z.B. bei einer Entzündung (so stelle ich mir das zumindest als Laie vor). Das soetwas passiert, hat man ja auch bei der PII gesehen... Elan hat deshalb die Dosierung in der PIII gesenkt.
Das grundsätzlich spannende ist aber, ob die Beta-Amyloid-Verklumpungen wirklich für Alzheimer verantwortlich sind. Das weiß nämlich bis heute keiner! Und meiner Meinung nach, sehe ich aus den bisherigen Ergebnissen eher, dass diese Theorie so nicht ganz stimmen kann. Trotzdem investieren die Pharmas Mrd in diese Sache. Dies muss kein Zeichen dafür sein, dass auch etwas dabei herauskommt... Lundbeck hat erst vor Kurzem 100 Mio USD an MYGN gezahlt und Flurizan ist trotzdem wenig später gefloppt. Die Sache ist insgesamt also ziemlich spannend! Es könnte auch sein, dass die Verklumpungen nur ein Nebenprodukt des Prozesses sind, der die Nervenzellen zerstört... dann bringt die Entfernung der Plaques nichts. Vielleicht sind die Plaques sogar eher eine Art Schutzmantel und die Zerstörung verstärkt Alzheimer sogar noch. Alles das ist bis jetzt unklar und unsicher... Elan verbreitet natürlich den Eindruck, dass alles bestens aussieht!
Gemessen an der Tatsache, dass jeder, der heute 85 oder älter wird mit grob 50% Wahrscheinlichkeit an Alzheimer erkrankt, wäre es natürlich sehr wünschenswert, wenn BAP funktioniert. Das ist keine Frage. Was mich nur stört, sind diese Darstellungen von Studienergebnissen: Nachträgliche Analysen bei vorliegenden Ergebnissen wie bei BAP oder auch bei Provenge grenzen für mich schon sehr an Manipulation und Täuschung der Anleger! Und das stört mich halt!
Wenn ihr in Elan investiert seid, dann hab ihr bis jetzt alles richtig gemacht... der Kurs gibt euch da eindeutig Recht!
mfg ipollit
und es sind noch zwei Dinge, ob die Idee bzw. fundamentalen Gründe hinter einer Investition richtig oder falsch sind oder ob man gut investiert und sich bezügl der Richtung des Aktienkurs richtig positioniert hat. Ich interessiere mich schon eher für die fundamentalen Daten... was die Kursbewegungen betrifft, so liege ich doch meistens daneben, das gebe ich gerne zu.
Ich habe doch schonmal gesagt, dass GPC für mich eine gravierende Fehlinvestition war, die eigentlich so nicht passieren sollte. Gerade wenn man sich zu sicher ist, dann geht es schnell schief. Vielleicht schreibe ich auch deshalb hier, um einfach eine andere Sichtweise darzustellen, so dass man vielleicht mal über seine Investition nachdenkt. Aber im wesentlichen ist es auch ein wenig Ärger darüber, dass die Kurse selten den Fundamentaldaten folgen: wie auch heute wieder ELN im Plus und MYGN's Abschlag ist so minimal, dass er kaum relevant ist (wobei MYGN ja nicht nur Flurizan ist und anscheinend das Scheitern weitgehend erwartet wurde)
zu deiner Frage: Flurizan und BAP sind natürlich unterschiedlich vom Ansatz. Beide zielen zwar darauf ab, Beta-Amyloid-Plaques im Gehirn zu reduzieren... dies ist die Beta-Amyloid-Theorie, bei der man davon ausgeht, dass die Verklumpungen von Beta-Amyloid Ursache für die Zerstörung der Nervenzellen und damit Alzheimer sind. BAP macht dies aktiv, indem es als Antikörper die Verklumpungen markiert, so dass die Immunabwehr darauf reagiert und die Plaques auflöst. Flurizan geht einen anderen Weg, indem es die Bildung von Beta-Amyloid-Plaques verhindern soll, also dass sich überhaupt Verklumpungen bilden. Flurizan kann von diesem Ansatz aus schonmal gar nicht gegen bereits vorhandene Plaques wirken, BAP dagegen schon. Bei Alzheimer im Anfangsstadium sollte aber auch Flurizan die Bildung von Beta-Amyloid-Plaques verhindern und somit die Entstehung von Alzheimer zumindest bremsen, wenn die Theorie richtig ist.
Ein Risiko bei BAP könnte darin bestehen, dass das Gehirn anschwillt, was fatal wäre. Dies liegt aber u.U. bereits am Wirkmechanismus selbst, denn BAP aktiviert ja die Körperabwehr, der Körper attakiert dabei die Plaques im Gehirn so wie irgendwelche Fremdkörper z.B. bei einer Entzündung (so stelle ich mir das zumindest als Laie vor). Das soetwas passiert, hat man ja auch bei der PII gesehen... Elan hat deshalb die Dosierung in der PIII gesenkt.
Das grundsätzlich spannende ist aber, ob die Beta-Amyloid-Verklumpungen wirklich für Alzheimer verantwortlich sind. Das weiß nämlich bis heute keiner! Und meiner Meinung nach, sehe ich aus den bisherigen Ergebnissen eher, dass diese Theorie so nicht ganz stimmen kann. Trotzdem investieren die Pharmas Mrd in diese Sache. Dies muss kein Zeichen dafür sein, dass auch etwas dabei herauskommt... Lundbeck hat erst vor Kurzem 100 Mio USD an MYGN gezahlt und Flurizan ist trotzdem wenig später gefloppt. Die Sache ist insgesamt also ziemlich spannend! Es könnte auch sein, dass die Verklumpungen nur ein Nebenprodukt des Prozesses sind, der die Nervenzellen zerstört... dann bringt die Entfernung der Plaques nichts. Vielleicht sind die Plaques sogar eher eine Art Schutzmantel und die Zerstörung verstärkt Alzheimer sogar noch. Alles das ist bis jetzt unklar und unsicher... Elan verbreitet natürlich den Eindruck, dass alles bestens aussieht!
Gemessen an der Tatsache, dass jeder, der heute 85 oder älter wird mit grob 50% Wahrscheinlichkeit an Alzheimer erkrankt, wäre es natürlich sehr wünschenswert, wenn BAP funktioniert. Das ist keine Frage. Was mich nur stört, sind diese Darstellungen von Studienergebnissen: Nachträgliche Analysen bei vorliegenden Ergebnissen wie bei BAP oder auch bei Provenge grenzen für mich schon sehr an Manipulation und Täuschung der Anleger! Und das stört mich halt!
Wenn ihr in Elan investiert seid, dann hab ihr bis jetzt alles richtig gemacht... der Kurs gibt euch da eindeutig Recht!
mfg ipollit
Antwort auf Beitrag Nr.: 34.405.406 von ipollit am 30.06.08 17:13:34Mittel gegen das Vergessen
Das Sterben von Nervenzellen soll aufgehalten werden: Eine neue Generation von Alzheimer-Medikamenten weckt Hoffnung.
Das erste Opfer, das wir kennen, starb vor 102 Jahren. Auguste Deter, eine 51 Jahre alte Frau kommt im November 1901 in ein psychiatrisches Krankenhaus in Frankfurt am Main. Sie ist orientierungslos, leidet an Paranoia und Halluzinationen und hat Gedächtnisstörungen. Als der Arzt sie am zweiten Tag fragt, wie lange sie schon hier sei, überlegt sie kurz und antwortet: drei Wochen. Als seine Patientin viereinhalb Jahre später stirbt, untersucht er ihr Gehirn. Unter dem Mikroskop entdeckt er überall kleine Klumpen, Plaques genannt. Das Gehirn von Auguste Deter ist davon durchsetzt. Es ist die erste Beobachtung der Krankheit, die wir heute unter dem Namen des Arztes kennen. Sein Name war Alois Alzheimer.
Die Plaques haben auch ein Jahrhundert später nichts von ihrer schrecklichen Faszination verloren. Im Gegenteil: Sie scheinen zum Schlüssel im Kampf gegen Alzheimer geworden zu sein. Denn eine wirkliche Wende in der medizinischen Schlacht gegen das große Vergessen bahnt sich an: Die ersten Medikamente, die spezifisch gegen Alzheimer gerichtet sind, durchlaufen zur Zeit die letzten Teststufen. Es könnte eine Revolution werden – und eine, die dringend gebraucht wird. Alleine in Deutschland sind nach Schätzungen mehr als 700 000 Menschen an Alzheimer erkrankt – und die vorhandenen Arzneien helfen nur wenig.
„Bisher gibt es eigentlich nur zwei Klassen von Medikamenten, die gegen Alzheimer eingesetzt werden können“, erklärt Sascha Weggen, der die Arbeitsgruppe Molekulare Neuropathologie an der Universität Düsseldorf leitet. Beide greifen in den Stoffwechsel des Gehirns ein. Die eine Klasse erhöht die Menge des wichtigen Botenstoffs Acetylcholin. Bei Alzheimerpatienten sterben die Zellen ab, die diesen Botenstoff ausschütten. Das führt zu verschiedenen Symptomen, wie etwa den frühen Gedächtnisstörungen. Der ständige Abbau des Acetylcholins, der normalerweise sehr schnell passiert, wird durch diese Medikamente verzögert. So können die abgestorbenen Zellen kompensiert werden. Ewig geht das aber auch nicht. „Wenn immer mehr von den Zellen sterben, sind irgendwann einfach keine Zellen mehr da, die Acetylcholin überhaupt produzieren“, erklärt Weggen. Auch Memantin, das einzige Medikament in der zweiten Klasse, setzt bei einem Botenstoff im Gehirn an. So richtig gut geklärt sei der Mechanismus aber nicht, gibt Weggen zu bedenken.
Immer wieder tauchen Zweifel an der Wirksamkeit dieser zugelassenen Medikamente auf. Und keines von ihnen packt die Krankheit an der Wurzel. Kein Wunder, denn lange Zeit war über die Ursachen so gut wie gar nichts bekannt. Man behandelte die Symptome und hoffte, die schlimmsten Auswirkungen ein wenig herauszögern zu können. Inzwischen weiß man mehr. Viele Rätsel der Alzheimerkrankheit sind in den letzten Jahren aufgeklärt worden, und die meisten Forscher sind sich mittlerweile einig, wie die Krankheit ungefähr entsteht. Das Schlagwort heißt Beta-Amyloid. Hinter diesem Namen verbirgt sich ein kleines Fragment eines wesentlich größeren Eiweißes namens APP. Im Gehirn wird aus dem APP das kleine Beta-Amyloid herausgeschnitten – durch entscheidende Moleküle, die Sekretasen. „Das sind im Grunde molekulare Scheren“, sagt Christian Haass, Biochemiker an der Universität München. Sie schneiden erst an einem Ende, dann am anderen das kleine Beta-Amyloid aus dem großen APP heraus. Was dann passiert, nennt Haass eine „tödliche Kaskade“: Nachdem das Beta-Amyloid herausgeschnitten wurde. verlässt es die Zelle, trifft auf andere Beta-Amyloid-Moleküle, mit denen es verklebt, und langsam bilden sich die gefürchteten Plaques, die schon Alois Alzheimer erkannt hat. Sie führen dann zum Absterben von Zellen und all den Symptomen, die aus einem geliebten Menschen plötzlich einen Fremden machen können.
Es ist nur eine These, dass alle wichtigen Veränderungen bei Alzheimer auf das kleine Beta-Amyloid zurückgehen. Aber die meisten Forscher sind davon überzeugt – und Pharmaunternehmen haben Milliarden darauf gewettet. An verschiedenen Stellen versuchen sie, die Entstehung der Plaques zu beeinflussen, um die Krankheit endlich da zu bekämpfen, wo ihre Ursachen liegen. Gegen beide Sekretasen, die molekularen Scheren, die das Beta-Amyloid erzeugen, sind Hemmstoffe in der Entwicklung. Sie sollen bewirken, dass diese Scheren weniger aktiv sind. So würde weniger Beta-Amyloid anfallen, die Plaques könnten sich nicht so schnell bilden, die Krankheit würde möglicherweise sogar vermieden. Einige dieser Hemmstoffe sind bereits in der letzten Phase der klinischen Erprobung. LY450139 heißt die Hoffnung des US-Pharmakonzerns Eli Lilly. Dieses Jahr hat eine Studie mit 1500 Patienten begonnen, um die Wirksamkeit der Substanz zu überprüfen. Sollte sie halten, was sie verspricht, könnte sie schon 2011 unter einem eingängigeren Namen Alzheimer-Patienten das Leben leichter machen.
Auch die US-Pharmafirma Myriad hat einen Sekretase-Hemmer in den Startlöchern: Flurizan. Dieser Wirkstoff, verwandt mit dem Schmerzmittel Ibuprofen, wird in zwei großen Studien getestet. Rund 2400 Patienten nehmen daran teil. Erste Ergebnisse will das Unternehmen noch diesen Monat bekannt geben. Sollten sie positiv sein, könnte Flurizan 2009 auf den Markt kommen.
Ob Flurizan wirklich die Sekretase hemmt, ist nach neuen Forschungsergebnissen, die diese Woche im Fachblatt „Nature“ (Band 453, S. 925) publiziert werden, unklar. Die Gruppe um den Neurowissenschaftler Thomas Kukar von der Mayo Klinik in Jacksonville, Florida, hat neben anderen Substanzen auch Flurizan untersucht und ist zu einem überraschenden Ergebnis gekommen. Ihre These: Das Medikament hemmt in erster Linie gar nicht die Sekretase. Stattdessen bindet es an das APP und verhindert so, dass es zerkleinert wird und verklebt. „Für die Wirksamkeit haben diese Befunde wenig Relevanz, aber sie zeigen, wie wenig auch jetzt noch über die kommende erste Generation von Alzheimer-Medikamenten bekannt ist“, sagt der Düsseldorfer Weggen. Dies gilt auch für andere neue Behandlungsansätze.
Die Firma Elan hat einen Impfstoff gegen Alzheimer entwickelt. Den Patienten wird ein Antikörper gespritzt, der das Immunsystem gegen die ungewollten Plaques richten soll. Ganz normale Zellen der Immunabwehr räumen dann das Beta-Amyloid weg. Die Tierversuche waren vielversprechend. Auch wenn das Unternehmen die Daten der ersten Versuche am Menschen noch nicht veröffentlicht hat, darf man hoffen. Nun hat eine große Studie des Impfstoffes in den USA und Kanada begonnen, die Klarheit bringen soll. Eine internationale Studie, an der auch Deutschland beteiligt sein soll, will das Unternehmen in den nächsten Wochen auf den Weg bringen. Erste Ergebnisse werden 2010 erwartet.
Sascha Weggen ist vorsichtig optimistisch: „In den nächsten ein bis vier Jahren wird es einen erheblichen Erkenntnisgewinn geben.“ Ob damit auch mehr Lebensqualität für Patienten einhergeht, hängt davon ab, ob die Amyloidhypothese richtig ist. „Wenn das Konzept nicht stimmen sollte, werden auch die Therapien nicht funktionieren“, sagt Weggen - und fügt hinzu: „Die Ergebnisse werden auf jeden Fall zeigen: Sind wir auf dem richtigen Weg? Ja oder Nein?“ Bei fast 20 Millionen Alzheimer-Patienten weltweit können wir nur hoffen, dass es der richtige Weg ist.
(Erschienen im gedruckten Tagesspiegel vom 12.06.2008)
Das Sterben von Nervenzellen soll aufgehalten werden: Eine neue Generation von Alzheimer-Medikamenten weckt Hoffnung.
Das erste Opfer, das wir kennen, starb vor 102 Jahren. Auguste Deter, eine 51 Jahre alte Frau kommt im November 1901 in ein psychiatrisches Krankenhaus in Frankfurt am Main. Sie ist orientierungslos, leidet an Paranoia und Halluzinationen und hat Gedächtnisstörungen. Als der Arzt sie am zweiten Tag fragt, wie lange sie schon hier sei, überlegt sie kurz und antwortet: drei Wochen. Als seine Patientin viereinhalb Jahre später stirbt, untersucht er ihr Gehirn. Unter dem Mikroskop entdeckt er überall kleine Klumpen, Plaques genannt. Das Gehirn von Auguste Deter ist davon durchsetzt. Es ist die erste Beobachtung der Krankheit, die wir heute unter dem Namen des Arztes kennen. Sein Name war Alois Alzheimer.
Die Plaques haben auch ein Jahrhundert später nichts von ihrer schrecklichen Faszination verloren. Im Gegenteil: Sie scheinen zum Schlüssel im Kampf gegen Alzheimer geworden zu sein. Denn eine wirkliche Wende in der medizinischen Schlacht gegen das große Vergessen bahnt sich an: Die ersten Medikamente, die spezifisch gegen Alzheimer gerichtet sind, durchlaufen zur Zeit die letzten Teststufen. Es könnte eine Revolution werden – und eine, die dringend gebraucht wird. Alleine in Deutschland sind nach Schätzungen mehr als 700 000 Menschen an Alzheimer erkrankt – und die vorhandenen Arzneien helfen nur wenig.
„Bisher gibt es eigentlich nur zwei Klassen von Medikamenten, die gegen Alzheimer eingesetzt werden können“, erklärt Sascha Weggen, der die Arbeitsgruppe Molekulare Neuropathologie an der Universität Düsseldorf leitet. Beide greifen in den Stoffwechsel des Gehirns ein. Die eine Klasse erhöht die Menge des wichtigen Botenstoffs Acetylcholin. Bei Alzheimerpatienten sterben die Zellen ab, die diesen Botenstoff ausschütten. Das führt zu verschiedenen Symptomen, wie etwa den frühen Gedächtnisstörungen. Der ständige Abbau des Acetylcholins, der normalerweise sehr schnell passiert, wird durch diese Medikamente verzögert. So können die abgestorbenen Zellen kompensiert werden. Ewig geht das aber auch nicht. „Wenn immer mehr von den Zellen sterben, sind irgendwann einfach keine Zellen mehr da, die Acetylcholin überhaupt produzieren“, erklärt Weggen. Auch Memantin, das einzige Medikament in der zweiten Klasse, setzt bei einem Botenstoff im Gehirn an. So richtig gut geklärt sei der Mechanismus aber nicht, gibt Weggen zu bedenken.
Immer wieder tauchen Zweifel an der Wirksamkeit dieser zugelassenen Medikamente auf. Und keines von ihnen packt die Krankheit an der Wurzel. Kein Wunder, denn lange Zeit war über die Ursachen so gut wie gar nichts bekannt. Man behandelte die Symptome und hoffte, die schlimmsten Auswirkungen ein wenig herauszögern zu können. Inzwischen weiß man mehr. Viele Rätsel der Alzheimerkrankheit sind in den letzten Jahren aufgeklärt worden, und die meisten Forscher sind sich mittlerweile einig, wie die Krankheit ungefähr entsteht. Das Schlagwort heißt Beta-Amyloid. Hinter diesem Namen verbirgt sich ein kleines Fragment eines wesentlich größeren Eiweißes namens APP. Im Gehirn wird aus dem APP das kleine Beta-Amyloid herausgeschnitten – durch entscheidende Moleküle, die Sekretasen. „Das sind im Grunde molekulare Scheren“, sagt Christian Haass, Biochemiker an der Universität München. Sie schneiden erst an einem Ende, dann am anderen das kleine Beta-Amyloid aus dem großen APP heraus. Was dann passiert, nennt Haass eine „tödliche Kaskade“: Nachdem das Beta-Amyloid herausgeschnitten wurde. verlässt es die Zelle, trifft auf andere Beta-Amyloid-Moleküle, mit denen es verklebt, und langsam bilden sich die gefürchteten Plaques, die schon Alois Alzheimer erkannt hat. Sie führen dann zum Absterben von Zellen und all den Symptomen, die aus einem geliebten Menschen plötzlich einen Fremden machen können.
Es ist nur eine These, dass alle wichtigen Veränderungen bei Alzheimer auf das kleine Beta-Amyloid zurückgehen. Aber die meisten Forscher sind davon überzeugt – und Pharmaunternehmen haben Milliarden darauf gewettet. An verschiedenen Stellen versuchen sie, die Entstehung der Plaques zu beeinflussen, um die Krankheit endlich da zu bekämpfen, wo ihre Ursachen liegen. Gegen beide Sekretasen, die molekularen Scheren, die das Beta-Amyloid erzeugen, sind Hemmstoffe in der Entwicklung. Sie sollen bewirken, dass diese Scheren weniger aktiv sind. So würde weniger Beta-Amyloid anfallen, die Plaques könnten sich nicht so schnell bilden, die Krankheit würde möglicherweise sogar vermieden. Einige dieser Hemmstoffe sind bereits in der letzten Phase der klinischen Erprobung. LY450139 heißt die Hoffnung des US-Pharmakonzerns Eli Lilly. Dieses Jahr hat eine Studie mit 1500 Patienten begonnen, um die Wirksamkeit der Substanz zu überprüfen. Sollte sie halten, was sie verspricht, könnte sie schon 2011 unter einem eingängigeren Namen Alzheimer-Patienten das Leben leichter machen.
Auch die US-Pharmafirma Myriad hat einen Sekretase-Hemmer in den Startlöchern: Flurizan. Dieser Wirkstoff, verwandt mit dem Schmerzmittel Ibuprofen, wird in zwei großen Studien getestet. Rund 2400 Patienten nehmen daran teil. Erste Ergebnisse will das Unternehmen noch diesen Monat bekannt geben. Sollten sie positiv sein, könnte Flurizan 2009 auf den Markt kommen.
Ob Flurizan wirklich die Sekretase hemmt, ist nach neuen Forschungsergebnissen, die diese Woche im Fachblatt „Nature“ (Band 453, S. 925) publiziert werden, unklar. Die Gruppe um den Neurowissenschaftler Thomas Kukar von der Mayo Klinik in Jacksonville, Florida, hat neben anderen Substanzen auch Flurizan untersucht und ist zu einem überraschenden Ergebnis gekommen. Ihre These: Das Medikament hemmt in erster Linie gar nicht die Sekretase. Stattdessen bindet es an das APP und verhindert so, dass es zerkleinert wird und verklebt. „Für die Wirksamkeit haben diese Befunde wenig Relevanz, aber sie zeigen, wie wenig auch jetzt noch über die kommende erste Generation von Alzheimer-Medikamenten bekannt ist“, sagt der Düsseldorfer Weggen. Dies gilt auch für andere neue Behandlungsansätze.
Die Firma Elan hat einen Impfstoff gegen Alzheimer entwickelt. Den Patienten wird ein Antikörper gespritzt, der das Immunsystem gegen die ungewollten Plaques richten soll. Ganz normale Zellen der Immunabwehr räumen dann das Beta-Amyloid weg. Die Tierversuche waren vielversprechend. Auch wenn das Unternehmen die Daten der ersten Versuche am Menschen noch nicht veröffentlicht hat, darf man hoffen. Nun hat eine große Studie des Impfstoffes in den USA und Kanada begonnen, die Klarheit bringen soll. Eine internationale Studie, an der auch Deutschland beteiligt sein soll, will das Unternehmen in den nächsten Wochen auf den Weg bringen. Erste Ergebnisse werden 2010 erwartet.
Sascha Weggen ist vorsichtig optimistisch: „In den nächsten ein bis vier Jahren wird es einen erheblichen Erkenntnisgewinn geben.“ Ob damit auch mehr Lebensqualität für Patienten einhergeht, hängt davon ab, ob die Amyloidhypothese richtig ist. „Wenn das Konzept nicht stimmen sollte, werden auch die Therapien nicht funktionieren“, sagt Weggen - und fügt hinzu: „Die Ergebnisse werden auf jeden Fall zeigen: Sind wir auf dem richtigen Weg? Ja oder Nein?“ Bei fast 20 Millionen Alzheimer-Patienten weltweit können wir nur hoffen, dass es der richtige Weg ist.
(Erschienen im gedruckten Tagesspiegel vom 12.06.2008)
Antwort auf Beitrag Nr.: 34.405.406 von ipollit am 30.06.08 17:13:34ipolit - vielen Dank, echt schön erklärt...
...und auch supi, dass du deine kleinen " emotionalen " Entgleisungen wieder zurechtrückst.....
...so macht`s richtig Spaß ......auf jeden Fall viel neues Wissen und Infos an der richtigen Stelle....
....well done, ipolit und nochmals THX...
....jetzt warten wir mal auf die Daten aus der PII....
Grüße bernie55

...und auch supi, dass du deine kleinen " emotionalen " Entgleisungen wieder zurechtrückst.....

...so macht`s richtig Spaß ......auf jeden Fall viel neues Wissen und Infos an der richtigen Stelle....
....well done, ipolit und nochmals THX...
....jetzt warten wir mal auf die Daten aus der PII....
Grüße bernie55

Antwort auf Beitrag Nr.: 34.405.494 von bernie55 am 30.06.08 17:28:30im Gegensatz zu Flurizan gibt es beim Ansatz von Elan/Wyeth bereits einen "proof of concept" und zwar mit dem Vorgängervaccin AN-1792, dessen Wirksamkeit bei der Plaque-Eliminierung nachgewiesen wurde.
http://archneur.ama-assn.org/cgi/content/abstract/64/4/583
Aufgrund von Nebenwirkungen (bei 6% der Studienteilnehmer wurden Entzündungen der Hirnhaut beobachtet) wurde diese Studie jedoch beendet. AAB-001 oder Bapineuzumab ist ein auf diesen Erkenntnissen modellierter Antikörper, der gegenüber dem Vaccin den Vorteil hat, nicht über eine aktive (Über)Stimulierung der Immunabwehr die Plaque anzugreifen, sondern passiv, also ohne körpereigene Immunreaktion, den degenerativen Prozess verhindern soll.
..anbei ein interessantes Video, in welchem Dale Schenk, Elans potentieller Nobelpreisträger (darauf würde ich wetten!), die Entwicklung über die letzten 20 Jahre beschreibt:
http://video.google.com/videoplay?docid=-7826073900349013452…
http://archneur.ama-assn.org/cgi/content/abstract/64/4/583
Aufgrund von Nebenwirkungen (bei 6% der Studienteilnehmer wurden Entzündungen der Hirnhaut beobachtet) wurde diese Studie jedoch beendet. AAB-001 oder Bapineuzumab ist ein auf diesen Erkenntnissen modellierter Antikörper, der gegenüber dem Vaccin den Vorteil hat, nicht über eine aktive (Über)Stimulierung der Immunabwehr die Plaque anzugreifen, sondern passiv, also ohne körpereigene Immunreaktion, den degenerativen Prozess verhindern soll.
..anbei ein interessantes Video, in welchem Dale Schenk, Elans potentieller Nobelpreisträger (darauf würde ich wetten!), die Entwicklung über die letzten 20 Jahre beschreibt:
http://video.google.com/videoplay?docid=-7826073900349013452…
Antwort auf Beitrag Nr.: 34.408.095 von Cyberhexe am 30.06.08 23:11:49Hallo Cybie,
....du konntest mich damals mit deinem " Know-How " von ELAN so überzeugen , dass ich ab April 05 zeit und kostengünstig einsteigen konnte.......
......dir volle THXs - mit vielen ********** .
..und nun warten wir alle natürlich auf die konkreten PII Daten........uiuiuiuiuiuiuiuiuiu ist das spannend...
Grüße bernie55
PS:
Schenk - Nominierung als potentieller Nobelpreisträger
Cyberhexe - Nominierung als potentieller Premium-Award-WO Boarder
....du konntest mich damals mit deinem " Know-How " von ELAN so überzeugen , dass ich ab April 05 zeit und kostengünstig einsteigen konnte.......

......dir volle THXs - mit vielen ********** .

..und nun warten wir alle natürlich auf die konkreten PII Daten........uiuiuiuiuiuiuiuiuiu ist das spannend...
Grüße bernie55

PS:
Schenk - Nominierung als potentieller Nobelpreisträger
Cyberhexe - Nominierung als potentieller Premium-Award-WO Boarder

Shots may rouse immune system to fight Alzheimer's
Posted on Tue, Jul. 01, 2008
BY ROGER SCHLUETER
News-Democrat
ST. LOUIS --St. Louis University doctors are hoping that a new drug study will be like a shot in the arm in the fight against Alzheimer's disease.
The research involves giving patients with mild to moderate Alzheimer's intravenous doses of an investigational drug. Like a flu shot or tetanus vaccination, the drug is designed to rev up the patient's own immune system, only this time to fight the mind-robbing disease.
"This therapy is the first treatment of its kind and holds great potential," said Dr. George Grossberg, director of the division of geriatric psychiatry at St. Louis University School of Medicine and the trial's principal investigator.
"Immune therapy has promise for being able to modify the course of Alzheimer's disease rather than simply treat the symptoms as current FDA-approved therapies do."
Grossberg calls the treatment a vaccination approach because it involves an antibody that doctors hope will attack the plaque that forms inside the brain of those with Alzheimer's. St. Louis University is the only St. Louis site taking part in the study, which will involve more than 2,000 patients at 200 sites across the United States and Canada.
Patients who participate in the research will receive an intravenous infusion of an antibody to the beta amyloid protein. This protein triggers the death of brain cells in Alzheimer's patients, scientists believe. Study participants will be randomly assigned to one of three groups: Two will receive different amounts of the real drug while a third group will receive a sham solution, or placebo.
Those eligible should be 50 to 88 years old and must have a caregiver to accompany them to all clinic visits. The caregivers also should see the volunteer at least five times a week during the study.
"It's important to note that these volunteers may stay on certain Alzheimer's medications," Grossberg said.
The study will last about 85 weeks. During 65 of those weeks, patients will receive hourlong infusions every 13 weeks at the St. Louis University Cancer Center. Researchers will look for physiological changes, such as whether MRIs show changes in the volume of the brain. They also will examine changes to mental health through various psychiatric screening tools.
About 40 volunteers are needed at the St. Louis site. For information, call (314) 977-4900.
The study is sponsored by Elan Pharmaceuticals and Wyeth Pharmaceuticals
Contact reporter Roger Schlueter at 239-2465 or rschlueter@bnd.com
--------------------------------------------------------------------------------
http://www.bnd.com/living/story/384821.html
Posted on Tue, Jul. 01, 2008
BY ROGER SCHLUETER
News-Democrat
ST. LOUIS --St. Louis University doctors are hoping that a new drug study will be like a shot in the arm in the fight against Alzheimer's disease.
The research involves giving patients with mild to moderate Alzheimer's intravenous doses of an investigational drug. Like a flu shot or tetanus vaccination, the drug is designed to rev up the patient's own immune system, only this time to fight the mind-robbing disease.
"This therapy is the first treatment of its kind and holds great potential," said Dr. George Grossberg, director of the division of geriatric psychiatry at St. Louis University School of Medicine and the trial's principal investigator.
"Immune therapy has promise for being able to modify the course of Alzheimer's disease rather than simply treat the symptoms as current FDA-approved therapies do."
Grossberg calls the treatment a vaccination approach because it involves an antibody that doctors hope will attack the plaque that forms inside the brain of those with Alzheimer's. St. Louis University is the only St. Louis site taking part in the study, which will involve more than 2,000 patients at 200 sites across the United States and Canada.
Patients who participate in the research will receive an intravenous infusion of an antibody to the beta amyloid protein. This protein triggers the death of brain cells in Alzheimer's patients, scientists believe. Study participants will be randomly assigned to one of three groups: Two will receive different amounts of the real drug while a third group will receive a sham solution, or placebo.
Those eligible should be 50 to 88 years old and must have a caregiver to accompany them to all clinic visits. The caregivers also should see the volunteer at least five times a week during the study.
"It's important to note that these volunteers may stay on certain Alzheimer's medications," Grossberg said.
The study will last about 85 weeks. During 65 of those weeks, patients will receive hourlong infusions every 13 weeks at the St. Louis University Cancer Center. Researchers will look for physiological changes, such as whether MRIs show changes in the volume of the brain. They also will examine changes to mental health through various psychiatric screening tools.
About 40 volunteers are needed at the St. Louis site. For information, call (314) 977-4900.
The study is sponsored by Elan Pharmaceuticals and Wyeth Pharmaceuticals
Contact reporter Roger Schlueter at 239-2465 or rschlueter@bnd.com
--------------------------------------------------------------------------------
http://www.bnd.com/living/story/384821.html
Pharmawire article by Kimberly Ha in New York
Wyeth/Elan's Alzheimer's candidate bapineuzumab faces potential Phase III hurdles - analysis
Story Wyeth/Elan's Alzheimer's candidate bapineuzumab faces potential Phase III hurdles, according to physicians and investigators.
Recently released data showed that bapineuzumab may improve cognitive functioning in patients who are non-carriers of the ApoE4 allele. Serious adverse events, such as vasogenic edema also occurred more frequently in bapineuzumab-treated APOE4 carriers.
Although the results for the subgroup were positive, the finding is inconclusive until it can be replicated and confirmed with larger follow-on studies, many of the physicians interviewed agreed.
“Many trials in the past reported findings that appeared highly promising, but were subsequently not confirmed in a duplicate trial,” said Dr. P. Murali Doraiswamy, chief of biological psychiatry at Duke University Medical.
APOE4 is only a correlating risk factor for Alzheimer’s, not necessarily a cause, and some physicians suggested that Wyeth and Elan’s bapineuzumab trials are just a “proof of principle” trials at best.
Scientists discovered the APOE4 correlation with Alzheimer's 15 years ago in the Roses Lab at Duke University, but have not yet established a causal link yet. Although 40-65% of AD patients have at least one copy of the 4 allele, APOE4 is not a determinant of the disease: at least a third of Alzheimer's patients are APOE4 negative and some APOE4 homozygotes never develop the disease.
Dr. G. William Rebeck, associate professor in the Department of Neuroscience at Georgetown University Medical Center, who has been conducting research on APOE since the early 1990s, said the study still needs to be duplicated as the higher frequency of vasogenic edema in patients carrying APOE4 was a post hoc analysis and there was no obvious underlying biological cause that would lead to such a reaction.
Dr Marwan Sabbagh, an investigator on Wyeth’s bapineuzumab trials and author of The Alzheimer’s Answer, said that so far the side-effects of vascular edema did not correlate with efficacy and are not predictive of a better response.
As APOE4 carriers (APOE4+) reported a higher incidence of adverse events, it is possible that bapineuzumab could be approved for non-APOE4 carriers. APOE4 status can be determined with a simple blood test.
Pfizer’s mild cognitive impairment (MCI) studies of Aricept (donepizil), a leading Alzheimer’s treatment, had a better effect in APOE4+ patients, while Wyeth’s bapineuzumab trial showed better effect in non-APOE4+ patients, according to Dr. Greg Jicha, an investigator on the Phase III trials, and professor of neurology at the University of Kentucky College of Medicine. Previous studies have found that the effect of the Aricept treatment lasted longer in those patients carrying the gene.
Studies have also shown that patients with the APOE4 gene have a higher propensity to develop Alzheimer's than the general population. "I query over this differential effect. I always took the Pfizer data to suggest that we can be a bit more definitive that patients with APOE4+ status had a higher likelihood of AD rather than an AD-mimicking disease," he added.
“The Phase IIb studies were clearly enough to make us understand we have to stratify dosing by genotype. It’s a first step in an area that will emerge as very common,” said Sabbagh.
When asked why Wyeth/Elan included APOE4+ patients on their Phase III trials, Rebeck said the companies were probably not "entirely convinced" from Phase II and would like to see it in a larger study.
Sabbagh said it is too late to change the trial design, even in light of the recent results. "My impression is that they were trying to just push ahead. It boils down to the first company to get an FDA-approved disease modifying agent, so there was this big push," added an anonymous investigator.
Another investigator speculated that a problem with Elan's Phase II trial was sample size calculation. "At least they got a signal in the non-APOE4 carriers which was enough for them to move forward," another investigator added.
Dr Neil Kurtz, CEO of Torreypines Therapeutics said Wyeth probably did not know about the APOE4 results, and that is why they recruited these patients into their larger Phase III trials.
An investigator who wished to remain anonymous explained that antibodies are specific for oligomers. Wyeth has said this drug is soluble for oligomers, he said, yet the drug was not designed to be specific. "I’m not sure whether they’re specific enough. If they could find any degree of activity, they can argue specificity. There are some antibodies that only recognize soluble oligomers, if you have a monoclonal cell line that produces a monoclonal antibody, you can humanize that and make it specific to a soluble amyloid."
He speculated that both companies are so invested in this antibody, "you don’t want to throw that out. But maybe we need to move to another approach."
In addition, the anonymous investigator said, "We had seen and heard there’s a great deal of skepticism about the agents that are pulling into Phase III trials. None of these agents have stellar early reports either. If I had Alzheimer's, I would enroll in Phase I and II studies rather than Phase III at this point."
Wyeth and Elan would not provide comment.
When asked whether Elan/Wyeth have the right Phase III trial design, such as patient characteristics and doses, the investigator said the companies were on target. Sabbagh added that protocol amendments can be done, but fundamental changes in trial design are usually not seen.
"APOE4 is definitely a risk factor and is found in roughly one-third of the US population. It seems to play an important role in the outcomes of clinical trials," said Dr Scott Turner, associate professor in the Department of Neurology at the University of Michigan Health System and incoming director of the Memory Disorders Program at Georgetown University Medical Center.
Only a subset of subjects will respond to certain medication, such as patients who are APOE4+. Myriad Genetics has data on the APOE4 status of patients on their recently failed Flurizan trial – but they haven’t released this specific data yet, said Turner.
"With the Flurizan trial, maybe some patients did tremendously on the drug, and some patients dragged down the overall results," said Jicha.
Myriad failed to meet its primary endpoint in a Phase III trial for a potential disease modifying agent named Flurizan earlier this week. "Flurizan tanked, just like Alzhemed. The site to site variation is plaguing the industry,” said Sabbagh.
The amount of resources needed for such large trials has become a problem, Rebeck noted. “When you’re analyzing data, sites ended up with statistically different results from each other. The assumption was that we could control for all the site to site variation,” he said.
Genotyping patients before deciding which therapy to give in the future may become a reality – and certain laws regarding APOE4 status to prevent genetic discrimination are currently underway, the physicians noted.
There is a clear need within the industry to develop and use a more objective quantifiable measure, such as the Pittsburgh Compound B (PIB), which binds to and can track levels of amyloid plaque in the brain.
“If we can quantify a reduction in amyloid load in the brain, that’s hard data that we as an industry have been sorely lacking. Many researchers in the field think it’s not good enough anymore, as we have surrogate markers now," said Sabbagh.
This is the first real step at looking at an objective measure in amyloid burden. Establishing a correlation with PIB would probably be the biggest finding of the year, and those works are underway, Sabbagh added. However, although the PIB principle is desirable, many physicians cautioned that proof is needed to show that PIB changes with disease progression. “I’ve heard some rumors that they have some studies that it changes with intervention,” said Sabbagh.
When asked whether PIB is at a stage where companies can start using that in trials, Sabbagh said, "theoretically, yes." One source close to Wyeth’s trials said PIB as an imaging agent was also proposed in the Phase III bapineuzumab trial, as well as many other studies. “Is PIB ready? No. Are we exploring it, yes,” he added.
If the correlation between amyloid plaque reduction and clinical improvement was shown by using the PIB imaging agent, it would be a significant move forward to Alzheimer's trials. PIB would be analogous to CD4 counts in HIV, said Turner.
"I think PIB will only give us a bit more biological data. We still need to find that surrogate marker," said Jicha.
There is currently no concrete correlation between amyloid plaque reduction and clinical benefit. "We don’t know that for a fact with human studies, but know that with mice. Plaques correlate with memory loss, and all of these are unanswered questions when it comes to human studies," said Turner.
"A lot of money is going into this. You can’t see individual plaques in living humans, but can see that on brain sections after death. You can see PIB binding in a nuclear medicine study, and this is one of the biomarkers being developed," said Turner.
The current industry goal is to stabilize the amount of plaque by the investigational drug, or even decrease the amount of plaque, and then conduct repeated PIB imaging scans as well as cognitive outcome measures to test for correlation, said Doraiswamy.
Some physicians also speculated that the recent failures may not have anything to do with the agent's efficacy, but a fundamental flaw in trial design, as there is no current standard set forth from the FDA on disease modifying agents for the treatment of Alzheimer's disease.
"On a practical level, as long as the patient is not worsening or improving, we might not care whether its disease modifying or not. In order to test that, we have to be able to determine disease burden while the patient is still alive. That’s why there is a lot of interest in this PIB scanning to determine this effect," said Turner.
But this would have to be validated by eventual autopsy studies and would take several years whether a drug is actually a disease modifying agent, physicians said.
"I think that we’re evolving into a field where we test for amyloid levels before giving a drug, and then test after for levels of PIB to show plaque reduction," said Turner.
However, most of the patient's on Wyeth's trials were also on Aricept - a drug which may also show a potential disease modifying effect. "Nowadays everyone is on Aricept and Namenda. Aricept might also have a disease modifying effect, and there is some evidence that it changes even some of the biological markers, and rate of brain atrophy," said Jicha.
Another potential reason behind both Neurochem’s Alzhemed and Myriad’s recent failure is the current gold standard endpoint used in Alzheimer’s trials – the ADAScog- may not be sensitive enough to pick up slight improvements in executive functioning in patients on trials, physicians noted.
"There is an interest in moving ADAScog out from being the primary outcome measure, and that the Flurizan data shows we don’t want to keep using ADAScog," said Sabbagh.
The ADAScog is not a perfect test, added Turner, but it’s a test that the FDA accepts as proof of efficacy. This is partially guided by what will convince the FDA and public that a drug has a positive outcome, he added.
"We’re certainly looking for better tests than the ADAScog. Other outcomes are used as well. It's certainly not a perfect test, but a standardized one," said Turner.
Jicha said the industry changed the entire treatment from symptomatic to disease modifying, but the FDA still depends on the symptomatic endpoints.
"There is some concern that we may have too many eggs in one basket, which is the amyloid basket. We still don’t understand the amyloid theory at a molecular level as much as we’d like to," added Doraiswamy.
Three different classes of drugs - the estrogens, statins, and anti-inflammatory class - were all promising in transgenic mice models, but physicians said have not shown success in human studies.
"This raises some questions about whether we’re approaching the amyloid pathway as precisely as we need to, or whether this is completely an epiphenomenon," added Doraiswamy.
“We know amyloid accumulates but we don’t know why. Is it the cause or effect? We also don’t understand the types of amyloid,” said Doraiswamy.
There are a lot of different subtypes of amyloid such as soluble and non-soluble types of amyloid. Amyloid plaques found in Alzheimer's patients can be in fibrillar form - long, insoluble fibers bound together in deposits called senile plaques. However, there are also soluble forms of amyloid beta, or oligomers, that may decisively contribute to neural degeneration.
Many physicians suggested that amyloid plaques may be analogous to cholesterol, where some types of amyloid are more protective - and some are harmful when removed.
Current agents such as Wyeth/Elan’s bapineuzumab are not selective enough to target a specific form of amyloid, said one trial investigator. “We’re targeting [amyloid] somewhat crudely, similar in the old days when we thought all cholesterol were bad,” supported Doraiswamy.
But at the end of the day, it will come down to risk-benefit, he added. The issue is not the side-effect, but the frequency of these events and whether they are dose dependent and predictable, he explained.
Elan found in their initial studies that there was a slightly higher frequency of vascular edema in APOE4+ subjects. The original theory was that APOE4 patients had more deposition in the brain, but there is still no hard evidence that these events are APOE4 status dependent.
“Nobody knows for sure what the right dose is still,” said Doraiswamy.
The company is being cautious, said Doraiswamy, noting that in the APOE4 non-carriers, the frequency was very low, so the company possibly felt a bit more confident with studying a broader dose range.
The next step in Alzheimer’s will be to develop a baseline PET scan that maps the level of amyloid load in the brain. The drug will then be combined to tailor the right dosing. Hopefully that will be the next step from companies 6-12 months from now, said Doraiswamy.
Doraiswamy noted that Wyeth currently needs two trials for approval, unless it’s expedited approval. "They would have to see very clear results, and minimal side effects. If the drug reported marginal side effects and continued to have the same risk of edema, I don’t think the field would be that excited," he added.
Rebeck said they are starting to incorporate the PET scan in trials, but this work is still preliminary.
Current Alzheimer's trials which are usually 18-months in length are too long, and are exposed to too many external variables and confounding effects. In order to get rid of that variation, there has to be a relatively quantifiable measure, some physicians said.
"The search for a biomarker where you can quantitatively measure something in a relatively short period of time is absolutely essential for the field to move forward," said Rebeck.
Ideally, a biomarker, or a ‘Factor X’ is identified in the mild cognitive impairment population. Therapies could then be developed to bring down Factor X. “We don’t know what Factor X is right now. We don’t know whether if we modify a-beta in the short term, whether it’ll even help,” said Rebeck.
Dr Ralph A. Nixon, associate professor of psychiatry and an amyloid expert at Harvard Medical School and McLean Hospital in Boston, said biomarkers in the cerebrospinal fluid (CSF) are an area that is urgently in need of more research because it is holding up some of the clinical trials.
The issue with measuring amyloid levels in the CSF as a surrogate endpoint is that actual progression of the disease causes amyloid levels to decrease over time, said Jicha. "They are reduced with the disease progression, so how do we know whether it’s the disease or the drug? You’ll never have any definitive proof for it. That’s a big problem."
"The fact that they may be doing the study may not necessarily be indicative of using it as an outcome measure. It may be a parallel analysis," added Nixon.
"The bottom line is this is the time where we have to do a lot more work. There are studies being designed to include PIB assessment before treatment and after treatment is part of the design. That is going to be a very important element in testing the efficacy of amyloid lowering as a viable therapy," Nixon said.
Nixon added that the current investigation is underway on patients from Wyeth's very first immunization trial that have now come to autopsy. "They have the clinical information after that trial as well as some pathologic information," he said.
"It's not that the current approaches are bad, but we need to take a fresh look at targeting the specific pathology of the disease," said Jicha.
Wyeth has a current market cap of USD 61.92bn. Elan has a current market cap of USD 16.58bn.
Wyeth/Elan's Alzheimer's candidate bapineuzumab faces potential Phase III hurdles - analysis
Story Wyeth/Elan's Alzheimer's candidate bapineuzumab faces potential Phase III hurdles, according to physicians and investigators.
Recently released data showed that bapineuzumab may improve cognitive functioning in patients who are non-carriers of the ApoE4 allele. Serious adverse events, such as vasogenic edema also occurred more frequently in bapineuzumab-treated APOE4 carriers.
Although the results for the subgroup were positive, the finding is inconclusive until it can be replicated and confirmed with larger follow-on studies, many of the physicians interviewed agreed.
“Many trials in the past reported findings that appeared highly promising, but were subsequently not confirmed in a duplicate trial,” said Dr. P. Murali Doraiswamy, chief of biological psychiatry at Duke University Medical.
APOE4 is only a correlating risk factor for Alzheimer’s, not necessarily a cause, and some physicians suggested that Wyeth and Elan’s bapineuzumab trials are just a “proof of principle” trials at best.
Scientists discovered the APOE4 correlation with Alzheimer's 15 years ago in the Roses Lab at Duke University, but have not yet established a causal link yet. Although 40-65% of AD patients have at least one copy of the 4 allele, APOE4 is not a determinant of the disease: at least a third of Alzheimer's patients are APOE4 negative and some APOE4 homozygotes never develop the disease.
Dr. G. William Rebeck, associate professor in the Department of Neuroscience at Georgetown University Medical Center, who has been conducting research on APOE since the early 1990s, said the study still needs to be duplicated as the higher frequency of vasogenic edema in patients carrying APOE4 was a post hoc analysis and there was no obvious underlying biological cause that would lead to such a reaction.
Dr Marwan Sabbagh, an investigator on Wyeth’s bapineuzumab trials and author of The Alzheimer’s Answer, said that so far the side-effects of vascular edema did not correlate with efficacy and are not predictive of a better response.
As APOE4 carriers (APOE4+) reported a higher incidence of adverse events, it is possible that bapineuzumab could be approved for non-APOE4 carriers. APOE4 status can be determined with a simple blood test.
Pfizer’s mild cognitive impairment (MCI) studies of Aricept (donepizil), a leading Alzheimer’s treatment, had a better effect in APOE4+ patients, while Wyeth’s bapineuzumab trial showed better effect in non-APOE4+ patients, according to Dr. Greg Jicha, an investigator on the Phase III trials, and professor of neurology at the University of Kentucky College of Medicine. Previous studies have found that the effect of the Aricept treatment lasted longer in those patients carrying the gene.
Studies have also shown that patients with the APOE4 gene have a higher propensity to develop Alzheimer's than the general population. "I query over this differential effect. I always took the Pfizer data to suggest that we can be a bit more definitive that patients with APOE4+ status had a higher likelihood of AD rather than an AD-mimicking disease," he added.
“The Phase IIb studies were clearly enough to make us understand we have to stratify dosing by genotype. It’s a first step in an area that will emerge as very common,” said Sabbagh.
When asked why Wyeth/Elan included APOE4+ patients on their Phase III trials, Rebeck said the companies were probably not "entirely convinced" from Phase II and would like to see it in a larger study.
Sabbagh said it is too late to change the trial design, even in light of the recent results. "My impression is that they were trying to just push ahead. It boils down to the first company to get an FDA-approved disease modifying agent, so there was this big push," added an anonymous investigator.
Another investigator speculated that a problem with Elan's Phase II trial was sample size calculation. "At least they got a signal in the non-APOE4 carriers which was enough for them to move forward," another investigator added.
Dr Neil Kurtz, CEO of Torreypines Therapeutics said Wyeth probably did not know about the APOE4 results, and that is why they recruited these patients into their larger Phase III trials.
An investigator who wished to remain anonymous explained that antibodies are specific for oligomers. Wyeth has said this drug is soluble for oligomers, he said, yet the drug was not designed to be specific. "I’m not sure whether they’re specific enough. If they could find any degree of activity, they can argue specificity. There are some antibodies that only recognize soluble oligomers, if you have a monoclonal cell line that produces a monoclonal antibody, you can humanize that and make it specific to a soluble amyloid."
He speculated that both companies are so invested in this antibody, "you don’t want to throw that out. But maybe we need to move to another approach."
In addition, the anonymous investigator said, "We had seen and heard there’s a great deal of skepticism about the agents that are pulling into Phase III trials. None of these agents have stellar early reports either. If I had Alzheimer's, I would enroll in Phase I and II studies rather than Phase III at this point."
Wyeth and Elan would not provide comment.
When asked whether Elan/Wyeth have the right Phase III trial design, such as patient characteristics and doses, the investigator said the companies were on target. Sabbagh added that protocol amendments can be done, but fundamental changes in trial design are usually not seen.
"APOE4 is definitely a risk factor and is found in roughly one-third of the US population. It seems to play an important role in the outcomes of clinical trials," said Dr Scott Turner, associate professor in the Department of Neurology at the University of Michigan Health System and incoming director of the Memory Disorders Program at Georgetown University Medical Center.
Only a subset of subjects will respond to certain medication, such as patients who are APOE4+. Myriad Genetics has data on the APOE4 status of patients on their recently failed Flurizan trial – but they haven’t released this specific data yet, said Turner.
"With the Flurizan trial, maybe some patients did tremendously on the drug, and some patients dragged down the overall results," said Jicha.
Myriad failed to meet its primary endpoint in a Phase III trial for a potential disease modifying agent named Flurizan earlier this week. "Flurizan tanked, just like Alzhemed. The site to site variation is plaguing the industry,” said Sabbagh.
The amount of resources needed for such large trials has become a problem, Rebeck noted. “When you’re analyzing data, sites ended up with statistically different results from each other. The assumption was that we could control for all the site to site variation,” he said.
Genotyping patients before deciding which therapy to give in the future may become a reality – and certain laws regarding APOE4 status to prevent genetic discrimination are currently underway, the physicians noted.
There is a clear need within the industry to develop and use a more objective quantifiable measure, such as the Pittsburgh Compound B (PIB), which binds to and can track levels of amyloid plaque in the brain.
“If we can quantify a reduction in amyloid load in the brain, that’s hard data that we as an industry have been sorely lacking. Many researchers in the field think it’s not good enough anymore, as we have surrogate markers now," said Sabbagh.
This is the first real step at looking at an objective measure in amyloid burden. Establishing a correlation with PIB would probably be the biggest finding of the year, and those works are underway, Sabbagh added. However, although the PIB principle is desirable, many physicians cautioned that proof is needed to show that PIB changes with disease progression. “I’ve heard some rumors that they have some studies that it changes with intervention,” said Sabbagh.
When asked whether PIB is at a stage where companies can start using that in trials, Sabbagh said, "theoretically, yes." One source close to Wyeth’s trials said PIB as an imaging agent was also proposed in the Phase III bapineuzumab trial, as well as many other studies. “Is PIB ready? No. Are we exploring it, yes,” he added.
If the correlation between amyloid plaque reduction and clinical improvement was shown by using the PIB imaging agent, it would be a significant move forward to Alzheimer's trials. PIB would be analogous to CD4 counts in HIV, said Turner.
"I think PIB will only give us a bit more biological data. We still need to find that surrogate marker," said Jicha.
There is currently no concrete correlation between amyloid plaque reduction and clinical benefit. "We don’t know that for a fact with human studies, but know that with mice. Plaques correlate with memory loss, and all of these are unanswered questions when it comes to human studies," said Turner.
"A lot of money is going into this. You can’t see individual plaques in living humans, but can see that on brain sections after death. You can see PIB binding in a nuclear medicine study, and this is one of the biomarkers being developed," said Turner.
The current industry goal is to stabilize the amount of plaque by the investigational drug, or even decrease the amount of plaque, and then conduct repeated PIB imaging scans as well as cognitive outcome measures to test for correlation, said Doraiswamy.
Some physicians also speculated that the recent failures may not have anything to do with the agent's efficacy, but a fundamental flaw in trial design, as there is no current standard set forth from the FDA on disease modifying agents for the treatment of Alzheimer's disease.
"On a practical level, as long as the patient is not worsening or improving, we might not care whether its disease modifying or not. In order to test that, we have to be able to determine disease burden while the patient is still alive. That’s why there is a lot of interest in this PIB scanning to determine this effect," said Turner.
But this would have to be validated by eventual autopsy studies and would take several years whether a drug is actually a disease modifying agent, physicians said.
"I think that we’re evolving into a field where we test for amyloid levels before giving a drug, and then test after for levels of PIB to show plaque reduction," said Turner.
However, most of the patient's on Wyeth's trials were also on Aricept - a drug which may also show a potential disease modifying effect. "Nowadays everyone is on Aricept and Namenda. Aricept might also have a disease modifying effect, and there is some evidence that it changes even some of the biological markers, and rate of brain atrophy," said Jicha.
Another potential reason behind both Neurochem’s Alzhemed and Myriad’s recent failure is the current gold standard endpoint used in Alzheimer’s trials – the ADAScog- may not be sensitive enough to pick up slight improvements in executive functioning in patients on trials, physicians noted.
"There is an interest in moving ADAScog out from being the primary outcome measure, and that the Flurizan data shows we don’t want to keep using ADAScog," said Sabbagh.
The ADAScog is not a perfect test, added Turner, but it’s a test that the FDA accepts as proof of efficacy. This is partially guided by what will convince the FDA and public that a drug has a positive outcome, he added.
"We’re certainly looking for better tests than the ADAScog. Other outcomes are used as well. It's certainly not a perfect test, but a standardized one," said Turner.
Jicha said the industry changed the entire treatment from symptomatic to disease modifying, but the FDA still depends on the symptomatic endpoints.
"There is some concern that we may have too many eggs in one basket, which is the amyloid basket. We still don’t understand the amyloid theory at a molecular level as much as we’d like to," added Doraiswamy.
Three different classes of drugs - the estrogens, statins, and anti-inflammatory class - were all promising in transgenic mice models, but physicians said have not shown success in human studies.
"This raises some questions about whether we’re approaching the amyloid pathway as precisely as we need to, or whether this is completely an epiphenomenon," added Doraiswamy.
“We know amyloid accumulates but we don’t know why. Is it the cause or effect? We also don’t understand the types of amyloid,” said Doraiswamy.
There are a lot of different subtypes of amyloid such as soluble and non-soluble types of amyloid. Amyloid plaques found in Alzheimer's patients can be in fibrillar form - long, insoluble fibers bound together in deposits called senile plaques. However, there are also soluble forms of amyloid beta, or oligomers, that may decisively contribute to neural degeneration.
Many physicians suggested that amyloid plaques may be analogous to cholesterol, where some types of amyloid are more protective - and some are harmful when removed.
Current agents such as Wyeth/Elan’s bapineuzumab are not selective enough to target a specific form of amyloid, said one trial investigator. “We’re targeting [amyloid] somewhat crudely, similar in the old days when we thought all cholesterol were bad,” supported Doraiswamy.
But at the end of the day, it will come down to risk-benefit, he added. The issue is not the side-effect, but the frequency of these events and whether they are dose dependent and predictable, he explained.
Elan found in their initial studies that there was a slightly higher frequency of vascular edema in APOE4+ subjects. The original theory was that APOE4 patients had more deposition in the brain, but there is still no hard evidence that these events are APOE4 status dependent.
“Nobody knows for sure what the right dose is still,” said Doraiswamy.
The company is being cautious, said Doraiswamy, noting that in the APOE4 non-carriers, the frequency was very low, so the company possibly felt a bit more confident with studying a broader dose range.
The next step in Alzheimer’s will be to develop a baseline PET scan that maps the level of amyloid load in the brain. The drug will then be combined to tailor the right dosing. Hopefully that will be the next step from companies 6-12 months from now, said Doraiswamy.
Doraiswamy noted that Wyeth currently needs two trials for approval, unless it’s expedited approval. "They would have to see very clear results, and minimal side effects. If the drug reported marginal side effects and continued to have the same risk of edema, I don’t think the field would be that excited," he added.
Rebeck said they are starting to incorporate the PET scan in trials, but this work is still preliminary.
Current Alzheimer's trials which are usually 18-months in length are too long, and are exposed to too many external variables and confounding effects. In order to get rid of that variation, there has to be a relatively quantifiable measure, some physicians said.
"The search for a biomarker where you can quantitatively measure something in a relatively short period of time is absolutely essential for the field to move forward," said Rebeck.
Ideally, a biomarker, or a ‘Factor X’ is identified in the mild cognitive impairment population. Therapies could then be developed to bring down Factor X. “We don’t know what Factor X is right now. We don’t know whether if we modify a-beta in the short term, whether it’ll even help,” said Rebeck.
Dr Ralph A. Nixon, associate professor of psychiatry and an amyloid expert at Harvard Medical School and McLean Hospital in Boston, said biomarkers in the cerebrospinal fluid (CSF) are an area that is urgently in need of more research because it is holding up some of the clinical trials.
The issue with measuring amyloid levels in the CSF as a surrogate endpoint is that actual progression of the disease causes amyloid levels to decrease over time, said Jicha. "They are reduced with the disease progression, so how do we know whether it’s the disease or the drug? You’ll never have any definitive proof for it. That’s a big problem."
"The fact that they may be doing the study may not necessarily be indicative of using it as an outcome measure. It may be a parallel analysis," added Nixon.
"The bottom line is this is the time where we have to do a lot more work. There are studies being designed to include PIB assessment before treatment and after treatment is part of the design. That is going to be a very important element in testing the efficacy of amyloid lowering as a viable therapy," Nixon said.
Nixon added that the current investigation is underway on patients from Wyeth's very first immunization trial that have now come to autopsy. "They have the clinical information after that trial as well as some pathologic information," he said.
"It's not that the current approaches are bad, but we need to take a fresh look at targeting the specific pathology of the disease," said Jicha.
Wyeth has a current market cap of USD 61.92bn. Elan has a current market cap of USD 16.58bn.
Antwort auf Beitrag Nr.: 34.408.095 von Cyberhexe am 30.06.08 23:11:49Info zu AN-1792 bzw. AD
http://www.elan.com/Images/2007ADPDAbetaImmunotherapy_tcm3-1…
http://www.elan.com/Images/2007ADPDCLINICALPROGRESSINTHEIMMU…
http://www.elan.com/Images/2007ADPDasynucleinclearancevaccin…
http://www.elan.com/Images/2007ADPDPOTENTIALMECHANISMSOFAbet…
Long-Term Follow Up of Patients Immunized with AN1792 (QS-21): Reduced Functional Decline in Antibody Responders
http://www.elan.com/Images/2007ADPDLongTermFollowUpofPatient…
http://www.elan.com/Images/2007ADPDAbetaImmunotherapy_tcm3-1…
http://www.elan.com/Images/2007ADPDCLINICALPROGRESSINTHEIMMU…
http://www.elan.com/Images/2007ADPDasynucleinclearancevaccin…
http://www.elan.com/Images/2007ADPDPOTENTIALMECHANISMSOFAbet…
Long-Term Follow Up of Patients Immunized with AN1792 (QS-21): Reduced Functional Decline in Antibody Responders
http://www.elan.com/Images/2007ADPDLongTermFollowUpofPatient…
Antwort auf Beitrag Nr.: 34.445.191 von Cyberhexe am 05.07.08 22:23:23Wie ich schon mehrmals gesagt habe, halte ich AN-1792 nicht für einen Beweis, dass Bapineuzumab funktioniert... gerade das Gegenteil ist der Fall: gerade einige Ergebnisse von AN-1792 haben mich dazu gebracht Bapineuzumab und alles, was mit dieser Beta-Amyloid-Theorie zusammenhängt kritisch zu sehen!!!
An einigen der mit AN-1792 behandelten Patienten wurden später nach dem Tode eine Autopsie durchgeführt. Es wurde eindeutig festgestellt, dass die Vakzine gewirkt hat! AN-1792 hat die Plaques fast vollständig beseitigt, wenn die Patienten auf die Vakzine ansprachen und Antikörper bildeten (Responsers). Und auch das kann man sicherlich sehr gut auch bei Bapineuzumab finden. Das Beta-Amyloid-Plaque wird durch den AK schön aufgelöst. Der Knackpunkt kommt aber doch erst... man weiß nicht, ob das Plaque überhaupt für Alzheimer verantwortlich ist, nur dass es im Zusammenhang mit Alzheimer (warum auch immer) auftritt, ist unstrittig. Wenn die Theorie "Auflösen des Plaques" gleich "Abmilderung oder gar Rückgängigmachen von Alzheimer" stimmen wurde, müsste man doch bei den mit AN-1792 behandelten Patienten, bei denen man klar die Entfernung der Plaques feststellen konnte, auch eine Verbesserung der Demenz bzw. der kognitiven Fähigkeiten feststellbar gewesen sein! Aber man hat eben das nicht feststellen können! Es gab keine Verbesserung bei den Plaque-freien mit AN-1792 behandelten Patienten... bei allen ging Alzheimer weiter, alle bildeten die Demenz aus und sind daran gestorben. Es waren zwar nur wenige, die so untersucht worden... doch für mich ist es absolut unerklärlich, wie Alzheimer bei diesen weiter fortschreiten konnte, obwohl das Plaque eindeutig beseitigt wurde, wenn das Plaque wirklich für Alzheimer verantwortlich ist. Daraus schließe ich, dass diese Theorie zumindest teilweise falsch ist und somit AN-1792 wie auch Bapineuzumab zwar das Plaque entfernen können, aber im entscheidenden Punkt, dem Stoppen von Alzheimer bzw. der Verbesserung der kognitiven Fähigkeiten, scheitern wird... so wie gerade Flurizan gescheitert ist. Die bisherigen PII-Ergebnisse von Bapineuzumab passen in dieses Bild!
btw... James Nicoll war soweit ich weiß in die von dir zitierte PII Studie zu AN-1792 und deren Auswertungen eingebunden. Also kein Dummschwätzer...
********
Plaque clearance: no panacea
In the final presentation of the session, James Nicoll of the University of Southampton, UK, delved more deeply into the halted trial, AN1792, and presented compelling evidence that the removal of Aβ plaques—the target of many current vaccine development efforts—may not, in and of itself, be sufficient to stop progression of dementia. :O
After the trial was halted, Nicoll and colleagues did a post-mortem evaluation of the first patient to be immunized and found complete plaque clearance in the temporal lobe and patchy clearance in the frontal lobe. [See Nicoll slide 12.]
Subsequent neuropathological evaluations of eight patients with confirmed Alzheimer's disease showed "substantial variation" in clearance—some had the same amount of plaque as unimmunized patients; others showed intermediate clearance; and others almost complete clearance, Nicoll said. Antibody titers correlated with plaque removal: about half of the patients generated Aβ antibodies, and those with the highest antibody titers showed the most extensive plaque removal.
Plaque clearance was associated with several histological features, Nicoll continued. These included residual cores of plaque in plaque-free areas; a "moth-eaten" appearance in the remaining plaques; phagocytosed Aβ within the microglia; cerebral amyloid angiopathy (CAA), which seemed to increase initially and then decrease; the elimination of tau-containing dystrophic neurites (tangles and neuropil threads remained); and accumulation of vascular Aβ.
Taken together, the findings suggested that Aβ immunization in a person with Alzheimer's disease induces disaggregation of plaques, associated with an increase in the severity of CAA, at least transiently, thereby increasing microhemorrhages.
At the same time, the group looked at clinical function and time to development of severe dementia in the immunized patients compared with non-immunized controls (Lancet, in press). It turned out that everyone, regardless of plaque status, declined to a score of zero on the Mini-Mental Status Exam (from 20–25 at baseline). Thus, even when all plaques are removed, "patients continue to have progressive terminal dementia," Nicoll stressed. This result is in contrast to transgenic mouse models, where plaque clearance is associated with improvements in cognitive function.
This result is in contrast to transgenic mouse models, where plaque clearance is associated with improvements in cognitive function.
The findings raise "a fundamental challenge" to the amyloid hypothesis, Nicoll said. It's possible that Aβ plaques are necessary to initiate the neurodegenerative process which includes other factors such as tau, synaptic degeneration, and neuroinflammation—but that once started, the process can perpetuate itself even in the presence of plaque removal, he suggested. Alternatively, perhaps non-plaque Aβ—oligomeric or intraneuronal—plays a more important role in cognitive decline.
The findings also have implications for the use of in vivo amyloid imaging in clinical studies of immunotherapy. "Several investigators are doing PET imaging of patients' brains before and after immunization. And yes, you may see reduction of plaques after immunization, but we now know that may not correlate with improved cognitive function. So imaging may not be a good readout to use to measure cognitive function, Nicoll concluded.
Vaccine approaches for Alzheimer's disease currently include different strategies that are yielding mixed results. Yet many investigators are convinced they are on the right track. For now, it seems likely that a future vaccine may need to target more than one key process involved in neurodegeneration, and would need to be started many years before symptoms might appear. Thus, long-term safety would have to be assured, and potential harm weighed against potential benefits.
*********
hat Elan diese Ergebnisse auch irgendwann mal publiziert?
Für mich ist Elan somit eine tickende Zeitbombe!
mfg ipollit
An einigen der mit AN-1792 behandelten Patienten wurden später nach dem Tode eine Autopsie durchgeführt. Es wurde eindeutig festgestellt, dass die Vakzine gewirkt hat! AN-1792 hat die Plaques fast vollständig beseitigt, wenn die Patienten auf die Vakzine ansprachen und Antikörper bildeten (Responsers). Und auch das kann man sicherlich sehr gut auch bei Bapineuzumab finden. Das Beta-Amyloid-Plaque wird durch den AK schön aufgelöst. Der Knackpunkt kommt aber doch erst... man weiß nicht, ob das Plaque überhaupt für Alzheimer verantwortlich ist, nur dass es im Zusammenhang mit Alzheimer (warum auch immer) auftritt, ist unstrittig. Wenn die Theorie "Auflösen des Plaques" gleich "Abmilderung oder gar Rückgängigmachen von Alzheimer" stimmen wurde, müsste man doch bei den mit AN-1792 behandelten Patienten, bei denen man klar die Entfernung der Plaques feststellen konnte, auch eine Verbesserung der Demenz bzw. der kognitiven Fähigkeiten feststellbar gewesen sein! Aber man hat eben das nicht feststellen können! Es gab keine Verbesserung bei den Plaque-freien mit AN-1792 behandelten Patienten... bei allen ging Alzheimer weiter, alle bildeten die Demenz aus und sind daran gestorben. Es waren zwar nur wenige, die so untersucht worden... doch für mich ist es absolut unerklärlich, wie Alzheimer bei diesen weiter fortschreiten konnte, obwohl das Plaque eindeutig beseitigt wurde, wenn das Plaque wirklich für Alzheimer verantwortlich ist. Daraus schließe ich, dass diese Theorie zumindest teilweise falsch ist und somit AN-1792 wie auch Bapineuzumab zwar das Plaque entfernen können, aber im entscheidenden Punkt, dem Stoppen von Alzheimer bzw. der Verbesserung der kognitiven Fähigkeiten, scheitern wird... so wie gerade Flurizan gescheitert ist. Die bisherigen PII-Ergebnisse von Bapineuzumab passen in dieses Bild!
btw... James Nicoll war soweit ich weiß in die von dir zitierte PII Studie zu AN-1792 und deren Auswertungen eingebunden. Also kein Dummschwätzer...
********
Plaque clearance: no panacea
In the final presentation of the session, James Nicoll of the University of Southampton, UK, delved more deeply into the halted trial, AN1792, and presented compelling evidence that the removal of Aβ plaques—the target of many current vaccine development efforts—may not, in and of itself, be sufficient to stop progression of dementia. :O
After the trial was halted, Nicoll and colleagues did a post-mortem evaluation of the first patient to be immunized and found complete plaque clearance in the temporal lobe and patchy clearance in the frontal lobe. [See Nicoll slide 12.]
Subsequent neuropathological evaluations of eight patients with confirmed Alzheimer's disease showed "substantial variation" in clearance—some had the same amount of plaque as unimmunized patients; others showed intermediate clearance; and others almost complete clearance, Nicoll said. Antibody titers correlated with plaque removal: about half of the patients generated Aβ antibodies, and those with the highest antibody titers showed the most extensive plaque removal.
Plaque clearance was associated with several histological features, Nicoll continued. These included residual cores of plaque in plaque-free areas; a "moth-eaten" appearance in the remaining plaques; phagocytosed Aβ within the microglia; cerebral amyloid angiopathy (CAA), which seemed to increase initially and then decrease; the elimination of tau-containing dystrophic neurites (tangles and neuropil threads remained); and accumulation of vascular Aβ.
Taken together, the findings suggested that Aβ immunization in a person with Alzheimer's disease induces disaggregation of plaques, associated with an increase in the severity of CAA, at least transiently, thereby increasing microhemorrhages.
At the same time, the group looked at clinical function and time to development of severe dementia in the immunized patients compared with non-immunized controls (Lancet, in press). It turned out that everyone, regardless of plaque status, declined to a score of zero on the Mini-Mental Status Exam (from 20–25 at baseline). Thus, even when all plaques are removed, "patients continue to have progressive terminal dementia," Nicoll stressed.
 This result is in contrast to transgenic mouse models, where plaque clearance is associated with improvements in cognitive function.
This result is in contrast to transgenic mouse models, where plaque clearance is associated with improvements in cognitive function.The findings raise "a fundamental challenge" to the amyloid hypothesis, Nicoll said. It's possible that Aβ plaques are necessary to initiate the neurodegenerative process which includes other factors such as tau, synaptic degeneration, and neuroinflammation—but that once started, the process can perpetuate itself even in the presence of plaque removal, he suggested. Alternatively, perhaps non-plaque Aβ—oligomeric or intraneuronal—plays a more important role in cognitive decline.
The findings also have implications for the use of in vivo amyloid imaging in clinical studies of immunotherapy. "Several investigators are doing PET imaging of patients' brains before and after immunization. And yes, you may see reduction of plaques after immunization, but we now know that may not correlate with improved cognitive function. So imaging may not be a good readout to use to measure cognitive function, Nicoll concluded.
Vaccine approaches for Alzheimer's disease currently include different strategies that are yielding mixed results. Yet many investigators are convinced they are on the right track. For now, it seems likely that a future vaccine may need to target more than one key process involved in neurodegeneration, and would need to be started many years before symptoms might appear. Thus, long-term safety would have to be assured, and potential harm weighed against potential benefits.
*********
hat Elan diese Ergebnisse auch irgendwann mal publiziert?

Für mich ist Elan somit eine tickende Zeitbombe!
mfg ipollit
Antwort auf Beitrag Nr.: 34.446.626 von ipollit am 06.07.08 17:03:03Du bist wirklich -sorry-der echte Oberschlaumeier---WIE bitteschön erklärst Du die Phase 2 Ergebnisse, die bei den Non Apoe-carriern in allen verfügbaren Tests statistisch signifikante Verbesserungen in den cognitiven und das Verhalten betreffende Variablen gezeigt haben...
DU solltest Dich wirklich von tickenden Zeitbomben fernhalten und Dich weiter mit den schon explodierten (und den materiellen Resten davon)beschäftigen.Schönen Gruss!
DU solltest Dich wirklich von tickenden Zeitbomben fernhalten und Dich weiter mit den schon explodierten (und den materiellen Resten davon)beschäftigen.Schönen Gruss!
Antwort auf Beitrag Nr.: 34.447.314 von Birgit.Tersteegen am 06.07.08 20:49:02Warum Oberschlaumeier? Ich habe nur die Ergebnisse von James Nicoll zitiert, darf man das etwa nicht?
Vielleicht kannst du mir ja erklären, warum bei den AN-1792 Respondern zwar das Beta-Amyloid-Plaque entfernt werden konnte (und da auch die Konzentration der Antikörper mit der Stärke der Plaque-Auflösung korreliert), aber der Verlauf der Alzheimer-Erkrankung dadurch nicht verbessert oder aufgehalten wurde?
du fragst mich warum ApoE4-Träger eine statistisch signifikante Verbesserung in der PII gezeigt haben? Ich habe das eigentlich schon einige Mal beantwortet... es ist reiner Zufall gewesen! Zufall und nichts mehr... es wird sich nicht wiederholen lassen, schon gar nicht bei einer großen PIII!
Zufall und nichts mehr... es wird sich nicht wiederholen lassen, schon gar nicht bei einer großen PIII!
ich könnte haufenweise Zitate bringen (und nicht von mir, sondern von Artikeln, die sich mit den PII-Daten beschäftigt haben), die das ebenfalls besagen... z.B. aus #1331
"Although the results for the subgroup were positive, the finding is inconclusive until it can be replicated and confirmed with larger follow-on studies, many of the physicians interviewed agreed." ... auf deutsch: viele Ärzte sind der Meinung, dass diese Ergebnisse wertlos sind, solange sie nicht durch eine PIII bestätigt werden
"Many trials in the past reported findings that appeared highly promising, but were subsequently not confirmed in a duplicate trial" ... letztes Beispiel war Flurizan. Es ist praktisch ein Spiegelbild zu Bapineuzumab... auch bei Flurizan gab es vielversprechende statistisch signifikante PII Daten zu einer Subgruppe, die sich dann nicht in der PIII (die bereits vor den PII-Daten begonnen wurde) bestätigt haben
"However, most of the patient's on Wyeth's trials were also on Aricept - a drug which may also show a potential disease modifying effect. "Nowadays everyone is on Aricept and Namenda. Aricept might also have a disease modifying effect, and there is some evidence that it changes even some of the biological markers, and rate of brain atrophy," said Jicha." ... die meisten Patienten haben während der Studie auch die Alzheimer-Medikamente Aricept und Namenda, die sich auf die Gedächnisleistung auswirken. Aricept könnte sogar selbst ein krankheitsveränderndes Medikament sein
oder #1295
"He says because the companies didn't decide to divide patients into those with and without APOE until after the study finished, there is a meaningful risk that the positive results are due solely to chance" ... auf deutsch... es gibt ein deutliches Risiko, dass die Ergebnisse der PII bezügl APOE reiner Zufall sind
"The news release also does not say how many different subsets of patients were analyzed, which is particularly important because the more subsets you analyze, the more you are likely to have a positive result merely by chance." ... zu deutsch... die Größe der Subgruppe ist unbekannt, was aber wichtig wäre, denn je kleiner die Subgruppe ist, umso höher ist die Wahrscheinlichkeit, dass sie rein zufällig statistisch signifikant positive Ergebnisse liefert
"One big mystery is why hitting amyloid with a drug would benefit people without the APOE4 gene but not those with it. There are few clinical differences, experts say, between those with APOE4 and those without it once they develop the disease." ... zu deutsch... es ist ein ziemliches Rätsel, warum mit und ohne APOE4 einen Unterschied bei einem Medikament machen soll, denn es gibt kaum einen Unterschied zwischen diesen Gruppen, sobald sie Alzheimer bekommen haben.
***************
zusammengefasst: Was spricht dafür, dass das Ergebnis rein zufällig ist... 8 Punkte!
1.) die angeblich signifikant positive Subgruppe enthält nur ein Bruchteil aller Patienten der PII. Je kleiner die Gruppe, umso weniger Aussagekraft hat das Ergebnis!
2.) sehr kritisch ist, dass es sich um eine nachträgliche Analyse handelt. Nachträgliche Analysen lassen sich beliebig manipulieren, um ein gewünschtes Ergebnis zu erhalten!
3.) die Subgruppen bezügl ApoE4 sind nicht randomisiert! D.h. sie enthalten völlig unterschiedliche Patienten, so dass es sehr wahrscheinlich ist, dass in eine der beiden Gruppen "mit ApoE4" und "ohne ApoE4" zufällig die gesünderen Patienten mit Bapineuzumab behandelt wurden und somit auch bessere Ergebnisse liefern als die Placebos. Korrekt müssten die beiden Gruppen randomisiert sein, d.h. jeweils vergleichbare Patienten enthalten, so dass der Patientenstatus zu Beginn der Studie das Ergebnis nicht verfälschen kann!
4.) die Patienten wurden anscheinend wahllos mit anderen Alzheimer-Medikamenten behandelt, was das Ergebnis wegen der fehlenden Randomisierung stark verfälschen kann
5.) das "ohne ApoE4" besser abschneiden soll, ist von der Theorie her eher unlogisch! Eigentlich sollten doch die am meisten von einem Alzheimer Medikament profitieren, die das höchste Risiko besitzen, Alzheimer zu bekommen (das sind die "mit ApoE4")!
6.) bei "ohne ApoE4" wird die Erkrankung vielleicht nicht so deutlich, da sie ein geringeres Risiko besitzen, an Alzheimer zu erkranken. Damit macht sich das statistische Rauschen bei diesen Patienten stärker bemerkbar!
7.) die Verbesserung dieser Subgruppe soll minimal sein, knapp an der Grenze zur klinischen Relevanz (also wo sich überhaupt ein Effekt bemerkbar macht)
8.) es gibt keine medizinische oder theoretische Erklärung, warum "ohne ApoE4" sich mit Bapineuzumab besser behandeln lassen sollte!
( 9.) der primäre Endpunkt der PII wurde verfehlt)
So, dass sind meine Argumente! Hast du auch Argumente, die für deine Position sprechen?
mfg ipollit
Vielleicht kannst du mir ja erklären, warum bei den AN-1792 Respondern zwar das Beta-Amyloid-Plaque entfernt werden konnte (und da auch die Konzentration der Antikörper mit der Stärke der Plaque-Auflösung korreliert), aber der Verlauf der Alzheimer-Erkrankung dadurch nicht verbessert oder aufgehalten wurde?

du fragst mich warum ApoE4-Träger eine statistisch signifikante Verbesserung in der PII gezeigt haben? Ich habe das eigentlich schon einige Mal beantwortet... es ist reiner Zufall gewesen!
 Zufall und nichts mehr... es wird sich nicht wiederholen lassen, schon gar nicht bei einer großen PIII!
Zufall und nichts mehr... es wird sich nicht wiederholen lassen, schon gar nicht bei einer großen PIII!ich könnte haufenweise Zitate bringen (und nicht von mir, sondern von Artikeln, die sich mit den PII-Daten beschäftigt haben), die das ebenfalls besagen... z.B. aus #1331
"Although the results for the subgroup were positive, the finding is inconclusive until it can be replicated and confirmed with larger follow-on studies, many of the physicians interviewed agreed." ... auf deutsch: viele Ärzte sind der Meinung, dass diese Ergebnisse wertlos sind, solange sie nicht durch eine PIII bestätigt werden
"Many trials in the past reported findings that appeared highly promising, but were subsequently not confirmed in a duplicate trial" ... letztes Beispiel war Flurizan. Es ist praktisch ein Spiegelbild zu Bapineuzumab... auch bei Flurizan gab es vielversprechende statistisch signifikante PII Daten zu einer Subgruppe, die sich dann nicht in der PIII (die bereits vor den PII-Daten begonnen wurde) bestätigt haben
"However, most of the patient's on Wyeth's trials were also on Aricept - a drug which may also show a potential disease modifying effect. "Nowadays everyone is on Aricept and Namenda. Aricept might also have a disease modifying effect, and there is some evidence that it changes even some of the biological markers, and rate of brain atrophy," said Jicha." ... die meisten Patienten haben während der Studie auch die Alzheimer-Medikamente Aricept und Namenda, die sich auf die Gedächnisleistung auswirken. Aricept könnte sogar selbst ein krankheitsveränderndes Medikament sein
oder #1295
"He says because the companies didn't decide to divide patients into those with and without APOE until after the study finished, there is a meaningful risk that the positive results are due solely to chance" ... auf deutsch... es gibt ein deutliches Risiko, dass die Ergebnisse der PII bezügl APOE reiner Zufall sind
"The news release also does not say how many different subsets of patients were analyzed, which is particularly important because the more subsets you analyze, the more you are likely to have a positive result merely by chance." ... zu deutsch... die Größe der Subgruppe ist unbekannt, was aber wichtig wäre, denn je kleiner die Subgruppe ist, umso höher ist die Wahrscheinlichkeit, dass sie rein zufällig statistisch signifikant positive Ergebnisse liefert
"One big mystery is why hitting amyloid with a drug would benefit people without the APOE4 gene but not those with it. There are few clinical differences, experts say, between those with APOE4 and those without it once they develop the disease." ... zu deutsch... es ist ein ziemliches Rätsel, warum mit und ohne APOE4 einen Unterschied bei einem Medikament machen soll, denn es gibt kaum einen Unterschied zwischen diesen Gruppen, sobald sie Alzheimer bekommen haben.
***************
zusammengefasst: Was spricht dafür, dass das Ergebnis rein zufällig ist... 8 Punkte!
1.) die angeblich signifikant positive Subgruppe enthält nur ein Bruchteil aller Patienten der PII. Je kleiner die Gruppe, umso weniger Aussagekraft hat das Ergebnis!
2.) sehr kritisch ist, dass es sich um eine nachträgliche Analyse handelt. Nachträgliche Analysen lassen sich beliebig manipulieren, um ein gewünschtes Ergebnis zu erhalten!
3.) die Subgruppen bezügl ApoE4 sind nicht randomisiert! D.h. sie enthalten völlig unterschiedliche Patienten, so dass es sehr wahrscheinlich ist, dass in eine der beiden Gruppen "mit ApoE4" und "ohne ApoE4" zufällig die gesünderen Patienten mit Bapineuzumab behandelt wurden und somit auch bessere Ergebnisse liefern als die Placebos. Korrekt müssten die beiden Gruppen randomisiert sein, d.h. jeweils vergleichbare Patienten enthalten, so dass der Patientenstatus zu Beginn der Studie das Ergebnis nicht verfälschen kann!
4.) die Patienten wurden anscheinend wahllos mit anderen Alzheimer-Medikamenten behandelt, was das Ergebnis wegen der fehlenden Randomisierung stark verfälschen kann
5.) das "ohne ApoE4" besser abschneiden soll, ist von der Theorie her eher unlogisch! Eigentlich sollten doch die am meisten von einem Alzheimer Medikament profitieren, die das höchste Risiko besitzen, Alzheimer zu bekommen (das sind die "mit ApoE4")!
6.) bei "ohne ApoE4" wird die Erkrankung vielleicht nicht so deutlich, da sie ein geringeres Risiko besitzen, an Alzheimer zu erkranken. Damit macht sich das statistische Rauschen bei diesen Patienten stärker bemerkbar!
7.) die Verbesserung dieser Subgruppe soll minimal sein, knapp an der Grenze zur klinischen Relevanz (also wo sich überhaupt ein Effekt bemerkbar macht)
8.) es gibt keine medizinische oder theoretische Erklärung, warum "ohne ApoE4" sich mit Bapineuzumab besser behandeln lassen sollte!
( 9.) der primäre Endpunkt der PII wurde verfehlt)
So, dass sind meine Argumente! Hast du auch Argumente, die für deine Position sprechen?
mfg ipollit
Antwort auf Beitrag Nr.: 34.447.314 von Birgit.Tersteegen am 06.07.08 20:49:02Du bist wirklich -sorry-der echte Oberschlaumeier---
Sorry, Birgit, aber ich finde " kritische Gesichtspunkte " bezüglich der Auswertungen von den PII Daten und die Erläuterungen von James Nicoll in diesem Zusammenhang als nicht unwichtig.....
Deshalb lasst uns gemeinsam auf die anstehende Konferenz im Juli schauen...
....dann werden viele Dinge vielleicht klarer zu sehen sein und Cyberhexe + ipolitt werden - hoffentlich !!- ihre statements sachlich dazu abgeben....
Grüße bernie55
Sorry, Birgit, aber ich finde " kritische Gesichtspunkte " bezüglich der Auswertungen von den PII Daten und die Erläuterungen von James Nicoll in diesem Zusammenhang als nicht unwichtig.....
Deshalb lasst uns gemeinsam auf die anstehende Konferenz im Juli schauen...
....dann werden viele Dinge vielleicht klarer zu sehen sein und Cyberhexe + ipolitt werden - hoffentlich !!- ihre statements sachlich dazu abgeben....
Grüße bernie55
Antwort auf Beitrag Nr.: 34.446.626 von ipollit am 06.07.08 17:03:03Es gab keine Verbesserung bei den Plaque-freien mit AN-1792 behandelten Patienten..
Also hier ziehst Du wohl die falschen Schlußfolgerungen. Richtig erscheint zur Zeit lediglich, ganz simpel ausgedrückt, daß der ( auch damals bei AN-1792) signifikante Antikörper nicht für alle an Demenz erkrankten Personen ein Allheilmittel ist.
Hier mal ein älterer Artikel zu AN-1792:
.........
Der Verlust kognitiver Funktionen wurde primär anhand der Mini-Mental- State-Examination gemessen und fiel im einjährigen Untersuchungszeitraum für die Responder signifikant niedriger aus als für die Non-Responder (minus 1,4 versus minus 6,4 Punkte). In der einjährigen Nachbeobachtungszeit verschlechterten sich unter den erfolgreich geimpften Personen lediglich drei von 19 (16 Prozent) um eine Stufe auf „ernsthafte Demenz“ (Severe Dementia, MMSE < 14). In der Kontrollgruppe waren es dagegen sechs von neun Patienten (67 Prozent). Eine „Intent-to-treat“-Auswertung wird erst nach der Entblindung der Daten möglich sein.
Die Stabilisierung der Responder unterscheidet sich deutlich vom natürlichen Verlauf der Alzheimer-Krankheit in diesem Stadium, schreiben Hock et al. Andererseits habe sich die Gedächtnisleistung der Non-Responder schneller als erwartet verschlechtert, bemerken in einem Editorial zur aktuellen Publikation Bengt Winblad vom Stockholms Karolinska Institute und Neuron-Herausgeber Kenneth Blum. Die potenziell tödliche Nebenwirkung der Impfung bleibe eine alles überragende Sorge, dennoch „zeigt dieser Artikel, dass das Konzept der Impfung am Leben ist“.
Nicht nur im MMSE schnitten die Responder besser ab. Auch die Beurteilung der Pfleger – gemessen anhand der Disability-Assessment-for-Dementia-Skala – ergab mit minus 2,9 versus minus 8,7 Punkten einen signifikanten Vorteil für jene Patientengruppe, bei der die Impfung angeschlagen hatte. Sie war wesentlich besser in der Lage, tägliche Aktivitäten auszuführen, zum Beispiel Körperpflege, Kochen, Telefonieren oder Einkaufen. „Die Stabilisierung der Gehirnleistung ist demnach auch für das Alltagsleben relevant“, bilanzieren Hock und Nitsch.
Zur anhaltenden wissenschaftlichen Debatte über die Ätiogenese der Alzheimer-Demenz bemerken sie, dies sei „der erste erfolgreiche klinische Nachweis für eine zentrale Bedeutung des
b-Amyloids als Ursache des kognitiven Verfalls und der Demenz bei Alzheimer-Patienten“.
........
http://www.aerzteblatt.de/v4/archiv/artikel.asp?id=37419
Also hier ziehst Du wohl die falschen Schlußfolgerungen. Richtig erscheint zur Zeit lediglich, ganz simpel ausgedrückt, daß der ( auch damals bei AN-1792) signifikante Antikörper nicht für alle an Demenz erkrankten Personen ein Allheilmittel ist.
Hier mal ein älterer Artikel zu AN-1792:
.........
Der Verlust kognitiver Funktionen wurde primär anhand der Mini-Mental- State-Examination gemessen und fiel im einjährigen Untersuchungszeitraum für die Responder signifikant niedriger aus als für die Non-Responder (minus 1,4 versus minus 6,4 Punkte). In der einjährigen Nachbeobachtungszeit verschlechterten sich unter den erfolgreich geimpften Personen lediglich drei von 19 (16 Prozent) um eine Stufe auf „ernsthafte Demenz“ (Severe Dementia, MMSE < 14). In der Kontrollgruppe waren es dagegen sechs von neun Patienten (67 Prozent). Eine „Intent-to-treat“-Auswertung wird erst nach der Entblindung der Daten möglich sein.
Die Stabilisierung der Responder unterscheidet sich deutlich vom natürlichen Verlauf der Alzheimer-Krankheit in diesem Stadium, schreiben Hock et al. Andererseits habe sich die Gedächtnisleistung der Non-Responder schneller als erwartet verschlechtert, bemerken in einem Editorial zur aktuellen Publikation Bengt Winblad vom Stockholms Karolinska Institute und Neuron-Herausgeber Kenneth Blum. Die potenziell tödliche Nebenwirkung der Impfung bleibe eine alles überragende Sorge, dennoch „zeigt dieser Artikel, dass das Konzept der Impfung am Leben ist“.
Nicht nur im MMSE schnitten die Responder besser ab. Auch die Beurteilung der Pfleger – gemessen anhand der Disability-Assessment-for-Dementia-Skala – ergab mit minus 2,9 versus minus 8,7 Punkten einen signifikanten Vorteil für jene Patientengruppe, bei der die Impfung angeschlagen hatte. Sie war wesentlich besser in der Lage, tägliche Aktivitäten auszuführen, zum Beispiel Körperpflege, Kochen, Telefonieren oder Einkaufen. „Die Stabilisierung der Gehirnleistung ist demnach auch für das Alltagsleben relevant“, bilanzieren Hock und Nitsch.
Zur anhaltenden wissenschaftlichen Debatte über die Ätiogenese der Alzheimer-Demenz bemerken sie, dies sei „der erste erfolgreiche klinische Nachweis für eine zentrale Bedeutung des
b-Amyloids als Ursache des kognitiven Verfalls und der Demenz bei Alzheimer-Patienten“.
........
http://www.aerzteblatt.de/v4/archiv/artikel.asp?id=37419
Antwort auf Beitrag Nr.: 34.447.856 von ipollit am 07.07.08 00:20:32zu 1:es betrifft nicht einen Bruchteil-sondern ca die Hälfte der Patienten der mit AAB001 Behandelten,nämlich die als Non-Carrier spezifiziert wurden.
zu 2:Wenn doppel-blind getestet wird,kann immer erst nachträglich ausgewertet werden.WER -um Himmels Willen-sollte Interesse daran haben,Forschungsergebnisse in Phase 2 zu manipulierern???Bestimmt nicht DIE FIRMA,die danach MIlliarden für die Phase 3 ausgibt,in der dann ja -schon vorher gewusst-die "Schummeleien auffliegen würden.WOZU??
zu 3: Schwachsinn--völlige Unterstellung + Mutmassung Deinerseits ,dass die "Gesünderen" zufällig in einer Gruppe auftauchen...ausserdem wieso sollten die Gesünderen bessere Entwicklungen aufzeigen ,wenn das Zeug eh nicht hilft?
zu 4:Woher nimmst Du die Behauptung,die Patienten wären "wahllos" mit anderen Medikamenten behandelt worden?Alle Patienten bekamen meines Wissens das gleiche gängige Alzheimer-Medikament Aricept -die Placebos wie die Behandelten.
zu 5:Was Dir unlogisch erscheint kann ich nicht nachvollziehen ,weil meines Wissens sich biologische Prozesse selten an der menschlichen Logik (und sicher sowieso nicht an der eines Besserwissers.... )orientieren.
)orientieren.
Es gibt hier übrigens die Vermutung in informierten Kreisen ,dass BAP bei den Carriern vielleicht ZU GUT funktioniert--was bedeutet,dass das Plaque zu schnell abgebaut wurde bei zu hoher Dosis und deshalb innerhalb der Phase 2 einige Teilnehmer aus dem Test ausschieden.Von daher sollten die Ergebnisse in Phase 3 SUPER sein ,wenn die Patienten dann mit geringeren Dosen behandelt werden
zu 6:die non Arrier machen lt Elan 60% der ALZ-Patienten aus-ist das eine für Dich unwesentliche Gruppe?
zu 7:Belege das bitte!Bisher sind die genauen Ergebnisse nicht veröffentlicht und Kelly Martin hielt die Ergebnisse der Phase2 für SPEKTAKULÄR und ohne ein Mikroskpp benutzen zu müssen(...er kannte Dich ja noch nicht... -ZWINGEND für die Initiierung von Phase 3.
-ZWINGEND für die Initiierung von Phase 3.
zu 8:Bei den Non Carriern gab es in allen Tests statistisch signifikante Ergebnisse!!!!Elan hat ALLES getroffen ,was man treffen wollte und sogar noch mehr, weil die Signifikanz gar nicht intendiert war mit Phase 2....
für Bernie :Kritische Anmerkungen sind auch mir stets WILLKOMMEN--aber nicht der Diktus eines Besserwissers----Elan arbeitet seit 25 Jahren mit den weltweit anerkanntesten Forschern an der Amyloid-Hypothese....und dann kommt ein Ipolit daher und meint ,dass die positiven Ergebnisse ,die sich daraus ergeben,alle Zufall sind,weil er Jemanden gelesen hat,der das auch meint...???
Ich bitte Dich...





In diesem Sinne grüsst Birgit
zu 2:Wenn doppel-blind getestet wird,kann immer erst nachträglich ausgewertet werden.WER -um Himmels Willen-sollte Interesse daran haben,Forschungsergebnisse in Phase 2 zu manipulierern???Bestimmt nicht DIE FIRMA,die danach MIlliarden für die Phase 3 ausgibt,in der dann ja -schon vorher gewusst-die "Schummeleien auffliegen würden.WOZU??
zu 3: Schwachsinn--völlige Unterstellung + Mutmassung Deinerseits ,dass die "Gesünderen" zufällig in einer Gruppe auftauchen...ausserdem wieso sollten die Gesünderen bessere Entwicklungen aufzeigen ,wenn das Zeug eh nicht hilft?
zu 4:Woher nimmst Du die Behauptung,die Patienten wären "wahllos" mit anderen Medikamenten behandelt worden?Alle Patienten bekamen meines Wissens das gleiche gängige Alzheimer-Medikament Aricept -die Placebos wie die Behandelten.
zu 5:Was Dir unlogisch erscheint kann ich nicht nachvollziehen ,weil meines Wissens sich biologische Prozesse selten an der menschlichen Logik (und sicher sowieso nicht an der eines Besserwissers....
 )orientieren.
)orientieren.Es gibt hier übrigens die Vermutung in informierten Kreisen ,dass BAP bei den Carriern vielleicht ZU GUT funktioniert--was bedeutet,dass das Plaque zu schnell abgebaut wurde bei zu hoher Dosis und deshalb innerhalb der Phase 2 einige Teilnehmer aus dem Test ausschieden.Von daher sollten die Ergebnisse in Phase 3 SUPER sein ,wenn die Patienten dann mit geringeren Dosen behandelt werden
zu 6:die non Arrier machen lt Elan 60% der ALZ-Patienten aus-ist das eine für Dich unwesentliche Gruppe?
zu 7:Belege das bitte!Bisher sind die genauen Ergebnisse nicht veröffentlicht und Kelly Martin hielt die Ergebnisse der Phase2 für SPEKTAKULÄR und ohne ein Mikroskpp benutzen zu müssen(...er kannte Dich ja noch nicht...
 -ZWINGEND für die Initiierung von Phase 3.
-ZWINGEND für die Initiierung von Phase 3.zu 8:Bei den Non Carriern gab es in allen Tests statistisch signifikante Ergebnisse!!!!Elan hat ALLES getroffen ,was man treffen wollte und sogar noch mehr, weil die Signifikanz gar nicht intendiert war mit Phase 2....
für Bernie :Kritische Anmerkungen sind auch mir stets WILLKOMMEN--aber nicht der Diktus eines Besserwissers----Elan arbeitet seit 25 Jahren mit den weltweit anerkanntesten Forschern an der Amyloid-Hypothese....und dann kommt ein Ipolit daher und meint ,dass die positiven Ergebnisse ,die sich daraus ergeben,alle Zufall sind,weil er Jemanden gelesen hat,der das auch meint...???
Ich bitte Dich...






In diesem Sinne grüsst Birgit

Antwort auf Beitrag Nr.: 34.448.522 von Birgit.Tersteegen am 07.07.08 09:31:07für Bernie :Kritische Anmerkungen sind auch mir stets WILLKOMMEN--aber nicht der Diktus eines Besserwissers----Elan arbeitet seit 25 Jahren mit den weltweit anerkanntesten Forschern an der Amyloid-Hypothese....und dann kommt ein Ipolit daher und meint ,dass die positiven Ergebnisse ,die sich daraus ergeben,alle Zufall sind,weil er Jemanden gelesen hat,der das auch meint...???
Ich bitte Dich...
Hi Birgit
..ok, dazu habe ich mich ja auch schon mal geäußert...
..ich denke, genauso wie Du , dass ELAN und WYETH genau wissen, was sie tun und dass ihre langjährige Erfahrungen für Entscheidungsprozesse hinsichtlich von Trials natürlich genaustens überdenken..
........deshalb sind wir ja auch schon sssoooo lange in ELAN investiert...
...aber trotz unserer Euphorie ( bisher hat ELAN ja alles richtig gemacht ) und unserer daraus resultierenden "emotionalen Verbundenheit"
) und unserer daraus resultierenden "emotionalen Verbundenheit"  sollten wir auf dem Teppich bleiben und immer einen möglichen negativen Ausgang eines PIII Trials im Auge haben......deshalb finde ich gewisse " kritsche " Anmerkungen zum Thema einfach ok.......that´s all....
sollten wir auf dem Teppich bleiben und immer einen möglichen negativen Ausgang eines PIII Trials im Auge haben......deshalb finde ich gewisse " kritsche " Anmerkungen zum Thema einfach ok.......that´s all....
...über die Art und Weise, die manchmal bei der Argumentation mitschwingt, mag ich mich jetzt einfach nicht mehr äußern....
DAMIT SEI DAS THEMA AN DIESER STELLE BEENDET
Ich bitte Dich......> Ich danke DICH <




Grüße bernie55
Ich bitte Dich...
Hi Birgit

..ok, dazu habe ich mich ja auch schon mal geäußert...
..ich denke, genauso wie Du , dass ELAN und WYETH genau wissen, was sie tun und dass ihre langjährige Erfahrungen für Entscheidungsprozesse hinsichtlich von Trials natürlich genaustens überdenken..
........deshalb sind wir ja auch schon sssoooo lange in ELAN investiert...

...aber trotz unserer Euphorie ( bisher hat ELAN ja alles richtig gemacht
 ) und unserer daraus resultierenden "emotionalen Verbundenheit"
) und unserer daraus resultierenden "emotionalen Verbundenheit"  sollten wir auf dem Teppich bleiben und immer einen möglichen negativen Ausgang eines PIII Trials im Auge haben......deshalb finde ich gewisse " kritsche " Anmerkungen zum Thema einfach ok.......that´s all....
sollten wir auf dem Teppich bleiben und immer einen möglichen negativen Ausgang eines PIII Trials im Auge haben......deshalb finde ich gewisse " kritsche " Anmerkungen zum Thema einfach ok.......that´s all.......über die Art und Weise, die manchmal bei der Argumentation mitschwingt, mag ich mich jetzt einfach nicht mehr äußern....
DAMIT SEI DAS THEMA AN DIESER STELLE BEENDET
Ich bitte Dich......> Ich danke DICH <





Grüße bernie55

Antwort auf Beitrag Nr.: 34.448.522 von Birgit.Tersteegen am 07.07.08 09:31:07zu 1... ja, in der Bevölkerung sollen 40-70% das ApoE4-Gen nicht besitzen ("estimated in the literature to be from 40 to 70 percent of the Alzheimer's disease population"). Aber das hatte ich hier ja nicht gemeint. Die Frage ist, wie groß diese Subgruppe in der PII war, auf die sich Elan stützt. In #1311 habe ich vorgerechnet, dass (wenn man deine 50% nimmt) man auf ca. 30 bis 60 Patienten kommt, die als Non-ApoE4 mit Bapineuzumab behandelt wurden. Dies ist keine allzu große Gruppe! Vielleicht sind es aber auch nur 20 Personen oder auch 100... niemand weiß das im Moment, da die Studie vorher ApoE4 nicht berücksichtigt und danach ausgesucht hat.
zu 2... "Wenn doppel-blind getestet wird,kann immer erst nachträglich ausgewertet werden"... die Auswertung erfolgt natürlich erst nach dem Ende der Studie. Allerdings wurde ja nicht nur die Auswertung nach dem Ende durchgeführt, sondern - und das ist das kritische - die Kriterien der Auswertung wurden auch erst nach dem Ende der Studie festgelegt. Aller Kriterien, die vorher ordentlich definiert wurden, wie der primäre Endpunkt, sind gescheitert. Wenn ich mir bei der Auswertung die Kriterien aussuchen kann, dann wähle ich natürlich die Kriterien so, dass sie mir die besten Ergebnisse liefern: und das nenne ich Manipulation! Daher werden retrospektive Analysen von der FDA auch nicht akzeptiert! Genausogut hätte Elan auch zu dem Ergebnis kommen können, dass Bapineuzumab bei Patienten mit blauen Augen besser wirkt, als bei braunen Augen... nur das wäre wohl etwas weniger glaubhaft gewesen wie mit und ohne ApoE4. Von der Sache her ist es aber gleich. Hätte Elan vor Beginn der Studie ApoE4 als Kriterium mit einbezogen und die Studie danach randomisiert, hätte ich weit weniger bedenken.
zu 3... Fakt(!) ist, dass die Studie nicht nach ApoE4 randomisiert wurde. Elan hat selbst(!) von Ungleichgewichten in der Studie gesprochen. "In this trial, there were imbalances in patient numbers and characteristics at baseline between subgroups studied that may or may not have affected these results." http://www.emaxhealth.com/91/23110.html "Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results" http://www.fool.com/investing/high-growth/2008/06/18/elans-a… Ich finde deine Position eher sehr gewagt, wenn du behauptest, dass diese Ungleichgewichte (die gemäß der Aussagen von Elan ebenfalls Fakt sind!) das Ergebnis nicht beeinflusst haben!
zu 4... wenn du mir das Belegen kannst, dass Aricept Teil der Studie war und alle Patienten es gleich erhalten haben, dann nehme ich das zurück. Mir ist es jedenfalls nicht bekannt und ich habe dazu auch nichts gefunden, außer das Patienten in der Studie auch andere Alzheimermedikamente erhalten haben
zu 5/8... Fakt ist, dass es keine wissenschaftliche Erklärung gibt, warum Bapineuzumab ohne ApoE4 besser wirken sollte! Das sagen selbst die Experten! "Es gibt hier übrigens die Vermutung in informierten Kreisen ,dass BAP bei den Carriern vielleicht ZU GUT funktioniert--was bedeutet,dass das Plaque zu schnell abgebaut wurde bei zu hoher Dosis und deshalb innerhalb der Phase 2 einige Teilnehmer aus dem Test ausschieden.Von daher sollten die Ergebnisse in Phase 3 SUPER sein ,wenn die Patienten dann mit geringeren Dosen behandelt werden" ... so kann man natürlich alles passend machen! Es hat einfach so toll gewirkt, dass die Patienten Bapineuzumab nicht mehr weiter nehmen wollten und aus der Studie ausgestiegen sind... das glaubst du doch wohl selber nicht!
Es hat einfach so toll gewirkt, dass die Patienten Bapineuzumab nicht mehr weiter nehmen wollten und aus der Studie ausgestiegen sind... das glaubst du doch wohl selber nicht!  - das die Dosierung in der PIII gesenkt wurde, wurde natürlich deshalb gemacht, damit die Patienten nicht dummerweise geheilt werden und dann nicht mehr weiter mitmachen wollen. Mmmm... der Grund für die Verringerung der Dosierung ist schon bekannt und kein positiver: bei den höheren Dosierungen kam es in der PII zu gefährlichen Gehirnschwellungen! (auch das ist ein Fakt!)
- das die Dosierung in der PIII gesenkt wurde, wurde natürlich deshalb gemacht, damit die Patienten nicht dummerweise geheilt werden und dann nicht mehr weiter mitmachen wollen. Mmmm... der Grund für die Verringerung der Dosierung ist schon bekannt und kein positiver: bei den höheren Dosierungen kam es in der PII zu gefährlichen Gehirnschwellungen! (auch das ist ein Fakt!)
zu 6... es ging mir nur um das statistische Rauschen bei den "ohne ApoE4"-Patienten. Natürlich sind 60% eine sehr wesentliche Gruppe. Wenn man nur ein paar Prozent der Alzheimer Patienten helfen könnte, wäre das schon riesig!
zu 7... "June 17, 2008... The drug called bapineuzumab helped some Alzheimer's patients reduce loss in cognitive functions by 2 to 2.5 points during an 18-month follow-up as measured by a test.
Normally, Alzheimer's patients would otherwise lose 6.5 points over 18 months.
Ian Sanderson, senior research analyst for Cowen & Company was quoted by the New York Times as saying that “Anything north of a 2 point spread would be considered clinically significant.”
The effect was only observed in patients who did not carry a genetic variation called ApoE4. An estimated 40 to 70 percent of Alzheimer’s patients are no carriers."
http://foodconsumer.org/7777/8888/D_rug_N_ews_50/06171222200…
********
"Elan arbeitet seit 25 Jahren mit den weltweit anerkanntesten Forschern an der Amyloid-Hypothese"... einer von denen ist eben dieser James Nicoll! "Mediziner der University of Southampton haben geklärt, warum der erste Impfversuch gegen Alzheimer fehlschlug und mit schweren Entzündungen im Gehirn der Patienten endete. Nach einer unter der Leitung von James Nicoll durchgeführten Autopsie wissen Ärzte nun, warum der hochgepriesene Impfstoff zu schweren Nebenwirkungen, die auch den Abbruch des Versuchs bedeuteten, führte. ... Im Jahr 2000 wollten Elan und das Nicoll-Team den Erfolg bei Menschen bestätigen. Der Versuch schlug aber fehl. Nicoll war also maßgeblich an der Entwicklung bei Elan mit beteiligt und ist Experte auf dem Gebiet. Und wie kommentiert er nun die aktuellen PII-Daten? "Nicoll called the news release this morning "tantalizing." However, he added: "There is quite a lot of information you would like to see that isn't there. If there is efficacy, it is not of a very large magnitude." - sehr überzeugend klingt das nicht!
mfg ipollit
zu 2... "Wenn doppel-blind getestet wird,kann immer erst nachträglich ausgewertet werden"... die Auswertung erfolgt natürlich erst nach dem Ende der Studie. Allerdings wurde ja nicht nur die Auswertung nach dem Ende durchgeführt, sondern - und das ist das kritische - die Kriterien der Auswertung wurden auch erst nach dem Ende der Studie festgelegt. Aller Kriterien, die vorher ordentlich definiert wurden, wie der primäre Endpunkt, sind gescheitert. Wenn ich mir bei der Auswertung die Kriterien aussuchen kann, dann wähle ich natürlich die Kriterien so, dass sie mir die besten Ergebnisse liefern: und das nenne ich Manipulation! Daher werden retrospektive Analysen von der FDA auch nicht akzeptiert! Genausogut hätte Elan auch zu dem Ergebnis kommen können, dass Bapineuzumab bei Patienten mit blauen Augen besser wirkt, als bei braunen Augen... nur das wäre wohl etwas weniger glaubhaft gewesen wie mit und ohne ApoE4. Von der Sache her ist es aber gleich. Hätte Elan vor Beginn der Studie ApoE4 als Kriterium mit einbezogen und die Studie danach randomisiert, hätte ich weit weniger bedenken.
zu 3... Fakt(!) ist, dass die Studie nicht nach ApoE4 randomisiert wurde. Elan hat selbst(!) von Ungleichgewichten in der Studie gesprochen. "In this trial, there were imbalances in patient numbers and characteristics at baseline between subgroups studied that may or may not have affected these results." http://www.emaxhealth.com/91/23110.html "Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results" http://www.fool.com/investing/high-growth/2008/06/18/elans-a… Ich finde deine Position eher sehr gewagt, wenn du behauptest, dass diese Ungleichgewichte (die gemäß der Aussagen von Elan ebenfalls Fakt sind!) das Ergebnis nicht beeinflusst haben!
zu 4... wenn du mir das Belegen kannst, dass Aricept Teil der Studie war und alle Patienten es gleich erhalten haben, dann nehme ich das zurück. Mir ist es jedenfalls nicht bekannt und ich habe dazu auch nichts gefunden, außer das Patienten in der Studie auch andere Alzheimermedikamente erhalten haben
zu 5/8... Fakt ist, dass es keine wissenschaftliche Erklärung gibt, warum Bapineuzumab ohne ApoE4 besser wirken sollte! Das sagen selbst die Experten! "Es gibt hier übrigens die Vermutung in informierten Kreisen ,dass BAP bei den Carriern vielleicht ZU GUT funktioniert--was bedeutet,dass das Plaque zu schnell abgebaut wurde bei zu hoher Dosis und deshalb innerhalb der Phase 2 einige Teilnehmer aus dem Test ausschieden.Von daher sollten die Ergebnisse in Phase 3 SUPER sein ,wenn die Patienten dann mit geringeren Dosen behandelt werden" ... so kann man natürlich alles passend machen!
 Es hat einfach so toll gewirkt, dass die Patienten Bapineuzumab nicht mehr weiter nehmen wollten und aus der Studie ausgestiegen sind... das glaubst du doch wohl selber nicht!
Es hat einfach so toll gewirkt, dass die Patienten Bapineuzumab nicht mehr weiter nehmen wollten und aus der Studie ausgestiegen sind... das glaubst du doch wohl selber nicht!  - das die Dosierung in der PIII gesenkt wurde, wurde natürlich deshalb gemacht, damit die Patienten nicht dummerweise geheilt werden und dann nicht mehr weiter mitmachen wollen. Mmmm... der Grund für die Verringerung der Dosierung ist schon bekannt und kein positiver: bei den höheren Dosierungen kam es in der PII zu gefährlichen Gehirnschwellungen! (auch das ist ein Fakt!)
- das die Dosierung in der PIII gesenkt wurde, wurde natürlich deshalb gemacht, damit die Patienten nicht dummerweise geheilt werden und dann nicht mehr weiter mitmachen wollen. Mmmm... der Grund für die Verringerung der Dosierung ist schon bekannt und kein positiver: bei den höheren Dosierungen kam es in der PII zu gefährlichen Gehirnschwellungen! (auch das ist ein Fakt!)zu 6... es ging mir nur um das statistische Rauschen bei den "ohne ApoE4"-Patienten. Natürlich sind 60% eine sehr wesentliche Gruppe. Wenn man nur ein paar Prozent der Alzheimer Patienten helfen könnte, wäre das schon riesig!
zu 7... "June 17, 2008... The drug called bapineuzumab helped some Alzheimer's patients reduce loss in cognitive functions by 2 to 2.5 points during an 18-month follow-up as measured by a test.
Normally, Alzheimer's patients would otherwise lose 6.5 points over 18 months.
Ian Sanderson, senior research analyst for Cowen & Company was quoted by the New York Times as saying that “Anything north of a 2 point spread would be considered clinically significant.”
The effect was only observed in patients who did not carry a genetic variation called ApoE4. An estimated 40 to 70 percent of Alzheimer’s patients are no carriers."
http://foodconsumer.org/7777/8888/D_rug_N_ews_50/06171222200…
********
"Elan arbeitet seit 25 Jahren mit den weltweit anerkanntesten Forschern an der Amyloid-Hypothese"... einer von denen ist eben dieser James Nicoll! "Mediziner der University of Southampton haben geklärt, warum der erste Impfversuch gegen Alzheimer fehlschlug und mit schweren Entzündungen im Gehirn der Patienten endete. Nach einer unter der Leitung von James Nicoll durchgeführten Autopsie wissen Ärzte nun, warum der hochgepriesene Impfstoff zu schweren Nebenwirkungen, die auch den Abbruch des Versuchs bedeuteten, führte. ... Im Jahr 2000 wollten Elan und das Nicoll-Team den Erfolg bei Menschen bestätigen. Der Versuch schlug aber fehl. Nicoll war also maßgeblich an der Entwicklung bei Elan mit beteiligt und ist Experte auf dem Gebiet. Und wie kommentiert er nun die aktuellen PII-Daten? "Nicoll called the news release this morning "tantalizing." However, he added: "There is quite a lot of information you would like to see that isn't there. If there is efficacy, it is not of a very large magnitude." - sehr überzeugend klingt das nicht!

mfg ipollit
Antwort auf Beitrag Nr.: 34.449.750 von ipollit am 07.07.08 12:13:35...warten wir mal die GANZ GENAUEN Ergebnisse in Ph2 und die der wahrscheinlich verkürzten Phase 3 ab.....dann kannst Du mir einen ausgeben, wenn die Ergebnisse ganz KLASSE sind.....

Antwort auf Beitrag Nr.: 34.450.162 von Birgit.Tersteegen am 07.07.08 13:11:16
http://www.bbbiotech.ch/fileadmin/user_upload/files/bbbiotec…
Ermutigende Ergebnisse der Phase-II-Studie des Alzheimer-Medikaments Bapineuzumab
Wyeth und Elan Corporation meldeten viel versprechende Resultate für$Bapineuzumab, einen monoklonalen Antikörper zur Behandlung von Alzheimer.
Zwar erreichte die 240 Patienten umfassende Studie nicht den für die gesamte Studienpopulation festgelegten Wirkungsendpunkt, jedoch konnte ein eindeutiger klinischer Nutzen für eine Subgruppe gezeigt werden.
Die beiden Firmen haben im vergangenen Dezember eine Phase-III-Studie mit 4000 Alzheimer-Patienten begonnen. Der mit Bapineuzumab verfolgte Wirkungsansatz stellt die aussichtsreichste Therapie dar, die derzeit entwickelt wird.
Wer sich nur etwas mit Statistik auskennt, der weiss, dass für derart kleine Phase-2-Studien die Power normalerweise nicht ausreicht, um statistisch signifikante Ergebnisse zu liefern. Hierfür sind die umfangreicheren ph3-Studien vorgesehen. Die Tatsache, dass bei den Apoe4-Nichtträgern jedoch Verbesserungen statistisch signifikant nachgewiesen wurde, ist das sensationelle an diesem Ergebnis. Allein schon der Vergleich eines derartigen AD-Merkmals mit dem Merkmal der Augenfarbe verbietet eine weitere Diskussion.
http://www.bbbiotech.ch/fileadmin/user_upload/files/bbbiotec…
Ermutigende Ergebnisse der Phase-II-Studie des Alzheimer-Medikaments Bapineuzumab
Wyeth und Elan Corporation meldeten viel versprechende Resultate für$Bapineuzumab, einen monoklonalen Antikörper zur Behandlung von Alzheimer.
Zwar erreichte die 240 Patienten umfassende Studie nicht den für die gesamte Studienpopulation festgelegten Wirkungsendpunkt, jedoch konnte ein eindeutiger klinischer Nutzen für eine Subgruppe gezeigt werden.
Die beiden Firmen haben im vergangenen Dezember eine Phase-III-Studie mit 4000 Alzheimer-Patienten begonnen. Der mit Bapineuzumab verfolgte Wirkungsansatz stellt die aussichtsreichste Therapie dar, die derzeit entwickelt wird.
Wer sich nur etwas mit Statistik auskennt, der weiss, dass für derart kleine Phase-2-Studien die Power normalerweise nicht ausreicht, um statistisch signifikante Ergebnisse zu liefern. Hierfür sind die umfangreicheren ph3-Studien vorgesehen. Die Tatsache, dass bei den Apoe4-Nichtträgern jedoch Verbesserungen statistisch signifikant nachgewiesen wurde, ist das sensationelle an diesem Ergebnis. Allein schon der Vergleich eines derartigen AD-Merkmals mit dem Merkmal der Augenfarbe verbietet eine weitere Diskussion.
Antwort auf Beitrag Nr.: 34.451.220 von Cyberhexe am 07.07.08 15:18:08Study Type: Interventional
Study Design: Treatment, Randomized, Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor), Placebo Control, Parallel Assignment
Official Title: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Non- Carriers.
http://www.clinicaltrials.gov/ct2/show/NCT00574132?term=bapi…
Man beachte, dass in dieser Studie nicht die Augenfarbe ein Unterscheidungsmerkmal ist, sondern eben die Trägerschaft eines ganz bestimmten Gens, welches für die Ausprägung von AD von besonderer Bedeutung ist.
Study Design: Treatment, Randomized, Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor), Placebo Control, Parallel Assignment
Official Title: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Non- Carriers.
http://www.clinicaltrials.gov/ct2/show/NCT00574132?term=bapi…
Man beachte, dass in dieser Studie nicht die Augenfarbe ein Unterscheidungsmerkmal ist, sondern eben die Trägerschaft eines ganz bestimmten Gens, welches für die Ausprägung von AD von besonderer Bedeutung ist.
Antwort auf Beitrag Nr.: 34.448.296 von Henrig am 07.07.08 08:54:40@bernie
Teilnehmer ipollit ist bereits in mehreren Foren durch pseudowissenschaftliche Kommentare aufgefallen, die, gepaart mit reichlich Stammtischmentalität, nur selten einer konstruktiven Auseinandersetzung dienlich waren.
Nicht dass ich der Meinung wäre, Bapineuzumab wäre bereits durch. Dies wird in den ph3-Studien zu beweisen sein, jedoch die oft lächerlichen bis dummen Argumente dieses Teilnehmers treiben jeden halbwegs Interessierten in andere Foren.
Teilnehmer ipollit ist bereits in mehreren Foren durch pseudowissenschaftliche Kommentare aufgefallen, die, gepaart mit reichlich Stammtischmentalität, nur selten einer konstruktiven Auseinandersetzung dienlich waren.
Nicht dass ich der Meinung wäre, Bapineuzumab wäre bereits durch. Dies wird in den ph3-Studien zu beweisen sein, jedoch die oft lächerlichen bis dummen Argumente dieses Teilnehmers treiben jeden halbwegs Interessierten in andere Foren.
Antwort auf Beitrag Nr.: 34.461.160 von Cyberhexe am 08.07.08 15:48:25auch wenn ich hier in der letzten Zeit nicht geschrieben habe, bin ich weiterhin interessierter Leser.


Antwort auf Beitrag Nr.: 34.461.160 von Cyberhexe am 08.07.08 15:48:25"pseudowissenschaftliche Kommentare"... "lächerlichen bis dummen Argumente" - soviel zur sachlichen Diskussion und Stammtischmentalität!  Aber das bin ich ja von dir schon gewohnt...
Aber das bin ich ja von dir schon gewohnt...
nimm dir mal lieber ein Beispiel an Bernie55... mit dem kann man sich auch unterhalten, wenn man nicht einer Meinung ist!
mfg ipollit
 Aber das bin ich ja von dir schon gewohnt...
Aber das bin ich ja von dir schon gewohnt...nimm dir mal lieber ein Beispiel an Bernie55... mit dem kann man sich auch unterhalten, wenn man nicht einer Meinung ist!
mfg ipollit
Antwort auf Beitrag Nr.: 34.451.220 von Cyberhexe am 07.07.08 15:18:08>>Wer sich nur etwas mit Statistik auskennt, der weiss, dass für derart kleine Phase-2-Studien die Power normalerweise nicht ausreicht, um statistisch signifikante Ergebnisse zu liefern. Hierfür sind die umfangreicheren ph3-Studien vorgesehen. Die Tatsache, dass bei den Apoe4-Nichtträgern jedoch Verbesserungen statistisch signifikant nachgewiesen wurde, ist das sensationelle an diesem Ergebnis. Allein schon der Vergleich eines derartigen AD-Merkmals mit dem Merkmal der Augenfarbe verbietet eine weitere Diskussion.<<
Und wer sich noch ein bisschen mehr mit Statistik auskennt, der weiß, dass die Aussage "statistisch signifikante Verbesserung" für eine winzigeSubgruppe abseits jeder Power bei fehlender Randomisierung und einer retrospektiven Analyse NAHEZU WERTLOS ist! Genauso könnten die die Korrelation mit der Augenfarbe oder der Schuhgröße feststellen.
"Die Tatsache, dass bei den Apoe4-Nichtträgern jedoch Verbesserungen statistisch signifikant nachgewiesen wurde, ist das sensationelle an diesem Ergebnis." - das ist ja gerade der aberwitzige Irrtum! Genauso wie bei dieser tollen Provenge-Geschichte! Ich kann ja auch nichts dafür, dass es hier wieder genauso läuft.
Zu sagen, dass etwas statistisch signifikant ist, heißt noch lange nicht, dass es auch wirklich statistisch signifikant ist! Derartige Dinge kann man leicht bereits bei wikipedia unter Statistik nachlesen...
Das Gegenteil von retrospektiv (wie die angeblich positive Subgruppen-Analyse ohne ApoE4) ist prospektiv (wie der negative primäre Endpunkt der PII)...
Der Sinn der Prospektivität liegt in der Tatsache, dass sich beim Aufnehmen von Versuchsdaten praktisch immer ein scheinbarer Zusammenhang zwischen zwei Parametern ergibt, ohne dass ein tatsächlicher Wirkungszusammenhang vorliegt. Ist die Untersuchungshypothese im Vorhinein festgelegt, so ist das Risiko viel geringer, dass sich für diese Hypothese ein solcher scheinbarer Zusammenhang ergibt. Weiterhin können mit prospektiven Studien Hypothesen sauber widerlegt werden. Nicht-prospektive Studien legen es hingegen nahe, neue Hypothesen aufzustellen.
http://de.wikipedia.org/wiki/Prospektive_Studie
ApoE4 ist nicht-prospektiv, also kann dies der scheinbare aber nicht reale Zusammenhang sein, denn Elan nachträglich gefunden hat!
************
das folgende ist alles zur statistischen Signifikanz...
http://de.wikipedia.org/wiki/Statistische_Signifikanz
Dennoch muss ein solcher Zusammenhang nicht zwingend vorhanden sein. Auch Unterschiede, die statistisch signifikant sind, können zufällig sein. Wie wahrscheinlich das ist, hängt von der Auswahl der untersuchten Meßgrößen ab: es können zwischen 0% und 100% der statistisch signifikanten Zusammenhänge zufälligen Ursprungs sein.
und weiter...
Auch bei tatsächlich oder vorgeblich statistisch signifikanten Aussagen ist immer eine kritische Überprüfung der Versuchsanordnung und -durchführung notwendig. Nur selten genügen wissenschaftliche Untersuchungen den mathematischen Anforderungen an einen aussagefähigen statistischen Test. Bei vielen Studien steht der Wunsch des oder der Studiendurchführenden (z. B. im Rahmen einer Doktorarbeit) nach einem 'signifikanten' Ergebnis bei der Studiendurchführung zu sehr im Vordergrund - Untersuchungen, bei denen die Nullhypothese bestätigt wird, werden nämlich gemeinhin als uninteressant und überflüssig angesehen. Als Hinweise auf die Qualität einer Studie können im medizinischen Umfeld die Eigenschaften "randomisiert" , "kontrolliert" und "doppelblind" gelten. Ohne diese sind Aussagen etwa zur Wirksamkeit von Therapien mit äußerster Vorsicht
, "kontrolliert" und "doppelblind" gelten. Ohne diese sind Aussagen etwa zur Wirksamkeit von Therapien mit äußerster Vorsicht  zu behandeln. Sehr schwierig und problematisch ist insbesondere die Interpretation signifikanter Korrelationen in retrospektiven Studien.
zu behandeln. Sehr schwierig und problematisch ist insbesondere die Interpretation signifikanter Korrelationen in retrospektiven Studien.  Zu bedenken ist darüber hinaus stets, dass aus statistisch signifikanten Korrelationen oft fälschlich auf eine vermeintliche Kausalität geschlossen wird (Beispiel: Zwischen 1960 und 1990 korrelierte die Zahl der Störche in Deutschland signifikant mit der menschlichen Geburtenrate, da beide Zahlen stark gesunken sind, dennoch ist die Kausalität zumindest fraglich).
Zu bedenken ist darüber hinaus stets, dass aus statistisch signifikanten Korrelationen oft fälschlich auf eine vermeintliche Kausalität geschlossen wird (Beispiel: Zwischen 1960 und 1990 korrelierte die Zahl der Störche in Deutschland signifikant mit der menschlichen Geburtenrate, da beide Zahlen stark gesunken sind, dennoch ist die Kausalität zumindest fraglich).
Wie sieht es mit den PII-Eregbnissen aus? Die statistisch signifikant positive Subgruppe "ohne ApoE4" ist...
- nicht randomisiert! :O
- retrospektiv! :O
- die Kausalität ist ungewiss, da es keine theoretische Erklärung für den Effekt gibt! :O
Aussagewert und Power (Beispiel klinische Forschung) Statistisch signifikante Studien können trotzdem einen geringen praktischen Aussagewert haben.
Studien mit großer Fallzahl führen aufgrund der hohen statistischen Power (Teststärke) oft zu hoch signifikanten Ergebnissen. Solche Studien können trotzdem einen geringen Aussagewert haben, wenn die Größe des beobachteten Effekts oder der gemessene Parameter nicht klinisch relevant sind. Statistische Signifikanz ist also ein notwendiges, aber noch kein hinreichendes Kriterium für eine praktisch auch relevante – d.h. hier: ausreichend starke – Wirkung eines Medikaments. Für die Beurteilung der Relevanz ist die Effektstärke (Effektgröße) ein wichtiges Hilfsmittel.
Weitere kritische Prüfsteine vom methodologischen Gesichtspunkt aus sind:
- die Korrektheit der statistischen Modellannahmen (beispielsweise die Verteilungsannahme)
- die Anzahl der durchgeführten statistischen Tests (bei mehreren Tests, von welchen nicht einer eindeutig als primärer Test gekennzeichnet ist, sollte eine Adjustierung des Signifikanzniveaus durchgeführt werden)
- die prospektive Definition der Analysemethoden vor der "Entblindung" doppelblinder Studien.
Und wie sieht es mit der PII aus?
Wenn die Zahlen stimmen, die ich zitiert habe, dann liegt das positive Ergebnis der subgruppe an der Grenze zur klinischen Relevanz! Das macht das Ergebnis unsicherer.
Die PII selbst war nicht ausreichend gepowert. Wenn das für die komplette Studie gilt, dann für eine Subgruppe erst recht! Die "ohne ApoE4"-Gruppe muss extrem underpowert sein! Dass trotzdem eine signifikante Verbesserung festgestellt werden konnte, lässt nur zwei Schlüsse zu:
1.) der Effekt ist für diese Subgruppe extrem groß, so dass trotz der geringen Anzahl und der fehlenden Power, das Eregbnis signifikant ist
2.) die Signifikanz ist eine irrtümliche Annahme, die sich nur zufällig ergeben hat.
Der erste Punkt steht aber im Wiederspruch zu allen bisherigen Aussagen, wie z.B. der Einschätzung von James Nicoll, dass wenn dann nur ein geringer Effekt existiert, oder wie die Autopsie-Berichte, der Fehlschlag des primären Endpunktes, den von mir gefundenen Zahlen zur klinischen Relevanz, usw...
Bleibt also nur Punkt 2!
letzterer Punkt "prospektive Definition der Analysemethode" wurde z.B. bei der Provenge-Studie verletzt!
***********
das folgende lässt sich auch verallgemeineren...
Üblicherweise werden nach einem geprüften Studiendesign die Werte in einer Datenbank erfaßt und statistisch ausgewertet. Die moderne Tendenz der halb- oder vollautomatischen Dokumentations- und Auswertungsprogramme soll vielfach den Wunsch von Medizinern befriedigen, sich möglichst wenig mit der Materie "Biometrie" auseinandersetzen zu müssen und "schnell Ergebnisse" erhalten zu können. Doch genau in diesen Forderungen liegt die große Gefahr weiterer statistischer Trugschlüsse. Wird die konkrete Fragestellung nicht im Vorfeld prospektiv definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können. Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit" target="_blank" rel="nofollow ugc noopener">http://www.aerzteblatt.de/V4/archiv/artikel.asp?id=2588
Üblicherweise werden nach einem geprüften Studiendesign die Werte in einer Datenbank erfaßt und statistisch ausgewertet. Die moderne Tendenz der halb- oder vollautomatischen Dokumentations- und Auswertungsprogramme soll vielfach den Wunsch von Medizinern befriedigen, sich möglichst wenig mit der Materie "Biometrie" auseinandersetzen zu müssen und "schnell Ergebnisse" erhalten zu können. Doch genau in diesen Forderungen liegt die große Gefahr weiterer statistischer Trugschlüsse. Wird die konkrete Fragestellung nicht im Vorfeld prospektiv definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können. Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit" target="_blank" rel="nofollow ugc noopener">Wird die konkrete Fragestellung nicht im Vorfeld prospektiv definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können. Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit" target="_blank" rel="nofollow ugc noopener">http://www.aerzteblatt.de/V4/archiv/artikel.asp?id=2588
Üblicherweise werden nach einem geprüften Studiendesign die Werte in einer Datenbank erfaßt und statistisch ausgewertet. Die moderne Tendenz der halb- oder vollautomatischen Dokumentations- und Auswertungsprogramme soll vielfach den Wunsch von Medizinern befriedigen, sich möglichst wenig mit der Materie "Biometrie" auseinandersetzen zu müssen und "schnell Ergebnisse" erhalten zu können. Doch genau in diesen Forderungen liegt die große Gefahr weiterer statistischer Trugschlüsse. Wird die konkrete Fragestellung nicht im Vorfeld prospektiv definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können. Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit
Und wer sich noch ein bisschen mehr mit Statistik auskennt, der weiß, dass die Aussage "statistisch signifikante Verbesserung" für eine winzigeSubgruppe abseits jeder Power bei fehlender Randomisierung und einer retrospektiven Analyse NAHEZU WERTLOS ist! Genauso könnten die die Korrelation mit der Augenfarbe oder der Schuhgröße feststellen.
"Die Tatsache, dass bei den Apoe4-Nichtträgern jedoch Verbesserungen statistisch signifikant nachgewiesen wurde, ist das sensationelle an diesem Ergebnis." - das ist ja gerade der aberwitzige Irrtum! Genauso wie bei dieser tollen Provenge-Geschichte! Ich kann ja auch nichts dafür, dass es hier wieder genauso läuft.
Zu sagen, dass etwas statistisch signifikant ist, heißt noch lange nicht, dass es auch wirklich statistisch signifikant ist! Derartige Dinge kann man leicht bereits bei wikipedia unter Statistik nachlesen...
Das Gegenteil von retrospektiv (wie die angeblich positive Subgruppen-Analyse ohne ApoE4) ist prospektiv (wie der negative primäre Endpunkt der PII)...
Der Sinn der Prospektivität liegt in der Tatsache, dass sich beim Aufnehmen von Versuchsdaten praktisch immer ein scheinbarer Zusammenhang zwischen zwei Parametern ergibt, ohne dass ein tatsächlicher Wirkungszusammenhang vorliegt. Ist die Untersuchungshypothese im Vorhinein festgelegt, so ist das Risiko viel geringer, dass sich für diese Hypothese ein solcher scheinbarer Zusammenhang ergibt. Weiterhin können mit prospektiven Studien Hypothesen sauber widerlegt werden. Nicht-prospektive Studien legen es hingegen nahe, neue Hypothesen aufzustellen.
http://de.wikipedia.org/wiki/Prospektive_Studie
ApoE4 ist nicht-prospektiv, also kann dies der scheinbare aber nicht reale Zusammenhang sein, denn Elan nachträglich gefunden hat!
************
das folgende ist alles zur statistischen Signifikanz...
http://de.wikipedia.org/wiki/Statistische_Signifikanz
Dennoch muss ein solcher Zusammenhang nicht zwingend vorhanden sein. Auch Unterschiede, die statistisch signifikant sind, können zufällig sein. Wie wahrscheinlich das ist, hängt von der Auswahl der untersuchten Meßgrößen ab: es können zwischen 0% und 100% der statistisch signifikanten Zusammenhänge zufälligen Ursprungs sein.
und weiter...
Auch bei tatsächlich oder vorgeblich statistisch signifikanten Aussagen ist immer eine kritische Überprüfung der Versuchsanordnung und -durchführung notwendig. Nur selten genügen wissenschaftliche Untersuchungen den mathematischen Anforderungen an einen aussagefähigen statistischen Test. Bei vielen Studien steht der Wunsch des oder der Studiendurchführenden (z. B. im Rahmen einer Doktorarbeit) nach einem 'signifikanten' Ergebnis bei der Studiendurchführung zu sehr im Vordergrund - Untersuchungen, bei denen die Nullhypothese bestätigt wird, werden nämlich gemeinhin als uninteressant und überflüssig angesehen. Als Hinweise auf die Qualität einer Studie können im medizinischen Umfeld die Eigenschaften "randomisiert"
 , "kontrolliert" und "doppelblind" gelten. Ohne diese sind Aussagen etwa zur Wirksamkeit von Therapien mit äußerster Vorsicht
, "kontrolliert" und "doppelblind" gelten. Ohne diese sind Aussagen etwa zur Wirksamkeit von Therapien mit äußerster Vorsicht  zu behandeln. Sehr schwierig und problematisch ist insbesondere die Interpretation signifikanter Korrelationen in retrospektiven Studien.
zu behandeln. Sehr schwierig und problematisch ist insbesondere die Interpretation signifikanter Korrelationen in retrospektiven Studien.  Zu bedenken ist darüber hinaus stets, dass aus statistisch signifikanten Korrelationen oft fälschlich auf eine vermeintliche Kausalität geschlossen wird (Beispiel: Zwischen 1960 und 1990 korrelierte die Zahl der Störche in Deutschland signifikant mit der menschlichen Geburtenrate, da beide Zahlen stark gesunken sind, dennoch ist die Kausalität zumindest fraglich).
Zu bedenken ist darüber hinaus stets, dass aus statistisch signifikanten Korrelationen oft fälschlich auf eine vermeintliche Kausalität geschlossen wird (Beispiel: Zwischen 1960 und 1990 korrelierte die Zahl der Störche in Deutschland signifikant mit der menschlichen Geburtenrate, da beide Zahlen stark gesunken sind, dennoch ist die Kausalität zumindest fraglich).Wie sieht es mit den PII-Eregbnissen aus? Die statistisch signifikant positive Subgruppe "ohne ApoE4" ist...
- nicht randomisiert! :O
- retrospektiv! :O
- die Kausalität ist ungewiss, da es keine theoretische Erklärung für den Effekt gibt! :O
Aussagewert und Power (Beispiel klinische Forschung) Statistisch signifikante Studien können trotzdem einen geringen praktischen Aussagewert haben.
Studien mit großer Fallzahl führen aufgrund der hohen statistischen Power (Teststärke) oft zu hoch signifikanten Ergebnissen. Solche Studien können trotzdem einen geringen Aussagewert haben, wenn die Größe des beobachteten Effekts oder der gemessene Parameter nicht klinisch relevant sind. Statistische Signifikanz ist also ein notwendiges, aber noch kein hinreichendes Kriterium für eine praktisch auch relevante – d.h. hier: ausreichend starke – Wirkung eines Medikaments. Für die Beurteilung der Relevanz ist die Effektstärke (Effektgröße) ein wichtiges Hilfsmittel.
Weitere kritische Prüfsteine vom methodologischen Gesichtspunkt aus sind:
- die Korrektheit der statistischen Modellannahmen (beispielsweise die Verteilungsannahme)
- die Anzahl der durchgeführten statistischen Tests (bei mehreren Tests, von welchen nicht einer eindeutig als primärer Test gekennzeichnet ist, sollte eine Adjustierung des Signifikanzniveaus durchgeführt werden)
- die prospektive Definition der Analysemethoden vor der "Entblindung" doppelblinder Studien.
Und wie sieht es mit der PII aus?
Wenn die Zahlen stimmen, die ich zitiert habe, dann liegt das positive Ergebnis der subgruppe an der Grenze zur klinischen Relevanz! Das macht das Ergebnis unsicherer.
Die PII selbst war nicht ausreichend gepowert. Wenn das für die komplette Studie gilt, dann für eine Subgruppe erst recht! Die "ohne ApoE4"-Gruppe muss extrem underpowert sein! Dass trotzdem eine signifikante Verbesserung festgestellt werden konnte, lässt nur zwei Schlüsse zu:
1.) der Effekt ist für diese Subgruppe extrem groß, so dass trotz der geringen Anzahl und der fehlenden Power, das Eregbnis signifikant ist
2.) die Signifikanz ist eine irrtümliche Annahme, die sich nur zufällig ergeben hat.
Der erste Punkt steht aber im Wiederspruch zu allen bisherigen Aussagen, wie z.B. der Einschätzung von James Nicoll, dass wenn dann nur ein geringer Effekt existiert, oder wie die Autopsie-Berichte, der Fehlschlag des primären Endpunktes, den von mir gefundenen Zahlen zur klinischen Relevanz, usw...
Bleibt also nur Punkt 2!
letzterer Punkt "prospektive Definition der Analysemethode" wurde z.B. bei der Provenge-Studie verletzt!
***********
das folgende lässt sich auch verallgemeineren...
Üblicherweise werden nach einem geprüften Studiendesign die Werte in einer Datenbank erfaßt und statistisch ausgewertet. Die moderne Tendenz der halb- oder vollautomatischen Dokumentations- und Auswertungsprogramme soll vielfach den Wunsch von Medizinern befriedigen, sich möglichst wenig mit der Materie "Biometrie" auseinandersetzen zu müssen und "schnell Ergebnisse" erhalten zu können. Doch genau in diesen Forderungen liegt die große Gefahr weiterer statistischer Trugschlüsse. Wird die konkrete Fragestellung nicht im Vorfeld prospektiv
 definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können.
 Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit" target="_blank" rel="nofollow ugc noopener">http://www.aerzteblatt.de/V4/archiv/artikel.asp?id=2588
Üblicherweise werden nach einem geprüften Studiendesign die Werte in einer Datenbank erfaßt und statistisch ausgewertet. Die moderne Tendenz der halb- oder vollautomatischen Dokumentations- und Auswertungsprogramme soll vielfach den Wunsch von Medizinern befriedigen, sich möglichst wenig mit der Materie "Biometrie" auseinandersetzen zu müssen und "schnell Ergebnisse" erhalten zu können. Doch genau in diesen Forderungen liegt die große Gefahr weiterer statistischer Trugschlüsse. Wird die konkrete Fragestellung nicht im Vorfeld prospektiv
 definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können.
 Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit" target="_blank" rel="nofollow ugc noopener">Wird die konkrete Fragestellung nicht im Vorfeld prospektiv
 definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können.
 Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit" target="_blank" rel="nofollow ugc noopener">http://www.aerzteblatt.de/V4/archiv/artikel.asp?id=2588
Üblicherweise werden nach einem geprüften Studiendesign die Werte in einer Datenbank erfaßt und statistisch ausgewertet. Die moderne Tendenz der halb- oder vollautomatischen Dokumentations- und Auswertungsprogramme soll vielfach den Wunsch von Medizinern befriedigen, sich möglichst wenig mit der Materie "Biometrie" auseinandersetzen zu müssen und "schnell Ergebnisse" erhalten zu können. Doch genau in diesen Forderungen liegt die große Gefahr weiterer statistischer Trugschlüsse. Wird die konkrete Fragestellung nicht im Vorfeld prospektiv
 definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"
definiert, sondern werden nur "möglichst viele" Patientendaten dokumentiert, um dann "mal zu sehen, was an Ergebnissen herauskommt"  , begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.
, begeht man bereits einen der wichtigsten Trugschlüsse [8], nämlich den des Multiplen Testens.Dieser zwar einfache, aber dennoch weitverbreitete Trugschluß Multiples Testen soll hier exemplifizierend erläutert werden, weil dessen Verbreitung mit dem Einsatz von EDV proportional zunimmt. Er tritt häufig auch in Kombination mit weiteren, zum Teil schwer erkennbaren Trugschlüssen auf, gehört aber zu denjenigen, die schon in der Designphase vermieden werden sollten, während andere erst in einer späteren Phase auffallen und dann zu einem Abbruch und Neustart der Studie führen müssen. Das Multiple Testen tritt inhaltlich meist in drei – hier beispielhaft vereinfachten – Varianten auf. Das Grundprinzip ist vergleichbar mit einem Glücksspiel, bei dem die Gewinnchance zum Beispiel 1 Prozent = 0,01 beträgt. Je öfter man spielt, um so größer ist die gesamte Wahrscheinlichkeit, in dieser Kette von Spielversuchen mindestens einen Gewinn zu erzielen. Spielt man zum Beispiel 500mal, gewinnt man "ziemlich sicher", nämlich mit einer Wahrscheinlichkeit von 1 bis 0,99500 = 99,3 Prozent. Anders als beim Glücksspiel, bei dem jeder Versuch (Ziehung) einen Einsatz kostet, dem Spieler also die finanziellen Mittel ausgehen würden, geht das Datenmaterial des Biometrikers niemals aus, und er kann "beliebig oft spielen".
Trugschluß 1: Es werden "möglichst viele" Werte dokumentiert, um hinterher (retrospektiv) möglichst "irgendein signifikantes Ergebnis" erreichen zu können.
 Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.
Dieses retrospektive Ausprobieren hat folgenden Trugschlußmechanismus: Je mehr (stochastisch unabhängige) Parameter wie Schmerz, Zufriedenheit, Blutverlust etc. (zum Beispiel 100) auf dem Signifikanzniveau (a = 0,01) ausgewertet werden, um so größer wird die Wahrscheinlichkeit, daß man ein irrtümlich signifikantes Ergebnis erhält. In diesem Beispiel mit 100 Tests beträgt die Wahrscheinlichkeit, irrtümlich mindestens ein signifikantes Ergebnis zufällig zu erhalten, immerhin schon 1 - 0,99100 = 63 Prozent. Wer also 100 Parameter multipel testet, erzielt mit einer Wahrscheinlichkeit von 63 Prozent ein – möglicherweise zufälliges und irrtümliches – signifikantes Ergebnis. Trugschluß 2: Es wird aus verschiedenen Datenbeständen (zum Beispiel 100 Stationen) derselbe Parameter (zum Beispiel Blutverlust im Vergleich zweier OP-Techniken) getestet. Die Wahrscheinlichkeit, daß mindestens ein Wissenschaftler irrtümlich ein signifikantes Ergebnis veröffentlichen wird, liegt ebenfalls bei 1 - 0,99100 = 63 Prozent.Und wie sieht es bei der PII aus?...
Elan hat klar den Trugschluss 1 begangen! Es wurden retrospektiv also nachträglich unterschiedlichste klinische Parameter wie ApoE4 mit ihren Subgruppen auf Signifikanz untersucht! Elan wird sicherlich sehr sehr viele unterschiedliche Parameter wie ApoE4 besitzen... je mehr getestet wurde, umso größer ist die Wahrscheinlichkeit, dass per Zufall ein signifikant positives Ergebnis dabei herauskommt! Daher legt man bei einer guten Studie die zu testenden Parameter prospektiv, also vor Beginn der Studie, fest, damit die Unternehmen nicht hinterher, so wie es Elan jetzt gemacht hat, "spielen" können!
Dendreon hat bei Provenge zusätzlich noch Trugschluss 2 begangen, indem unterschiedliche Studien miteinander vermischt wurden.
es liesen sich noch endlos derartige Aussagen zur Statistik anbringen.
Ich weiß aus erster Hand, dass nicht-randomisierte retrospektive Studien über kleine Patientengruppen, völlig wertlos sind!
mfg ipollit
Antwort auf Beitrag Nr.: 34.462.593 von ipollit am 08.07.08 17:30:55nur so viel zu deinen unqualifizierten Äusserungen:
http://www.clinicaltrials.gov/ct2/show/NCT00112073?term=AAB-…
Official Title: A Phase IIA, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose, Safety, Tolerability, Pharmacokinetic, Pharmacodynamic, and Immunogenicity Trial of AAB-001 in Patients With Mild to Moderate AD
diese Studie wird nun als eine "open label study" weitergheführt und bietet somit den Placebos die Möglichkeit, ebenfalls das Verum zu erhalten!
http://www.clinicaltrials.gov/ct2/show/NCT00112073?term=AAB-…
Official Title: A Phase IIA, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Multiple Ascending Dose, Safety, Tolerability, Pharmacokinetic, Pharmacodynamic, and Immunogenicity Trial of AAB-001 in Patients With Mild to Moderate AD
diese Studie wird nun als eine "open label study" weitergheführt und bietet somit den Placebos die Möglichkeit, ebenfalls das Verum zu erhalten!
Antwort auf Beitrag Nr.: 34.461.582 von ipollit am 08.07.08 16:17:31...die Guten ins Töpfchen,die Bösen ins Kröpfchen...ach nee....
Ist ja wie Deine Argumentation, dass die (mit der lila Augenfarbe... )non Carrier wahrscheinlich zufällig viel weniger schwere Fälle waren als die Carrier(die mit den roten Pupillen
)non Carrier wahrscheinlich zufällig viel weniger schwere Fälle waren als die Carrier(die mit den roten Pupillen in der Ph2 und deshalb scheinbar und zufällig gute Ergebnisse brachten...-
in der Ph2 und deshalb scheinbar und zufällig gute Ergebnisse brachten...-

Ist ja wie Deine Argumentation, dass die (mit der lila Augenfarbe...
 )non Carrier wahrscheinlich zufällig viel weniger schwere Fälle waren als die Carrier(die mit den roten Pupillen
)non Carrier wahrscheinlich zufällig viel weniger schwere Fälle waren als die Carrier(die mit den roten Pupillen in der Ph2 und deshalb scheinbar und zufällig gute Ergebnisse brachten...-
in der Ph2 und deshalb scheinbar und zufällig gute Ergebnisse brachten...-
Antwort auf Beitrag Nr.: 34.463.930 von Cyberhexe am 08.07.08 19:22:56About the Trial
The Phase 2 trial was a randomized, double-blind, placebo
controlled, multiple ascending dose study of patients with mild to moderate Alzheimer's disease. The study was designed to enroll approximately 240 participants at 29 sites in the United States. The study tested four doses of bapineuzumab (0.15 mg/kg, 0.5 mg/kg, 1.0 mg/kg and 2.0 mg/kg) with approximately 60 patients in each dose cohort. Patients were randomized on an 8:7 ratio to receive bapineuzumab or placebo, resulting in approximately 32 participants receiving bapineuzumab and 28 participants receiving placebo in each dose group.
The study assessed the safety of bapineuzumab as well as impact of treatment on cognitive and functional endpoints, biomarkers and blood serum levels. The pre-specified primary efficacy endpoints were change from baseline in ADAS-cog and DAD in the 0.5 mg/kg, 1.0 mg/kg and 2.0 mg/kg dose groups against their placebo cohorts. The study was not powered to detect changes from baseline for the ADAS-Cog or DAD scales. Change in concentrations of tau in cerebral spinal fluid (CSF) was a secondary endpoint. The Neuropsychological Test Battery, Clinical Dementia Rating Sum of Boxes and Mini Mental State Examination (MMSE) were included as tertiary efficacy endpoints.
Efficacy was assessed from baseline for 18 months.
The Phase 2 trial was a randomized, double-blind, placebo
controlled, multiple ascending dose study of patients with mild to moderate Alzheimer's disease. The study was designed to enroll approximately 240 participants at 29 sites in the United States. The study tested four doses of bapineuzumab (0.15 mg/kg, 0.5 mg/kg, 1.0 mg/kg and 2.0 mg/kg) with approximately 60 patients in each dose cohort. Patients were randomized on an 8:7 ratio to receive bapineuzumab or placebo, resulting in approximately 32 participants receiving bapineuzumab and 28 participants receiving placebo in each dose group.
The study assessed the safety of bapineuzumab as well as impact of treatment on cognitive and functional endpoints, biomarkers and blood serum levels. The pre-specified primary efficacy endpoints were change from baseline in ADAS-cog and DAD in the 0.5 mg/kg, 1.0 mg/kg and 2.0 mg/kg dose groups against their placebo cohorts. The study was not powered to detect changes from baseline for the ADAS-Cog or DAD scales. Change in concentrations of tau in cerebral spinal fluid (CSF) was a secondary endpoint. The Neuropsychological Test Battery, Clinical Dementia Rating Sum of Boxes and Mini Mental State Examination (MMSE) were included as tertiary efficacy endpoints.
Efficacy was assessed from baseline for 18 months.
Antwort auf Beitrag Nr.: 34.463.930 von Cyberhexe am 08.07.08 19:22:56die PII war natürlich bezügl. des primären Endpunkts, über die einzelnen Arme und über ALLE Patienten randomisiert, kontrolliert und doppelt-verblindet!
der primäre Endpunkt wurde NICHT erreicht!
Elan Corporation, Plc and Wyeth announced preliminary results from a Phase II study of bapineuzumab for treatment in patients with mild to moderate Alzheimer's disease.
The Phase II trial was a randomized, double-blind, placebo controlled, multiple ascending dose study ...
The study did not meet the primary efficacy endpoints, however, post-hoc analyses showed clinically meaningful benefits in some subgroups.
*********
die Subgruppe der ApoE4 Non-Carrier ist eine post-hoc Analyse, damit nicht teil des primären und sekundären Endpunkts und damit nicht im Studienprotokoll definiert! Eine zuvor unbekannte Subgruppe kann schon per Definition implizit niemals randomisiert worden sein! Wie soll das denn gehen???? Es wäre ein absoluter Zufall, wenn ich diese beliebige Gruppe der ApoE4 Non-Carrier aus der randomisierten Gesamtmenge rausgreife und diese zufällige Subgruppe immer noch bzw. ebenfalls randomisiert ist!
Daher spricht Elan von Unausgewogenheiten ("imbalances") in der Subgruppe? Ist für dich eine randomisierte Gruppe etwa unausgewogen????
"In this trial, there were imbalances in patient numbers and characteristics at baseline between subgroups studied that may or may not have affected these results."
http://www.emaxhealth.com/91/23110.html
"Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results"
http://www.fool.com/investing/high-growth/2008/06/18/elans-a…
"Imbalances" in den Subgruppen und "Randomisierung" der Subgruppen passt wohl nicht so ganz!
dein plumper Versuch, Fakten vom Tisch zu fegen, ist wohl leider fehlgeschlagen!
mfg ipollit
der primäre Endpunkt wurde NICHT erreicht!
Elan Corporation, Plc and Wyeth announced preliminary results from a Phase II study of bapineuzumab for treatment in patients with mild to moderate Alzheimer's disease.
The Phase II trial was a randomized, double-blind, placebo controlled, multiple ascending dose study ...
The study did not meet the primary efficacy endpoints, however, post-hoc analyses showed clinically meaningful benefits in some subgroups.
*********
die Subgruppe der ApoE4 Non-Carrier ist eine post-hoc Analyse, damit nicht teil des primären und sekundären Endpunkts und damit nicht im Studienprotokoll definiert! Eine zuvor unbekannte Subgruppe kann schon per Definition implizit niemals randomisiert worden sein! Wie soll das denn gehen???? Es wäre ein absoluter Zufall, wenn ich diese beliebige Gruppe der ApoE4 Non-Carrier aus der randomisierten Gesamtmenge rausgreife und diese zufällige Subgruppe immer noch bzw. ebenfalls randomisiert ist!
Daher spricht Elan von Unausgewogenheiten ("imbalances") in der Subgruppe? Ist für dich eine randomisierte Gruppe etwa unausgewogen????

"In this trial, there were imbalances in patient numbers and characteristics at baseline between subgroups studied that may or may not have affected these results."
http://www.emaxhealth.com/91/23110.html
"Elan and Wyeth also noted that "there were imbalances" in this phase 2 trial that could have skewed its results"
http://www.fool.com/investing/high-growth/2008/06/18/elans-a…
"Imbalances" in den Subgruppen und "Randomisierung" der Subgruppen passt wohl nicht so ganz!
dein plumper Versuch, Fakten vom Tisch zu fegen, ist wohl leider fehlgeschlagen!

mfg ipollit
Antwort auf Beitrag Nr.: 34.464.087 von Cyberhexe am 08.07.08 19:37:54...wer derart zugängliche Informationen in Frage stellt und darüber seitenweise Aufsätze schreibt, der vergeudet schlichtweg seine Zeit und beansprucht die Geduld der Forumsteilnehmer.
Gegensätzliche Meinungen sind immer erlaubt, nein sie sind sogar erwünscht, jedoch sollte man sich an den Fakten orientieren und zuvor ein Minimum an Zeit investieren. Mit derart falschen Fakten pseudwissenschaftlich aufzutrumpfen und möglicherweise mehr Verwirrung als Aufklärung zu stiften, ist kontraproduktiv.
Noch einmal: Bapineuzumab ist sicherlich noch nicht durch, die bisher publizierten Ergebnisse und das Studiendesign der ph3-Studien geben jedoch Anlass zu berechtigten Hoffnungen. Die Martteilnehmer scheinen dies ähnlich zu beurteilen. Mit Spannung werden detaillierte Ergebnisse auf der Ende Juli stattfindenden Alzheimer-Konferenz in Chicago erwartet.
http://www.alz.org/icad/abstract.asp
ICAD is the world's leading forum on dementia research. Join more than 5,000 world-renowned researchers for an invited program not to be missed. The 2008 conference will take place July 26-31, 2008 in Chicago.
Presentation Number: O3-04-05
Presentation Time: 7/29/2008 4:00:00 PM
Title: Clinical trials of bapineuzumab a beta-amyloid-targeted immunotherapy in patients with mild-to-moderate Alzheimers disease
Category: Therapeutic strategies, amyloid-based
Author(s): Michael Grundman1, Ronald Black2, 1Elan Pharmaceuticals, South San Francisco, CA, USA; 2Wyeth Research, Philadelphia, PA, USA. Contact e-mail: michael.grundman@elan.com
Background:
Immunotherapy has been shown to reduce beta-amyloid (Aβ), a protein that accumulates in Alzheimer’s disease (AD) brain and is central to the neuropathology of AD. Preclinical studies have shown that immunotherapy with antibodies raised to the N-terminus of Aβ reduce amyloid burden and have favorable effects on synaptic density and memory.
Methods:
A phase 1 study of bapineuzumab, a fully-humanized monoclonal antibody raised against the N-terminus of Aβ, demonstrated single dose safety and tolerability of bapineuzumab in patients with mild-to-moderate AD. Based on phase 1 results, a phase 2, multiple ascending dose study of bapineuzumab was initiated. This randomized, multicenter, double-blind study in patients with mild-to-moderate AD was designed to explore the potential efficacy and safety of bapineuzumab at various doses compared with placebo.
Results:
Patients were randomized to receive either 0.15 mg/kg, 0.5 mg/kg, 1.0 mg/kg, or 2.0 mg/kg bapineuzumab or placebo in a 1:1 fashion. Patients are being followed for 18 months to determine the safety of bapineuzumab, as well as bapineuzumab’s efficacy as measured by cognitive measures (ADAS-Cog; NTB) and functional assessments (DAD). Additional analyses of brain volume by MRI, amyloid brain imaging, and measurement of cerebrospinal fluid Aβ and tau protein levels are planned.
Conclusions:
Information regarding the bapineuzumab development program will be presented.
Disclosures: M. Grundman, Elan Pharmaceuticals, Employee; Elan Pharmaceuticals, Stock Shareholder (directly purchased); R. Black, Wyeth Research, Employee; Wyeth Research, Stock Shareholder (directly purchased).
Gegensätzliche Meinungen sind immer erlaubt, nein sie sind sogar erwünscht, jedoch sollte man sich an den Fakten orientieren und zuvor ein Minimum an Zeit investieren. Mit derart falschen Fakten pseudwissenschaftlich aufzutrumpfen und möglicherweise mehr Verwirrung als Aufklärung zu stiften, ist kontraproduktiv.
Noch einmal: Bapineuzumab ist sicherlich noch nicht durch, die bisher publizierten Ergebnisse und das Studiendesign der ph3-Studien geben jedoch Anlass zu berechtigten Hoffnungen. Die Martteilnehmer scheinen dies ähnlich zu beurteilen. Mit Spannung werden detaillierte Ergebnisse auf der Ende Juli stattfindenden Alzheimer-Konferenz in Chicago erwartet.
http://www.alz.org/icad/abstract.asp
ICAD is the world's leading forum on dementia research. Join more than 5,000 world-renowned researchers for an invited program not to be missed. The 2008 conference will take place July 26-31, 2008 in Chicago.
Presentation Number: O3-04-05
Presentation Time: 7/29/2008 4:00:00 PM
Title: Clinical trials of bapineuzumab a beta-amyloid-targeted immunotherapy in patients with mild-to-moderate Alzheimers disease
Category: Therapeutic strategies, amyloid-based
Author(s): Michael Grundman1, Ronald Black2, 1Elan Pharmaceuticals, South San Francisco, CA, USA; 2Wyeth Research, Philadelphia, PA, USA. Contact e-mail: michael.grundman@elan.com
Background:
Immunotherapy has been shown to reduce beta-amyloid (Aβ), a protein that accumulates in Alzheimer’s disease (AD) brain and is central to the neuropathology of AD. Preclinical studies have shown that immunotherapy with antibodies raised to the N-terminus of Aβ reduce amyloid burden and have favorable effects on synaptic density and memory.
Methods:
A phase 1 study of bapineuzumab, a fully-humanized monoclonal antibody raised against the N-terminus of Aβ, demonstrated single dose safety and tolerability of bapineuzumab in patients with mild-to-moderate AD. Based on phase 1 results, a phase 2, multiple ascending dose study of bapineuzumab was initiated. This randomized, multicenter, double-blind study in patients with mild-to-moderate AD was designed to explore the potential efficacy and safety of bapineuzumab at various doses compared with placebo.
Results:
Patients were randomized to receive either 0.15 mg/kg, 0.5 mg/kg, 1.0 mg/kg, or 2.0 mg/kg bapineuzumab or placebo in a 1:1 fashion. Patients are being followed for 18 months to determine the safety of bapineuzumab, as well as bapineuzumab’s efficacy as measured by cognitive measures (ADAS-Cog; NTB) and functional assessments (DAD). Additional analyses of brain volume by MRI, amyloid brain imaging, and measurement of cerebrospinal fluid Aβ and tau protein levels are planned.
Conclusions:
Information regarding the bapineuzumab development program will be presented.
Disclosures: M. Grundman, Elan Pharmaceuticals, Employee; Elan Pharmaceuticals, Stock Shareholder (directly purchased); R. Black, Wyeth Research, Employee; Wyeth Research, Stock Shareholder (directly purchased).
Antwort auf Beitrag Nr.: 34.464.259 von ipollit am 08.07.08 19:53:35die PII war natürlich bezügl. des primären Endpunkts, über die einzelnen Arme und über ALLE Patienten randomisiert, kontrolliert und doppelt-verblindet!
...so ein Schmarrn: eine Studie ist entweder randomisiert und doppelt-verblindet oder eben nicht. Das hat mit dem primären Endpunkt aber auch gar nichts zu tun.
...so ein Schmarrn: eine Studie ist entweder randomisiert und doppelt-verblindet oder eben nicht. Das hat mit dem primären Endpunkt aber auch gar nichts zu tun.
Antwort auf Beitrag Nr.: 34.464.417 von Cyberhexe am 08.07.08 20:09:58http://biz.yahoo.com/ap/080708/elan_analyst_note.html?.v=1
Cowen analyst: Elan drug could be a blockbuster
Tuesday July 8, 2:13 pm ET
Cowen sees Elan's Alzheimer's disease drug as potential blockbuster, but years from market
NEW YORK (AP) -- Biotechnology company Elan Corp. could reap blockbuster rewards from its developing Alzheimer's disease drug bapineuzumab, but the drug likely won't hit the market for years, a Cowen and Co. analyst said Tuesday.
ADVERTISEMENT
American Depository Shares of the Irish company rose 74 cents, or 2.2 percent, to $34.95 in afternoon trading.
The stock has been on the upswing since the middle of June, when the company said results of a midstage trial showed bapineuzumab helped Alzheimer's patients who lack a certain gene -- a large subgroup of the patient population. Shares reached $36.08 on July 2, their highest point in more than seven years.
"Elan's lead drug development candidate, bapineuzumab, could be the first disease modifying treatment for Alzheimer's disease, targeting a multibillion dollar market opportunity," Cowen analyst Ian Sanderson said in a note to investors. He estimates potential sales of $7 billion to $8 billion in 2015.
However, with the drug only midway through development and not likely to reach market until 2011, Sanderson said Elan's stock has limited near-term upside potential.
He started coverage of shares at "Neutral."
Shares of Elan's development partner, Madison, N.J.-based Wyeth, rose $1.38, or 2.9 percent, to $48.96. The stock has traded between $38.39 and $58 over the last 52 weeks.
...falls Bapineuzumab tatsächlich die Marktzulassung erhalten sollte, dann würde ich diese Umsatzprognosen als sehr konservativ beurteilen:
According to the Alzheimer's Association (alz.org), there are more than 5 million Americans living with Alzheimer's disease. The direct and indirect costs of Alzheimer's and other dementias amount to more than $148 billion per year.
The lack of effective treatments for Alzheimer's disease and an aging population open up an enormous opportunity for disease-modifying medicines, according to analysis from Decision Resources Inc. (dresources.com). Competition to develop new drugs for Alzheimer's disease is fierce, and the efficacy bar for current Alzheimer's disease therapies is low. Therefore, Decision Resources analysts believe that market penetration will hinge on safety profiles.
"The launch of bapineuzumab will be the most-important factor driving growth in the Alzheimer's disease drug market," says Nitasha Manchanda, Ph.D., analyst, Decision Resources. "Despite being priced considerably lower than monoclonal antibodies in other markets, we expect bapineuzumab to enter the market priced nearly eightfold higher than current small-molecule therapies. Nevertheless, we anticipate significant uptake of this agent -- particularly in patients with mild Alzheimer's disease -- despite the safety concerns."
Cowen analyst: Elan drug could be a blockbuster
Tuesday July 8, 2:13 pm ET
Cowen sees Elan's Alzheimer's disease drug as potential blockbuster, but years from market
NEW YORK (AP) -- Biotechnology company Elan Corp. could reap blockbuster rewards from its developing Alzheimer's disease drug bapineuzumab, but the drug likely won't hit the market for years, a Cowen and Co. analyst said Tuesday.
ADVERTISEMENT
American Depository Shares of the Irish company rose 74 cents, or 2.2 percent, to $34.95 in afternoon trading.
The stock has been on the upswing since the middle of June, when the company said results of a midstage trial showed bapineuzumab helped Alzheimer's patients who lack a certain gene -- a large subgroup of the patient population. Shares reached $36.08 on July 2, their highest point in more than seven years.
"Elan's lead drug development candidate, bapineuzumab, could be the first disease modifying treatment for Alzheimer's disease, targeting a multibillion dollar market opportunity," Cowen analyst Ian Sanderson said in a note to investors. He estimates potential sales of $7 billion to $8 billion in 2015.
However, with the drug only midway through development and not likely to reach market until 2011, Sanderson said Elan's stock has limited near-term upside potential.
He started coverage of shares at "Neutral."
Shares of Elan's development partner, Madison, N.J.-based Wyeth, rose $1.38, or 2.9 percent, to $48.96. The stock has traded between $38.39 and $58 over the last 52 weeks.
...falls Bapineuzumab tatsächlich die Marktzulassung erhalten sollte, dann würde ich diese Umsatzprognosen als sehr konservativ beurteilen:
According to the Alzheimer's Association (alz.org), there are more than 5 million Americans living with Alzheimer's disease. The direct and indirect costs of Alzheimer's and other dementias amount to more than $148 billion per year.
The lack of effective treatments for Alzheimer's disease and an aging population open up an enormous opportunity for disease-modifying medicines, according to analysis from Decision Resources Inc. (dresources.com). Competition to develop new drugs for Alzheimer's disease is fierce, and the efficacy bar for current Alzheimer's disease therapies is low. Therefore, Decision Resources analysts believe that market penetration will hinge on safety profiles.
"The launch of bapineuzumab will be the most-important factor driving growth in the Alzheimer's disease drug market," says Nitasha Manchanda, Ph.D., analyst, Decision Resources. "Despite being priced considerably lower than monoclonal antibodies in other markets, we expect bapineuzumab to enter the market priced nearly eightfold higher than current small-molecule therapies. Nevertheless, we anticipate significant uptake of this agent -- particularly in patients with mild Alzheimer's disease -- despite the safety concerns."
Antwort auf Beitrag Nr.: 34.464.417 von Cyberhexe am 08.07.08 20:09:58die Subgruppe ist entweder randomisiert oder nicht randomisiert... im Falle von Elan's PII ist die Subgruppe nicht randomisiert!
(ich glaube jetzt langsam sind wir wieder an dem Punkt, wo mir wieder Lügen unterstellt werden... )
)
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
http://www.ncchta.org/pdfexecs/summ533.pdf
RECOMMENDATIONS FOR SUBGROUP ANALYSES AND THEIR INTERPRETATION:
(1) Subgroup analyses should, as far as possible, be restricted to those proposed before data collection. Any subgroups chosen after this time should be clearly identified.
retrospektive Wahl der Subgruppe ohne-ApoE4... gegen (1) hat Elan also schonmal verstoßen!
(2) Trials should ideally be powered with subgroup analyses in mind. However, for modest interactions, this may not be feasible.
die PII war ja nichtmal selbst genug gepowered! ... ganz zu schweigen von der Power der Subgruppe... Elan hat sich nicht gemäß (2) verhalten!
... ganz zu schweigen von der Power der Subgruppe... Elan hat sich nicht gemäß (2) verhalten!
(3) Subgroup-specific analyses are particularly unreliable and are affected by many factors. Subgroup analyses should always be based on formal tests of interaction although even these should be interpreted with caution.
and are affected by many factors. Subgroup analyses should always be based on formal tests of interaction although even these should be interpreted with caution.
oho... Subgruppen-Analysen sind besonders unzuverlässig und sollen nur, wenn überhaupt, mit großer Vorsicht interpretiert werden. Naja, ist es eine große Vorsicht, wenn Elan daraus direkt ein sehr vielversprechendes Ergebnis macht, ein Ergebnis, das "kristallklar" für den sofortigen Beginn der PIII spricht??? - ich würde sagen... eine klare Überinterpretation und damit Verstoß Nr (3)!
- ich würde sagen... eine klare Überinterpretation und damit Verstoß Nr (3)!
(4) The results from any subgroup analyses should not be over-interpreted. Unless there is strong supporting evidence, they are best viewed as a hypothesis-generation exercise. In particular, one should be wary of evidence suggesting that treatment is effective in one subgroup only.
Natürlich weiß Elan genau, warum ApoE4 Non-Carrier so super abschneiden und die anderen nicht. Was unterstützt diese These? Es gibt keine Theorie, nichts... nur eine Zahl: "statistisch signifikant!" Ich finde auch, Elan sollte sich davor hüten, diese Zahl für real zu halten! Tun sie leider nicht und damit der (4). Verstoß!
(5) Any apparent lack of differential effect should be regarded with caution unless the study was specifically powered with interactions in mind.
das spielt hier eigentlich keine Rolle
Und wie lautet das Ergebnis? Elan hat gegen 4 dieser 5 Empfehlungen zur Subgruppenanalyse verstoßen! ... aber warum? Vielleicht, weil es um viel Geld geht???? Weil es keine Alternative gibt????? Weil zu scheitern schmerzhaft ist????? Wer weiß...
... aber warum? Vielleicht, weil es um viel Geld geht???? Weil es keine Alternative gibt????? Weil zu scheitern schmerzhaft ist????? Wer weiß...
mfg ipollit
(ich glaube jetzt langsam sind wir wieder an dem Punkt, wo mir wieder Lügen unterstellt werden...
 )
)Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
http://www.ncchta.org/pdfexecs/summ533.pdf
RECOMMENDATIONS FOR SUBGROUP ANALYSES AND THEIR INTERPRETATION:
(1) Subgroup analyses should, as far as possible, be restricted to those proposed before data collection. Any subgroups chosen after this time should be clearly identified.
retrospektive Wahl der Subgruppe ohne-ApoE4... gegen (1) hat Elan also schonmal verstoßen!
(2) Trials should ideally be powered with subgroup analyses in mind. However, for modest interactions, this may not be feasible.
die PII war ja nichtmal selbst genug gepowered!
 ... ganz zu schweigen von der Power der Subgruppe... Elan hat sich nicht gemäß (2) verhalten!
... ganz zu schweigen von der Power der Subgruppe... Elan hat sich nicht gemäß (2) verhalten!(3) Subgroup-specific analyses are particularly unreliable
 and are affected by many factors. Subgroup analyses should always be based on formal tests of interaction although even these should be interpreted with caution.
and are affected by many factors. Subgroup analyses should always be based on formal tests of interaction although even these should be interpreted with caution.oho... Subgruppen-Analysen sind besonders unzuverlässig und sollen nur, wenn überhaupt, mit großer Vorsicht interpretiert werden. Naja, ist es eine große Vorsicht, wenn Elan daraus direkt ein sehr vielversprechendes Ergebnis macht, ein Ergebnis, das "kristallklar" für den sofortigen Beginn der PIII spricht???
 - ich würde sagen... eine klare Überinterpretation und damit Verstoß Nr (3)!
- ich würde sagen... eine klare Überinterpretation und damit Verstoß Nr (3)!(4) The results from any subgroup analyses should not be over-interpreted. Unless there is strong supporting evidence, they are best viewed as a hypothesis-generation exercise. In particular, one should be wary of evidence suggesting that treatment is effective in one subgroup only.

Natürlich weiß Elan genau, warum ApoE4 Non-Carrier so super abschneiden und die anderen nicht. Was unterstützt diese These? Es gibt keine Theorie, nichts... nur eine Zahl: "statistisch signifikant!" Ich finde auch, Elan sollte sich davor hüten, diese Zahl für real zu halten! Tun sie leider nicht und damit der (4). Verstoß!
(5) Any apparent lack of differential effect should be regarded with caution unless the study was specifically powered with interactions in mind.
das spielt hier eigentlich keine Rolle
Und wie lautet das Ergebnis? Elan hat gegen 4 dieser 5 Empfehlungen zur Subgruppenanalyse verstoßen!
 ... aber warum? Vielleicht, weil es um viel Geld geht???? Weil es keine Alternative gibt????? Weil zu scheitern schmerzhaft ist????? Wer weiß...
... aber warum? Vielleicht, weil es um viel Geld geht???? Weil es keine Alternative gibt????? Weil zu scheitern schmerzhaft ist????? Wer weiß...mfg ipollit

Antwort auf Beitrag Nr.: 34.464.714 von Cyberhexe am 08.07.08 20:43:14This randomized, multicenter, double-blind study in patients with mild-to-moderate AD was designed to explore the potential efficacy and safety of bapineuzumab at various doses compared with placebo.
...falls dies nicht von allen verstanden wird, eine ph2-Studie ist die Grundlage für optimal entworfene ph3-Studien. Und genau dies haben Elan/Wyeth auch umgesetzt. Aufgrund der Zwischenergebnisse der ph2-Studien wurden die ph-3-Studien entsprechend optimiert, sprich man hat die Studien unterteilt in Apoe4-Träger und Nichtträger und dies in Absprache bzw. Kooperation mit den FDA-Verantwortlichen.
Ein Blick in die entsprechend dokumentierten Studien genügt:
http://www.clinicaltrials.gov/ct2/show/NCT00575055?term=bapi…
Official Title: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Carriers.
http://www.clinicaltrials.gov/ct2/show/NCT00667810?term=bapi…
Official Title: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Non-Carriers
...und ich bin mir ziemlich sicher, dass Bapineuzumab eine Zulassungschance hat , bevor die ph3-Studien abgeschlossen sind.
Genauere Prognosen sind jedoch erst nach Bekanntgabe der detaillierten ph-2-Ergebnisse möglich.
Die haarsträubenden Versuche, die bisher veröffentlichten Fakten mit für ph-2-Studien unrelevanten Regelwerken zu diskreditieren, bestätigt das Bild von pseudowissenschaftlicher Aufschneiderei.
...falls dies nicht von allen verstanden wird, eine ph2-Studie ist die Grundlage für optimal entworfene ph3-Studien. Und genau dies haben Elan/Wyeth auch umgesetzt. Aufgrund der Zwischenergebnisse der ph2-Studien wurden die ph-3-Studien entsprechend optimiert, sprich man hat die Studien unterteilt in Apoe4-Träger und Nichtträger und dies in Absprache bzw. Kooperation mit den FDA-Verantwortlichen.
Ein Blick in die entsprechend dokumentierten Studien genügt:
http://www.clinicaltrials.gov/ct2/show/NCT00575055?term=bapi…
Official Title: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Carriers.
http://www.clinicaltrials.gov/ct2/show/NCT00667810?term=bapi…
Official Title: A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Trial of Bapineuzumab in Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E ε4 Non-Carriers
...und ich bin mir ziemlich sicher, dass Bapineuzumab eine Zulassungschance hat , bevor die ph3-Studien abgeschlossen sind.
Genauere Prognosen sind jedoch erst nach Bekanntgabe der detaillierten ph-2-Ergebnisse möglich.
Die haarsträubenden Versuche, die bisher veröffentlichten Fakten mit für ph-2-Studien unrelevanten Regelwerken zu diskreditieren, bestätigt das Bild von pseudowissenschaftlicher Aufschneiderei.
Antwort auf Beitrag Nr.: 34.466.083 von Cyberhexe am 08.07.08 23:41:42"Die haarsträubenden Versuche, die bisher veröffentlichten Fakten mit für ph-2-Studien unrelevanten Regelwerken zu diskreditieren, bestätigt das Bild von pseudowissenschaftlicher Aufschneiderei." 

********
gut, die Ergebnisse haben Elan dazu veranlasst, die PIII danach auszurichten (das ist ja noch lange kein Beweis, dass das auch klug war)
aber die Subgruppe der ApoE4 Non-Carrier war definitiv nicht randomisiert, zudem retrospektiv und stark underpowered! Damit ist die gefundene Signifikanz des Ergebnisses für diese Gruppe äußerst fraglich!!!
Selbst die Optimisten unter den Analysten sehen nur eine Zulassungschance von weit unter 50%! Aber Cyberhexe gehörte ja schon immer zu den Oberoptimisten...
mfg ipollit


********
gut, die Ergebnisse haben Elan dazu veranlasst, die PIII danach auszurichten (das ist ja noch lange kein Beweis, dass das auch klug war)
aber die Subgruppe der ApoE4 Non-Carrier war definitiv nicht randomisiert, zudem retrospektiv und stark underpowered! Damit ist die gefundene Signifikanz des Ergebnisses für diese Gruppe äußerst fraglich!!!
Selbst die Optimisten unter den Analysten sehen nur eine Zulassungschance von weit unter 50%! Aber Cyberhexe gehörte ja schon immer zu den Oberoptimisten...
mfg ipollit
Antwort auf Beitrag Nr.: 34.466.177 von ipollit am 09.07.08 00:06:39gut, die Ergebnisse haben Elan dazu veranlasst, die PIII danach auszurichten (das ist ja noch lange kein Beweis, dass das auch klug war)
...es wäre unklug, die ph-3-Studien nicht nach den Ergebnissen der ph2 zu entwerfen. Damit wird eine nachträgliche, also retrospektive Analyse dieses Merkmals überflüssig!
aber die Subgruppe der ApoE4 Non-Carrier war definitiv nicht randomisiert, zudem retrospektiv und stark underpowered! Damit ist die gefundene Signifikanz des Ergebnisses für diese Gruppe äußerst fraglich!!!
das Merkmal Apoe4-Träger oder Nichtträger war im ph2-Studienentwurf nicht vorgesehen. Dies hat jedoch mit randomisiert oder nicht wenig zu tun!!
Selbst die Optimisten unter den Analysten sehen nur eine Zulassungschance von weit unter 50%! Aber Cyberhexe gehörte ja schon immer zu den Oberoptimisten...
ich habe mich bisher nicht über Zulassungswahrscheinlichkeiten geäussert - aber derartzige Unterstellungen sind von pseudowissenschaftlichen Teilnehmern nicht Neues!
...es wäre unklug, die ph-3-Studien nicht nach den Ergebnissen der ph2 zu entwerfen. Damit wird eine nachträgliche, also retrospektive Analyse dieses Merkmals überflüssig!
aber die Subgruppe der ApoE4 Non-Carrier war definitiv nicht randomisiert, zudem retrospektiv und stark underpowered! Damit ist die gefundene Signifikanz des Ergebnisses für diese Gruppe äußerst fraglich!!!
das Merkmal Apoe4-Träger oder Nichtträger war im ph2-Studienentwurf nicht vorgesehen. Dies hat jedoch mit randomisiert oder nicht wenig zu tun!!
Selbst die Optimisten unter den Analysten sehen nur eine Zulassungschance von weit unter 50%! Aber Cyberhexe gehörte ja schon immer zu den Oberoptimisten...
ich habe mich bisher nicht über Zulassungswahrscheinlichkeiten geäussert - aber derartzige Unterstellungen sind von pseudowissenschaftlichen Teilnehmern nicht Neues!
Antwort auf Beitrag Nr.: 34.466.198 von Cyberhexe am 09.07.08 00:16:33"ich habe mich bisher nicht über Zulassungswahrscheinlichkeiten geäussert" ... tut mir Leid! Du siehst also auch nur eine Zulassungschance von weit unter 50%? Hatte ich jetzt nicht erwartet... deine Postings haben meistens etwas anders geklungen. Nun gut, wir werden es ja bald sehen...
mfg ipollit
mfg ipollit
Antwort auf Beitrag Nr.: 34.465.986 von ipollit am 08.07.08 23:18:39Also bei GPC hat Dich alles statistische Wissen (leider) trotzdem daneben liegen lassen. Manchmal muß man die Parameter auch werten und kann nicht das ApoE4 Gen in der Bedeutung einfach mit der Augenfarbe gleich setzen. Man sollte vor lauter Wald (statistische Masse) nicht die einzelnen vielleicht eindeutig besonderen Bäume übersehen, auch wenn man sich ihre Besonderheit vielleicht nicht erklären kann. 

Antwort auf Beitrag Nr.: 34.466.219 von ipollit am 09.07.08 00:23:23Also, ich denke hier gibt es jede Menge Verunsicherung und Unwissenheit.
Das mit der Plaqueauflösung und dem Fortschreiten der ALzheimererkrankung hat mich am Anfang auch erst irritiert. Es gibt jedoch Beweise das nicht die monomere oder polymere Form des b-Amyloids, sondern dimere oder trimere From die neurotoxische ist.
Dale Schenk darauf hingewiesen, daß BAP gegen jede From von b-Amyloid wirkt.
BAP kann also durchaus die polymere Form auflösen (Plaques), ohne das eine Besserung der Alzheimererkrankung erreicht wird. Entscheidend ist nicht das Entfernen der polymeren Form, sondern das Entfernen der Dimere und Trimere.
Das Entfernen der Plaques (polymere Form) führt jedoch noch zu einer besseren Durchblutung des Gehirns, was den Alzheimer zusätzlich bremsen kann.
Zu den statistischen Haarspaltereien kann ich nur sagen, warten wir ab was Ende Juli vorgelegt wird.
Ich denke das bei den Phase II Design schon eine gute Durchmischung stattgefunden hat. Da ApoE4-Carrier 40% der Bevölkerung ausmachen müssen die Sub-Gruppen unterschiedlich groß sein (96 / 144 Personen bei 240 Personen gesamt, wovon Idealerweise in jeder Gruppe 48 bzw 72 mit BAP behandelt wurden).
Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ....
Das mit der Plaqueauflösung und dem Fortschreiten der ALzheimererkrankung hat mich am Anfang auch erst irritiert. Es gibt jedoch Beweise das nicht die monomere oder polymere Form des b-Amyloids, sondern dimere oder trimere From die neurotoxische ist.
Dale Schenk darauf hingewiesen, daß BAP gegen jede From von b-Amyloid wirkt.
BAP kann also durchaus die polymere Form auflösen (Plaques), ohne das eine Besserung der Alzheimererkrankung erreicht wird. Entscheidend ist nicht das Entfernen der polymeren Form, sondern das Entfernen der Dimere und Trimere.
Das Entfernen der Plaques (polymere Form) führt jedoch noch zu einer besseren Durchblutung des Gehirns, was den Alzheimer zusätzlich bremsen kann.
Zu den statistischen Haarspaltereien kann ich nur sagen, warten wir ab was Ende Juli vorgelegt wird.
Ich denke das bei den Phase II Design schon eine gute Durchmischung stattgefunden hat. Da ApoE4-Carrier 40% der Bevölkerung ausmachen müssen die Sub-Gruppen unterschiedlich groß sein (96 / 144 Personen bei 240 Personen gesamt, wovon Idealerweise in jeder Gruppe 48 bzw 72 mit BAP behandelt wurden).
Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ....
 Dann schauen wir mal in die Zukunft
Dann schauen wir mal in die Zukunft 
.....Mit Spannung werden detaillierte Ergebnisse auf der Ende Juli stattfindenden Alzheimer-Konferenz in Chicago erwartet.
.....Deshalb lasst uns gemeinsam auf die anstehende Konferenz im Juli schauen.......dann werden viele Dinge vielleicht klarer zu sehen sein
...warten wir mal die GANZ GENAUEN Ergebnisse in Ph2
.....Zu den statistischen Haarspaltereien kann ich nur sagen, warten wir ab was Ende Juli vorgelegt wird.
Antwort auf Beitrag Nr.: 34.466.328 von 2CB_06 am 09.07.08 02:25:06 ....."Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ."
....."Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ." ....Da bist Du aber SEHR optimistisch...
....Da bist Du aber SEHR optimistisch...
Danke für diese Klarstellung!
für diese Klarstellung!
 ....."Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ."
....."Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ." ....Da bist Du aber SEHR optimistisch...
....Da bist Du aber SEHR optimistisch...
Danke
 für diese Klarstellung!
für diese Klarstellung!
Antwort auf Beitrag Nr.: 34.466.328 von 2CB_06 am 09.07.08 02:25:06"Es gibt jedoch Beweise das nicht die monomere oder polymere Form des b-Amyloids, sondern dimere oder trimere From die neurotoxische ist.
Dale Schenk darauf hingewiesen, daß BAP gegen jede From von b-Amyloid wirkt."
stimmt schon... z.B.
Amyloid-Beta Dimers Induce Alzheimer's-Like Findings in Rodents
By bmartin on June 24, 2008 4:51 PM
For the first time, researchers showed that amyloid beta (Aβ) dimers from the brains of individuals with Alzheimer's disease induce several AD-like changes in normal rodents. The results of a series of related experiments were reported in the latest online edition of Nature Medicine.
AD-consistent pathophysiologic changes were observed in the normal rodent hippocampus after it was exposed to soluble (but not insoluble) Aβ. The investigators, from Boston and Ireland, discovered
- the inhibition of long-term potentiation (LTP) of synaptic transmission (a cellular model for learning and memory) in a dose-dependent fashion;
- enhanced long-term depression (LTD) (a marker of weakening synaptic transmission);and
- reduced dendritic spine density (a marker of synaptic loss).*
The administration of the Aβ dimer also disrupted the learning of a standard avoidance task in rats. Antibodies to the N-terminus of Aβ prevented the dimer's effects on LTP and LTD (which requires metabotropic glutamate receptors); however, the effect of antibodies to other regions of Aβ was not as remarkable.
The fact that AD-like changes were not detected with insoluble Aβ or other oligomers of Aβ may explain the disconnect between relatively preserved cognitive function during life and a high burden of Aβ in some brains at autopsy —as Marcelle Morrison-Bogorad, director of the neuroscience division at the National Insititute on Aging (NIA), told the AP. The Nature Medicine study was funded, in part, by the NIA.
* The authors note that decreased synaptic density is the strongest neuropathologic correlate of dementia in AD.
andererseits, wenn Bapineuzumab Beta-Amyloid allgemein beseitigt, könnte es auch passieren, dass neben dem löslichen Dimer vielleicht Plaque entfernt wird, der vielleicht eine Art Schutzfunktion besitzt, was dann eher schädlich wäre.
Ich denke das bei den Phase II Design schon eine gute Durchmischung stattgefunden hat. Da ApoE4-Carrier 40% der Bevölkerung ausmachen müssen die Sub-Gruppen unterschiedlich groß sein (96 / 144 Personen bei 240 Personen gesamt, wovon Idealerweise in jeder Gruppe 48 bzw 72 mit BAP behandelt wurden).
Unterschiedlich groß ist das eine. 72 Non-Carriers mit BAP... gut, aber es sind ja auch noch 4 Kohorten mit unterschiedlichen Dosierungen. Wurden nicht höhere Dosierungen aus der PIII herausgenommen? Konsequenterweise müsste man dann auch die Statistik in der PII ohne diese Dosierungsguppe nehmen, oder zumindest den Effekt einer höheren Dosierung und damit einer potentiell höheren Wirkung auf das beobachtete Ergebnis der gesamten Subgruppe quantifizieren.
Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ...
So meinte ich das ja auch nicht. Die Randomisierung ist notwendig, um andere Einflussfaktoren als BAP auf ein Ergebnis ausschließen zu können. Es wäre z.B. unfair (nur ein extremes Beispiel!), wenn alle Placebos sehr alte Patienten wären und alle BAP noch recht jung wären. Da liegt ein positives Ergebnis nicht vielleicht nicht an BAP sondern am Alter. Daher müssen diese Einflussgrößen (und derartige sollte es einige geben) etwa in allen Gruppen gleich verteilt (also zufällig sein), damit diese einen möglichst geringen Einfluss auf das Ergebnis haben. Wenn die PII ein paar 1000 Patienten beinhaltet hätte, wäre eine Untermenge von ein paar hundert Patienten nach einer Größe die mit den anderen Einlussfaktoren nicht korreliert ist, vielleicht auch noch gut randomisiert. Aber bei so kleinen Untergruppen kann ich mir das nicht vorstellen? Meinst du die Non-Carrier BAP-Patienten sind jetzt im Schnitt genauso alt (und das Alter genauso verteilt) wie die Non-Carrier Placebos bzw die ApoE4-Carrier BAP und ApoE4-Carrier Placebo-Gruppe?
mfg ipollit
Dale Schenk darauf hingewiesen, daß BAP gegen jede From von b-Amyloid wirkt."
stimmt schon... z.B.
Amyloid-Beta Dimers Induce Alzheimer's-Like Findings in Rodents
By bmartin on June 24, 2008 4:51 PM
For the first time, researchers showed that amyloid beta (Aβ) dimers from the brains of individuals with Alzheimer's disease induce several AD-like changes in normal rodents. The results of a series of related experiments were reported in the latest online edition of Nature Medicine.
AD-consistent pathophysiologic changes were observed in the normal rodent hippocampus after it was exposed to soluble (but not insoluble) Aβ. The investigators, from Boston and Ireland, discovered
- the inhibition of long-term potentiation (LTP) of synaptic transmission (a cellular model for learning and memory) in a dose-dependent fashion;
- enhanced long-term depression (LTD) (a marker of weakening synaptic transmission);and
- reduced dendritic spine density (a marker of synaptic loss).*
The administration of the Aβ dimer also disrupted the learning of a standard avoidance task in rats. Antibodies to the N-terminus of Aβ prevented the dimer's effects on LTP and LTD (which requires metabotropic glutamate receptors); however, the effect of antibodies to other regions of Aβ was not as remarkable.
The fact that AD-like changes were not detected with insoluble Aβ or other oligomers of Aβ may explain the disconnect between relatively preserved cognitive function during life and a high burden of Aβ in some brains at autopsy —as Marcelle Morrison-Bogorad, director of the neuroscience division at the National Insititute on Aging (NIA), told the AP. The Nature Medicine study was funded, in part, by the NIA.
* The authors note that decreased synaptic density is the strongest neuropathologic correlate of dementia in AD.
andererseits, wenn Bapineuzumab Beta-Amyloid allgemein beseitigt, könnte es auch passieren, dass neben dem löslichen Dimer vielleicht Plaque entfernt wird, der vielleicht eine Art Schutzfunktion besitzt, was dann eher schädlich wäre.
Ich denke das bei den Phase II Design schon eine gute Durchmischung stattgefunden hat. Da ApoE4-Carrier 40% der Bevölkerung ausmachen müssen die Sub-Gruppen unterschiedlich groß sein (96 / 144 Personen bei 240 Personen gesamt, wovon Idealerweise in jeder Gruppe 48 bzw 72 mit BAP behandelt wurden).
Unterschiedlich groß ist das eine. 72 Non-Carriers mit BAP... gut, aber es sind ja auch noch 4 Kohorten mit unterschiedlichen Dosierungen. Wurden nicht höhere Dosierungen aus der PIII herausgenommen? Konsequenterweise müsste man dann auch die Statistik in der PII ohne diese Dosierungsguppe nehmen, oder zumindest den Effekt einer höheren Dosierung und damit einer potentiell höheren Wirkung auf das beobachtete Ergebnis der gesamten Subgruppe quantifizieren.
Kleinere Verschiebungen sind durchaus möglich, aber es ist sehr unwahrscheinlich, daß bei einer völligen Randomisierung plötzlich alle BAP behandelten Patienten (120) in der ApoE4 non Carrier Gruppe (144) zu finden sind. Das müsste eigentlich auch dem größten Idioten klar sein ...
So meinte ich das ja auch nicht. Die Randomisierung ist notwendig, um andere Einflussfaktoren als BAP auf ein Ergebnis ausschließen zu können. Es wäre z.B. unfair (nur ein extremes Beispiel!), wenn alle Placebos sehr alte Patienten wären und alle BAP noch recht jung wären. Da liegt ein positives Ergebnis nicht vielleicht nicht an BAP sondern am Alter. Daher müssen diese Einflussgrößen (und derartige sollte es einige geben) etwa in allen Gruppen gleich verteilt (also zufällig sein), damit diese einen möglichst geringen Einfluss auf das Ergebnis haben. Wenn die PII ein paar 1000 Patienten beinhaltet hätte, wäre eine Untermenge von ein paar hundert Patienten nach einer Größe die mit den anderen Einlussfaktoren nicht korreliert ist, vielleicht auch noch gut randomisiert. Aber bei so kleinen Untergruppen kann ich mir das nicht vorstellen? Meinst du die Non-Carrier BAP-Patienten sind jetzt im Schnitt genauso alt (und das Alter genauso verteilt) wie die Non-Carrier Placebos bzw die ApoE4-Carrier BAP und ApoE4-Carrier Placebo-Gruppe?
mfg ipollit
...die Inzidenz von Hautkrebs scheint unter Natalizumab nicht erhöht:
http://content.nejm.org/cgi/content/short/359/1/99
More on Melanoma with Transdifferentiation
Mullen et al. (Feb. 7 issue)1 report on two cases of melanoma in patients with multiple sclerosis who received natalizumab. Their letter shows the limited interpretability of individual case reports in establishing cause-and-effect relationships. In response to this letter, we performed a meta-analysis of safety data from clinical trials of natalizumab. The incidence of melanoma was similar among patients who received natalizumab (3 of 4250 [0.07%]) as compared with those who received placebo (2 of 2059 [0.10%]). Rates of melanoma followed a similar pattern: 0.419 per 1000 patient-years among persons who received natalizumab versus 0.823 per 1000 patient-years among persons who received placebo. In addition, postmarketing surveillance data as of December 2007 do not indicate an increased risk of melanoma among more than 21,000 patients who received natalizumab (unpublished data).
Studies suggest that 4β1–vascular-cell adhesion molecule 1 interactions are important for the metastatic capacity of melanoma cells and that blockade may prevent metastasis.2,3 In addition, natalizumab at saturating levels had no effect on tumor growth or metastasis in a xenograft model involving an 4-integrin–positive human melanoma tumor line implanted in nude mice.
http://content.nejm.org/cgi/content/short/359/1/99
More on Melanoma with Transdifferentiation
Mullen et al. (Feb. 7 issue)1 report on two cases of melanoma in patients with multiple sclerosis who received natalizumab. Their letter shows the limited interpretability of individual case reports in establishing cause-and-effect relationships. In response to this letter, we performed a meta-analysis of safety data from clinical trials of natalizumab. The incidence of melanoma was similar among patients who received natalizumab (3 of 4250 [0.07%]) as compared with those who received placebo (2 of 2059 [0.10%]). Rates of melanoma followed a similar pattern: 0.419 per 1000 patient-years among persons who received natalizumab versus 0.823 per 1000 patient-years among persons who received placebo. In addition, postmarketing surveillance data as of December 2007 do not indicate an increased risk of melanoma among more than 21,000 patients who received natalizumab (unpublished data).
Studies suggest that 4β1–vascular-cell adhesion molecule 1 interactions are important for the metastatic capacity of melanoma cells and that blockade may prevent metastasis.2,3 In addition, natalizumab at saturating levels had no effect on tumor growth or metastasis in a xenograft model involving an 4-integrin–positive human melanoma tumor line implanted in nude mice.
Antwort auf Beitrag Nr.: 34.469.858 von Cyberhexe am 09.07.08 13:45:01http://www.ncbi.nlm.nih.gov/pubmed/18322388?ordinalpos=13&it…
Progress in the active immunotherapeutic approach to Alzheimer's disease: clinical investigations into AN1792-associated meningoencephalitis.Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS.
Wyeth Research, Collegeville, PA 19426, USA.
BACKGROUND: In a phase 2a clinical trial of AN1792 (Study 201), a potential immunotherapeutic agent for use in Alzheimer's disease (AD), approximately 6% of the treated AD patients (18/300) developed meningoencephalitis (ME). OBJECTIVE: To elucidate potential immune mechanisms of treatment-induced ME. METHODS: Peripheral blood mononuclear cells obtained from patients who received AN1792 were stimulated in vitro either with beta-amyloid (Abeta) or various overlapping peptides of Abeta(1-42), followed by quantification of cytokine-secreting cells by enzyme-linked immunosorbent spot assay. RESULTS: A significant difference in the quality of the T-cell responses between patients in Study 201 and those in earlier studies of AN1792 was noted. T-cell responses specific to the carboxy terminus of Abeta elicited from patients' peripheral blood mononuclear cells in an earlier multiple dose study (Study 102) were Th2 biased, while those from Study 201 were biased toward a proinflammatory Th1 response. Antibody responses in both studies were quantitatively and qualitatively similar (as determined by epitope mapping). The addition of polysorbate 80 to the formulation used in Study 201 is the most likely explanation for the difference in the T-cell response. CONCLUSION: ME following injection of AN1792 may be related to immune response differences driven by a formulation change. To address this, a novel peptide-carrier protein conjugate using an amino-terminal fragment of Abeta (ACC-001) has been developed to avoid potentially harmful T-cell responses, while maintaining a similar antibody response to that of AN1792. Immunotherapeutic trials using this treatment approach in AD patients are in progress. 2008 S. Karger AG, Basel
PMID: 18322388 [PubMed - indexed for MEDLINE]
Progress in the active immunotherapeutic approach to Alzheimer's disease: clinical investigations into AN1792-associated meningoencephalitis.Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS.
Wyeth Research, Collegeville, PA 19426, USA.
BACKGROUND: In a phase 2a clinical trial of AN1792 (Study 201), a potential immunotherapeutic agent for use in Alzheimer's disease (AD), approximately 6% of the treated AD patients (18/300) developed meningoencephalitis (ME). OBJECTIVE: To elucidate potential immune mechanisms of treatment-induced ME. METHODS: Peripheral blood mononuclear cells obtained from patients who received AN1792 were stimulated in vitro either with beta-amyloid (Abeta) or various overlapping peptides of Abeta(1-42), followed by quantification of cytokine-secreting cells by enzyme-linked immunosorbent spot assay. RESULTS: A significant difference in the quality of the T-cell responses between patients in Study 201 and those in earlier studies of AN1792 was noted. T-cell responses specific to the carboxy terminus of Abeta elicited from patients' peripheral blood mononuclear cells in an earlier multiple dose study (Study 102) were Th2 biased, while those from Study 201 were biased toward a proinflammatory Th1 response. Antibody responses in both studies were quantitatively and qualitatively similar (as determined by epitope mapping). The addition of polysorbate 80 to the formulation used in Study 201 is the most likely explanation for the difference in the T-cell response. CONCLUSION: ME following injection of AN1792 may be related to immune response differences driven by a formulation change. To address this, a novel peptide-carrier protein conjugate using an amino-terminal fragment of Abeta (ACC-001) has been developed to avoid potentially harmful T-cell responses, while maintaining a similar antibody response to that of AN1792. Immunotherapeutic trials using this treatment approach in AD patients are in progress. 2008 S. Karger AG, Basel
PMID: 18322388 [PubMed - indexed for MEDLINE]
FDA News
July 9, 2008
FDA Revises Process for Responding to Drug Applications
The U.S. Food and Drug Administration today announced that it is revising the way it communicates to drug companies when a marketing application cannot be approved as submitted.
Under new regulations that govern the drug approval process, FDA's Center for Drug Evaluation and Research (CDER) will no longer issue "approvable" or "not approvable" letters when a drug application is not approved. Instead, CDER will issue a "complete response" letter at the end of the review period to let a drug company know of the agency's decision on the application.
"These new regulations will help the FDA adopt a more consistent and neutral way of conveying information to a company when we cannot approve a drug application in its present form," said Janet Woodcock, M.D., director of the agency's Center for Drug Evaluation and Research (CDER). "Thorough and timely review of drug applications is a priority of the FDA, and these new processes will make our communications with sponsors of applications more consistent."
Taking the place of "approvable" and "not approvable" letters, a "complete response" letter will be issued to let a company know that the review period for a drug is complete and that the application is not yet ready for approval. The letter will describe specific deficiencies and, when possible, will outline recommended actions the applicant might take to get the application ready for approval.
Currently, when assessing new drug applications, the FDA can respond to a sponsor in one of three types of letters: an "approval" letter, meaning the drug has met agency standards for safety and efficacy and the drug can be marketed for sale in the United States; an "approvable" letter, which generally indicates that the drug can probably be approved at a later date provided that the applicant provides certain additional information or makes specified changes (such as to labeling); or a "not approvable" letter, meaning the application has deficiencies generally requiring the submission of substantial additional data before the application can be approved.
"Complete response" letters are already used to respond to companies that submit biologic license applications. The process for drugs and biologics will be consistent under the new regulations.
The revision should not affect the overall time it takes the FDA to review new or generic drug applications or biologic license applications. These changes, which will become effective on Aug. 11, 2008, are not expected to directly affect consumers.
In July 2004, the FDA issued a proposed rule on these topics. At that time the agency asked for comments on the proposal. Today's final rule addresses comments submitted to the agency.
For more information, see:
Link to the Complete Response Final Rule
http://www.fda.gov/cder/regulatory/complete_response_FR/defa…
Link to the drug approval process page
http://www.fda.gov/fdac/special/testtubetopatient/default.ht…
http://www.fda.gov/bbs/topics/NEWS/2008/NEW01859.html
July 9, 2008
FDA Revises Process for Responding to Drug Applications
The U.S. Food and Drug Administration today announced that it is revising the way it communicates to drug companies when a marketing application cannot be approved as submitted.
Under new regulations that govern the drug approval process, FDA's Center for Drug Evaluation and Research (CDER) will no longer issue "approvable" or "not approvable" letters when a drug application is not approved. Instead, CDER will issue a "complete response" letter at the end of the review period to let a drug company know of the agency's decision on the application.
"These new regulations will help the FDA adopt a more consistent and neutral way of conveying information to a company when we cannot approve a drug application in its present form," said Janet Woodcock, M.D., director of the agency's Center for Drug Evaluation and Research (CDER). "Thorough and timely review of drug applications is a priority of the FDA, and these new processes will make our communications with sponsors of applications more consistent."
Taking the place of "approvable" and "not approvable" letters, a "complete response" letter will be issued to let a company know that the review period for a drug is complete and that the application is not yet ready for approval. The letter will describe specific deficiencies and, when possible, will outline recommended actions the applicant might take to get the application ready for approval.
Currently, when assessing new drug applications, the FDA can respond to a sponsor in one of three types of letters: an "approval" letter, meaning the drug has met agency standards for safety and efficacy and the drug can be marketed for sale in the United States; an "approvable" letter, which generally indicates that the drug can probably be approved at a later date provided that the applicant provides certain additional information or makes specified changes (such as to labeling); or a "not approvable" letter, meaning the application has deficiencies generally requiring the submission of substantial additional data before the application can be approved.
"Complete response" letters are already used to respond to companies that submit biologic license applications. The process for drugs and biologics will be consistent under the new regulations.
The revision should not affect the overall time it takes the FDA to review new or generic drug applications or biologic license applications. These changes, which will become effective on Aug. 11, 2008, are not expected to directly affect consumers.
In July 2004, the FDA issued a proposed rule on these topics. At that time the agency asked for comments on the proposal. Today's final rule addresses comments submitted to the agency.
For more information, see:
Link to the Complete Response Final Rule
http://www.fda.gov/cder/regulatory/complete_response_FR/defa…
Link to the drug approval process page
http://www.fda.gov/fdac/special/testtubetopatient/default.ht…
http://www.fda.gov/bbs/topics/NEWS/2008/NEW01859.html

Presentation Number: O3-04-05
Presentation Time: 7/29/2008 4:00:00 PM
Title:
Clinical trials of bapineuzumab a beta-amyloid-targeted immunotherapy in patients with mild-to-moderate Alzheimers disease
Category: Therapeutic strategies, amyloid-based
Author(s): Michael Grundman1, Ronald Black2, 1Elan Pharmaceuticals, South San Francisco, CA, USA; 2Wyeth Research, Philadelphia, PA, USA. Contact e-mail: michael.grundman@elan.com
Background:
Immunotherapy has been shown to reduce beta-amyloid (Aβ), a protein that accumulates in Alzheimer’s disease (AD) brain and is central to the neuropathology of AD. Preclinical studies have shown that immunotherapy with antibodies raised to the N-terminus of Aβ reduce amyloid burden and have favorable effects on synaptic density and memory.
Methods:
A phase 1 study of bapineuzumab, a fully-humanized monoclonal antibody raised against the N-terminus of Aβ, demonstrated single dose safety and tolerability of bapineuzumab in patients with mild-to-moderate AD. Based on phase 1 results, a phase 2, multiple ascending dose study of bapineuzumab was initiated. This randomized, multicenter, double-blind study in patients with mild-to-moderate AD was designed to explore the potential efficacy and safety of bapineuzumab at various doses compared with placebo.
Results:
Patients were randomized to receive either 0.15 mg/kg, 0.5 mg/kg, 1.0 mg/kg, or 2.0 mg/kg bapineuzumab or placebo in a 1:1 fashion. Patients are being followed for 18 months to determine the safety of bapineuzumab, as well as bapineuzumab’s efficacy as measured by cognitive measures (ADAS-Cog; NTB) and functional assessments (DAD). Additional analyses of brain volume by MRI, amyloid brain imaging, and measurement of cerebrospinal fluid Aβ and tau protein levels are planned.
Conclusions:
Information regarding the bapineuzumab development program will be presented.
Disclosures: M. Grundman, Elan Pharmaceuticals, Employee; Elan Pharmaceuticals, Stock Shareholder (directly purchased); R. Black, Wyeth Research, Employee; Wyeth Research, Stock Shareholder (directly purchased).
http://www.abstractsonline.com/viewer/SearchResults.asp
http://www.abstractsonline.com/viewer/viewAbstractPrintFrien…
tick tack tick tack... 
http://www.msnbc.msn.com/id/25722244
*********
Vaccine failure deepens Alzheimer's mystery
Experimental shot stopped plaque, but not dementia, researchers say :O
The Associated Press
Thurs July 17, 2008, 7:00 p.m. ET
LONDON - Some doctors have long suspected that if the plaque that builds up in the brains of patients with Alzheimer\'s disease could be removed, they could be saved. But a new vaccine that did just that suggests the theory is wrong.
British researchers gave 64 patients with moderate Alzheimer\'s disease an experimental vaccine designed to eliminate plaque from their brains. Some patients were followed for up to six years.
Autopsies on seven patients who died of Alzheimer\'s during the study showed that nearly all of the sticky beta-amyloid protein thought to be dangerous had been removed. But all patients still had severe dementia.
\"It may be that these toxic plaques trigger the neurodegeneration, but don\'t have an ongoing role,\" said Clive Holmes of the University of Southampton, lead author, in a press statement. The study was published Friday in the medical journal, The Lancet. It was paid for by the Alzheimer\'s Research Trust, a British charity.
Alzheimer\'s disease is the most common cause of dementia and affects about 25 million people worldwide.
Experts said that the study\'s findings pointed to a major gap in our understanding of the disease. Doctors have never been sure whether the brain plaques are the cause of Alzheimer\'s disease or just a side effect.
\"We still don\'t have enough understanding of what we should target,\" said Dr. Bengt Winblad, director of the Alzheimer\'s Centre at Sweden\'s Karolinska Institute. Winblad was not connected to the study.
Brain tangles may play a role
Aside from the plaque build-up, scientists also think that tangles of another brain protein called tau play a major role in Alzheimer\'s. Because those tangles form later than the plaque, some experts think they should be the focus instead.
\"It may be harder to get a response from targeting plaque because that forms years before people actually have Alzheimer\'s,\" said Dr. Simon Lovestone, professor of Old Age Psychiatry at King\'s College in London. \"By the time you do something, it may be too late.\"
Winblad said there was a better connection between brain tangles and Alzheimer\'s symptoms, but that no studies so far had looked at whether removing tangles might improve or even reverse Alzheimer\'s disease in patients.
Still, experts say that attacking toxic plaque in the brain shouldn\'t be abandoned just yet, since the formation of such plaques might be what sparks Alzheimer\'s disease in the first place.
\"Removal of the initial motor for the disease might slow progression,\" wrote Peter H. St. George-Hyslop and John C. Morris of the University of Cambridge and the University of Toronto in an accompanying commentary in the Lancet.
mfg ipollit

http://www.msnbc.msn.com/id/25722244
*********
Vaccine failure deepens Alzheimer's mystery
Experimental shot stopped plaque, but not dementia, researchers say :O
The Associated Press
Thurs July 17, 2008, 7:00 p.m. ET
LONDON - Some doctors have long suspected that if the plaque that builds up in the brains of patients with Alzheimer\'s disease could be removed, they could be saved. But a new vaccine that did just that suggests the theory is wrong.
British researchers gave 64 patients with moderate Alzheimer\'s disease an experimental vaccine designed to eliminate plaque from their brains. Some patients were followed for up to six years.
Autopsies on seven patients who died of Alzheimer\'s during the study showed that nearly all of the sticky beta-amyloid protein thought to be dangerous had been removed. But all patients still had severe dementia.
\"It may be that these toxic plaques trigger the neurodegeneration, but don\'t have an ongoing role,\" said Clive Holmes of the University of Southampton, lead author, in a press statement. The study was published Friday in the medical journal, The Lancet. It was paid for by the Alzheimer\'s Research Trust, a British charity.
Alzheimer\'s disease is the most common cause of dementia and affects about 25 million people worldwide.
Experts said that the study\'s findings pointed to a major gap in our understanding of the disease. Doctors have never been sure whether the brain plaques are the cause of Alzheimer\'s disease or just a side effect.
\"We still don\'t have enough understanding of what we should target,\" said Dr. Bengt Winblad, director of the Alzheimer\'s Centre at Sweden\'s Karolinska Institute. Winblad was not connected to the study.
Brain tangles may play a role
Aside from the plaque build-up, scientists also think that tangles of another brain protein called tau play a major role in Alzheimer\'s. Because those tangles form later than the plaque, some experts think they should be the focus instead.
\"It may be harder to get a response from targeting plaque because that forms years before people actually have Alzheimer\'s,\" said Dr. Simon Lovestone, professor of Old Age Psychiatry at King\'s College in London. \"By the time you do something, it may be too late.\"
Winblad said there was a better connection between brain tangles and Alzheimer\'s symptoms, but that no studies so far had looked at whether removing tangles might improve or even reverse Alzheimer\'s disease in patients.
Still, experts say that attacking toxic plaque in the brain shouldn\'t be abandoned just yet, since the formation of such plaques might be what sparks Alzheimer\'s disease in the first place.
\"Removal of the initial motor for the disease might slow progression,\" wrote Peter H. St. George-Hyslop and John C. Morris of the University of Cambridge and the University of Toronto in an accompanying commentary in the Lancet.
mfg ipollit
Antwort auf Beitrag Nr.: 34.537.031 von ipollit am 18.07.08 13:17:19soviel zum Erfolg von AN1792, Cyberhexe... es gibt weitere deutliche Anzeichen, dass die Beta-Amyloid-Plaque Theorie in der Praxis nicht so funktioniert wie gedacht...
http://www.reuters.com/article/marketsNews/idINN174531762008…
Studies show mixed success in Alzheimer's fight
Thu Jul 17, 2008 6:30pm EDT
By Julie Steenhuysen
CHICAGO, July 17 (Reuters) - A new drug showed promise at treating Alzheimer's disease, but an experimental vaccine that cleared brain-clogging plaques failed to improve memory or help patients live longer, researchers said on Thursday.
One of two studies, conducted in Russia and published in the journal Lancet, showed Medivation Inc's (MDVN.O: Quote, Profile, Research, Stock Buzz) drug Dimebon, first approved in Russia as an antihistamine, improved thinking processes and ability to function in patients with mild to moderate Alzheimer's disease.
A second showed that an experimental vaccine by Elan Corp (ELN.I: Quote, Profile, Research, Stock Buzz) and Wyeth (WYE.N: Quote, Profile, Research, Stock Buzz) known as AN1792 may have removed signature plaques from patients' brains, but they all developed severe dementia anyway.
Dimebon helped keep Alzheimer's from progressing for more than a year. "It's a strong signal," said Dr. Rachelle Doody of Baylor College of Medicine in Houston.
Doody said the drug helped improve five measures of memory and function.
It is not yet clear how the drug works, but Doody said it appears to protect the energy powerhouses in cells known as mitochondria, which are often destroyed in neurodegenerative diseases.
Medivation, which helped design the study, earlier this month said Dimebon significantly improved cognitive function in patients with Huntington's disease, which causes uncontrolled movement and loss of thinking ability.
If proven to work in a larger study, the drug could add to a limited pool of approved drugs, which include Eisai (4523.T: Quote, Profile, Research, Stock Buzz) and Pfizer's (PFE.N: Quote, Profile, Research, Stock Buzz) Aricept, Forest Laboratories' FRX.N Namenda, Novartis' (NOVN.VX: Quote, Profile, Research, Stock Buzz) Exelon and Johnson & Johnson's (JNJ.N: Quote, Profile, Research, Stock Buzz) Razadyne.
All affect message-carrying chemicals in the brain called neurotransmitters. The Medivation drug would offer a different approach. "It's a unique mechanism," Doody said by telephone.
TARGETING PLAQUE
A study by Dr. Clive Holmes of Moorgreen Hospital in Southampton in Britain, involved six-year follow-up of AN1792. The vaccine was one of the first attempts to to reverse formation of plaques in the brain associated with Alzheimer's.
Studies in mice showed immunization against a protein called amyloid beta helped remove plaques, but a study in humans was halted early because some patients developed severe brain inflammation.
Holmes and colleagues followed 80 patients who had been in the study. Tests showed no signs it had any effect on cognitive function. They also did autopsies on eight people who died. "In some cases there was a virtually complete removal of plaques," Holmes said in an e-mail.
But seven out of the eight, including those with virtually complete plaque removal, had severe end-stage dementia before death."It strongly suggests that plaques are not sufficient on their own to account for disease progression," Holmes said.
Dr. Peter St George-Hyslop of the University of Cambridge said in a commentary, "This study will undoubtedly evoke concern that anti-amyloid therapies will be ineffectual, and that two decades of experimental work supporting their development were spent barking up the wrong tree." :O
While not a cure, he said the approach may still slow progression of the disease.
Elan and Wyeth now have hopes for bapineuzumab, an antibody drug that fights beta amyloid plaques, in late-stage clinical trials after success in some patients in a smaller study. Alzheimer's affects 26 million people worldwide. (Editing by Maggie Fox and Jackie Frank)
*********
tick tack...
mfg ipollit
http://www.reuters.com/article/marketsNews/idINN174531762008…
Studies show mixed success in Alzheimer's fight
Thu Jul 17, 2008 6:30pm EDT
By Julie Steenhuysen
CHICAGO, July 17 (Reuters) - A new drug showed promise at treating Alzheimer's disease, but an experimental vaccine that cleared brain-clogging plaques failed to improve memory or help patients live longer, researchers said on Thursday.
One of two studies, conducted in Russia and published in the journal Lancet, showed Medivation Inc's (MDVN.O: Quote, Profile, Research, Stock Buzz) drug Dimebon, first approved in Russia as an antihistamine, improved thinking processes and ability to function in patients with mild to moderate Alzheimer's disease.
A second showed that an experimental vaccine by Elan Corp (ELN.I: Quote, Profile, Research, Stock Buzz) and Wyeth (WYE.N: Quote, Profile, Research, Stock Buzz) known as AN1792 may have removed signature plaques from patients' brains, but they all developed severe dementia anyway.
Dimebon helped keep Alzheimer's from progressing for more than a year. "It's a strong signal," said Dr. Rachelle Doody of Baylor College of Medicine in Houston.
Doody said the drug helped improve five measures of memory and function.
It is not yet clear how the drug works, but Doody said it appears to protect the energy powerhouses in cells known as mitochondria, which are often destroyed in neurodegenerative diseases.
Medivation, which helped design the study, earlier this month said Dimebon significantly improved cognitive function in patients with Huntington's disease, which causes uncontrolled movement and loss of thinking ability.
If proven to work in a larger study, the drug could add to a limited pool of approved drugs, which include Eisai (4523.T: Quote, Profile, Research, Stock Buzz) and Pfizer's (PFE.N: Quote, Profile, Research, Stock Buzz) Aricept, Forest Laboratories' FRX.N Namenda, Novartis' (NOVN.VX: Quote, Profile, Research, Stock Buzz) Exelon and Johnson & Johnson's (JNJ.N: Quote, Profile, Research, Stock Buzz) Razadyne.
All affect message-carrying chemicals in the brain called neurotransmitters. The Medivation drug would offer a different approach. "It's a unique mechanism," Doody said by telephone.
TARGETING PLAQUE
A study by Dr. Clive Holmes of Moorgreen Hospital in Southampton in Britain, involved six-year follow-up of AN1792. The vaccine was one of the first attempts to to reverse formation of plaques in the brain associated with Alzheimer's.
Studies in mice showed immunization against a protein called amyloid beta helped remove plaques, but a study in humans was halted early because some patients developed severe brain inflammation.
Holmes and colleagues followed 80 patients who had been in the study. Tests showed no signs it had any effect on cognitive function. They also did autopsies on eight people who died. "In some cases there was a virtually complete removal of plaques," Holmes said in an e-mail.
But seven out of the eight, including those with virtually complete plaque removal, had severe end-stage dementia before death."It strongly suggests that plaques are not sufficient on their own to account for disease progression," Holmes said.
Dr. Peter St George-Hyslop of the University of Cambridge said in a commentary, "This study will undoubtedly evoke concern that anti-amyloid therapies will be ineffectual, and that two decades of experimental work supporting their development were spent barking up the wrong tree." :O
While not a cure, he said the approach may still slow progression of the disease.
Elan and Wyeth now have hopes for bapineuzumab, an antibody drug that fights beta amyloid plaques, in late-stage clinical trials after success in some patients in a smaller study. Alzheimer's affects 26 million people worldwide. (Editing by Maggie Fox and Jackie Frank)
*********
tick tack...

mfg ipollit
Antwort auf Beitrag Nr.: 34.537.229 von ipollit am 18.07.08 13:39:30...das ist doch kalter Kaffee...Bap wirkt anders-(klärt nicht nur die Plaques) und dass es wirkt, sagen die P2 Daten (für Alle ausser für Dich)deutlich.....wenn es soweit bei Dir ist,kannst Du ja Dimebon nehmen (ist vielleicht besser als nur Placebo....)Das kannst Du noch ein Jahr länger Deine wertvollen Beiträge in den Boards liefern...
PS.Bap hat sich gegen Aricept bewiesen,nicht nur gegen Placebos.....
PS.Bap hat sich gegen Aricept bewiesen,nicht nur gegen Placebos.....
Antwort auf Beitrag Nr.: 34.537.229 von ipollit am 18.07.08 13:39:30Author: living_with_elan aus dem IV-Board
Wwilson's post on the AN-1792 article, to refresh our memories
Note the date, this study came out in February:
2/25/2008 6:37:51 PM
Author: wwilson_2003
AN-1792 News
Comments:
1. Eagle eyes here have noted that this is not the halted trial. This is the 80 patient AN-1792 Phase 1. So let's hit the re-wind. This Phase 1 had multiple doses (5 without the PS80 surfactant, then approx three more with the polysorbate 80 surfactant). They eventually achieved approx 55% response rate. A woman in this trial suffered the encephalitis side effect after she was switched to the PS80 formulation. The trial went on to completion. So multiple doses, decent response rates, and results that show very little.
If we have been watching this movie we'd know that there are reasons why AAB-001 should be very much superior to AN-1792 and that there are indications in the limited data that tend to support this.
2. This Phase 1 trial showed benefits as measured by DAD. Nothing else.
3. Nicholl, Wilkerson, Fox, at others at Southhampton are the good guys. Southhampton had the charge of measuring all the brain volumes from the Phase 2. Wilkerson gave the improved Phase 1 DAD paper in Philladelphia in 2004. We know about the autopsy work by Nicholl. They are respected and trusted by Elan, I believe. They are not pulling a Nitche/Hock and trying to be the first to press.
4. The abstract provided by allineln indicated that a retrospective showed that there was no difference in the time to severe dementia and time to death for the patients studied. These seem to be all patients, live and deceased. Thanks to the posters who have surmised that the living patients have probably stubbornly resisted autopsy. It seems unreasonable, but they might have to wait until they are dead. No sig advantage on cognition. No sig advantages for AN-1792.
This is pretty consistent with what the AN-1792 Phase 2 RESPONDERS have showed us. They went from 21 to 13 on MMSE while the Phase 2 placebos went from 20 to 10 after 4.5 years. Not good at all, but it COULD be indicative of some good effects. It could simply mean that better immune systems hang in there better (the differences are so small).
5. Some possible disadvantages of AN-1792 RESPONDERS vs. AAB-001 - possibilities:
low antibody levels
slow to develop decent antibody levels (months)
increased soluble (dangerous) AB in brain - prevented efflux
T-cell activation, inflamation
worsened CAA for a period of time (blocked brain vessels with AB)
no bump in cognition
safety problems with T-cell activation
loss of gray matter volume
TEVA hates AN-1792 more
Wwilson's post on the AN-1792 article, to refresh our memories
Note the date, this study came out in February:
2/25/2008 6:37:51 PM
Author: wwilson_2003
AN-1792 News
Comments:
1. Eagle eyes here have noted that this is not the halted trial. This is the 80 patient AN-1792 Phase 1. So let's hit the re-wind. This Phase 1 had multiple doses (5 without the PS80 surfactant, then approx three more with the polysorbate 80 surfactant). They eventually achieved approx 55% response rate. A woman in this trial suffered the encephalitis side effect after she was switched to the PS80 formulation. The trial went on to completion. So multiple doses, decent response rates, and results that show very little.
If we have been watching this movie we'd know that there are reasons why AAB-001 should be very much superior to AN-1792 and that there are indications in the limited data that tend to support this.
2. This Phase 1 trial showed benefits as measured by DAD. Nothing else.
3. Nicholl, Wilkerson, Fox, at others at Southhampton are the good guys. Southhampton had the charge of measuring all the brain volumes from the Phase 2. Wilkerson gave the improved Phase 1 DAD paper in Philladelphia in 2004. We know about the autopsy work by Nicholl. They are respected and trusted by Elan, I believe. They are not pulling a Nitche/Hock and trying to be the first to press.
4. The abstract provided by allineln indicated that a retrospective showed that there was no difference in the time to severe dementia and time to death for the patients studied. These seem to be all patients, live and deceased. Thanks to the posters who have surmised that the living patients have probably stubbornly resisted autopsy. It seems unreasonable, but they might have to wait until they are dead. No sig advantage on cognition. No sig advantages for AN-1792.
This is pretty consistent with what the AN-1792 Phase 2 RESPONDERS have showed us. They went from 21 to 13 on MMSE while the Phase 2 placebos went from 20 to 10 after 4.5 years. Not good at all, but it COULD be indicative of some good effects. It could simply mean that better immune systems hang in there better (the differences are so small).
5. Some possible disadvantages of AN-1792 RESPONDERS vs. AAB-001 - possibilities:
low antibody levels
slow to develop decent antibody levels (months)
increased soluble (dangerous) AB in brain - prevented efflux
T-cell activation, inflamation
worsened CAA for a period of time (blocked brain vessels with AB)
no bump in cognition
safety problems with T-cell activation
loss of gray matter volume
TEVA hates AN-1792 more
Author: Fishman2001us
This is what KM said about AAB-001-- Transcript
For me, this was the highlight of the cc. Thanks to Jive for asking this question!
Cheers to all,
Cynthia
------------------------------------------------------------------
Jive: What you have mentioned, the ph3 issue for aab, any chance we can file for a BLA at that time?
KM: It would depend on the data... What we're trying to do with AAB-001, just to be clear... is we have an opportunity to have the ph2 be a pivotal trial. At the same time, we have to do a ph3 because the size of ph2 isn't big enough from a safety point of view.
We're trying to understand 2 things in as much specificity as we can-- one is the dose, and one is the length of the dose. Those 2 things are incredibly important for 2 reasons -- one is obvious, one is less obvious... The dose and the length of the dose-- you need to understand the clinical response... How much time do you need at what dose... and, you know, this is an ascending study. So we have different doses at different times, so we have different sets of data, there's different sets of patients that have had different doses for different periods of time.
Why the AN-1792 data is so important, cause that gives you all the clues of what to look for, cause that is an immunotheraputic approach to Alzheimer's... so that is rich with data that we look at all the time, because that gives us clues as far as how to look at.... if we're looking at ph2, having these people look at it, what you're supposed to look at....
So doses are important because when you go for a disease-modifying drug or even a symptomatic drug, you need to understand what are the endpoints that the regulator needs to see in order to give you approval... And the endpoints move at different times for different reasons, and to add to that, you have Alzheimer's patients who are all slightly different...
So dose is important for that reason, and dose is very important for manufacturing-- because there's a gigantic difference between lowest dose and highest dose from a manufacturing point of view-- then --and you have 10 million patients-- so you couldn't build enough biological capacity fast enough if you got the highest dose at the shortest interval, and it's a fantastic drug from an alzheimer's point of view, you're gonna have a hard time meeting capacity fairly shortly.
So, you know, there's no other 2 companies on Earth, Wyeth and Elan, that want to move this forward, but again, we've been working on this from Elan point of view since the late '80s, in research, and Wyeth's a great partner, but getting it right-- it's certainly in our collective, including shareholders' best interest to get it right as precisely as we can.
We will move to ph3 when we have enough confirmatory data to do that and to make reasonable judgements about those 2 things, and we need the ph3 because we need more safety data... So ph2, it may stand on its own with the data, the data will drive it, and if it does we will file... and we will file, but we'll also need a ph3 going, because you'll need more patients from a safety point of view for the FDA... The FDA is not gonna make a decision on 10 million patients based on a 240 patient trial, this is not gonna do it, I don't think they should do it...
So that's were we are.... Immunotherapy works... It's not new information... AN-1792 works-- after 2 doses-- so, we're fine-tuning what we already know, in the public domain... and when we reach the 95th to 100th percentile, in comfort, because of those reasons, then Wyeth and ourselves will proceed... but we could file with ph2 if the data allows us to do that.
Jive: And when will you look at the ph2 data?
KM: Mid-year... and the mid-year correlates to where the doses are with different dose groups... the mid-year is gonna be rich with data... and again, what we know is the immunotheraputic approach to Alzheimer's works... That's not a debate, the debate is what's the dose, and what's the time interval....
And you're making a decision for 10 million patients... and 10 million patients, by the way, have on average 3 caregivers... so when we say we're gonna move forward, we're talking about 50 million people who are gonna call Elan or Wyeth and say, "How do I get in?" I mean it's.... it's big...
And, we understand, from a shareholder point of view it's important... and we're not, in the big scheme of things timeline-wise, you know, we're not that far away from having a lot, all the clarity we need to move forward...
If we get up and stand up here and say, "We're in ph3" people are gonna say, "How'd you get to ph3?" I wanna be able to say here's how we got here, and here's what we're doing, and here's why, and that credibility is very important to us... There's lots of companies in ph2 or 3 in Alzheimer's, haven't seen any data, but they're in ph2, ph3... We don't wanna be like that... That's a stock market drug development strategy...
This is what KM said about AAB-001-- Transcript
For me, this was the highlight of the cc. Thanks to Jive for asking this question!
Cheers to all,
Cynthia
------------------------------------------------------------------
Jive: What you have mentioned, the ph3 issue for aab, any chance we can file for a BLA at that time?
KM: It would depend on the data... What we're trying to do with AAB-001, just to be clear... is we have an opportunity to have the ph2 be a pivotal trial. At the same time, we have to do a ph3 because the size of ph2 isn't big enough from a safety point of view.
We're trying to understand 2 things in as much specificity as we can-- one is the dose, and one is the length of the dose. Those 2 things are incredibly important for 2 reasons -- one is obvious, one is less obvious... The dose and the length of the dose-- you need to understand the clinical response... How much time do you need at what dose... and, you know, this is an ascending study. So we have different doses at different times, so we have different sets of data, there's different sets of patients that have had different doses for different periods of time.
Why the AN-1792 data is so important, cause that gives you all the clues of what to look for, cause that is an immunotheraputic approach to Alzheimer's... so that is rich with data that we look at all the time, because that gives us clues as far as how to look at.... if we're looking at ph2, having these people look at it, what you're supposed to look at....
So doses are important because when you go for a disease-modifying drug or even a symptomatic drug, you need to understand what are the endpoints that the regulator needs to see in order to give you approval... And the endpoints move at different times for different reasons, and to add to that, you have Alzheimer's patients who are all slightly different...
So dose is important for that reason, and dose is very important for manufacturing-- because there's a gigantic difference between lowest dose and highest dose from a manufacturing point of view-- then --and you have 10 million patients-- so you couldn't build enough biological capacity fast enough if you got the highest dose at the shortest interval, and it's a fantastic drug from an alzheimer's point of view, you're gonna have a hard time meeting capacity fairly shortly.
So, you know, there's no other 2 companies on Earth, Wyeth and Elan, that want to move this forward, but again, we've been working on this from Elan point of view since the late '80s, in research, and Wyeth's a great partner, but getting it right-- it's certainly in our collective, including shareholders' best interest to get it right as precisely as we can.
We will move to ph3 when we have enough confirmatory data to do that and to make reasonable judgements about those 2 things, and we need the ph3 because we need more safety data... So ph2, it may stand on its own with the data, the data will drive it, and if it does we will file... and we will file, but we'll also need a ph3 going, because you'll need more patients from a safety point of view for the FDA... The FDA is not gonna make a decision on 10 million patients based on a 240 patient trial, this is not gonna do it, I don't think they should do it...
So that's were we are.... Immunotherapy works... It's not new information... AN-1792 works-- after 2 doses-- so, we're fine-tuning what we already know, in the public domain... and when we reach the 95th to 100th percentile, in comfort, because of those reasons, then Wyeth and ourselves will proceed... but we could file with ph2 if the data allows us to do that.
Jive: And when will you look at the ph2 data?
KM: Mid-year... and the mid-year correlates to where the doses are with different dose groups... the mid-year is gonna be rich with data... and again, what we know is the immunotheraputic approach to Alzheimer's works... That's not a debate, the debate is what's the dose, and what's the time interval....
And you're making a decision for 10 million patients... and 10 million patients, by the way, have on average 3 caregivers... so when we say we're gonna move forward, we're talking about 50 million people who are gonna call Elan or Wyeth and say, "How do I get in?" I mean it's.... it's big...
And, we understand, from a shareholder point of view it's important... and we're not, in the big scheme of things timeline-wise, you know, we're not that far away from having a lot, all the clarity we need to move forward...
If we get up and stand up here and say, "We're in ph3" people are gonna say, "How'd you get to ph3?" I wanna be able to say here's how we got here, and here's what we're doing, and here's why, and that credibility is very important to us... There's lots of companies in ph2 or 3 in Alzheimer's, haven't seen any data, but they're in ph2, ph3... We don't wanna be like that... That's a stock market drug development strategy...
Antwort auf Beitrag Nr.: 34.537.229 von ipollit am 18.07.08 13:39:30.....es gibt weitere deutliche Anzeichen, dass die Beta-Amyloid-Plaque Theorie in der Praxis nicht so funktioniert wie gedacht...
..... But a new vaccine that did just that suggests the theory is wrong...
...Autopsies on seven patients who died of Alzheimer\'s during the study showed that nearly all of the sticky beta-amyloid protein thought to be dangerous had been removed. But all patients still had severe dementia....
...have never been sure whether the brain plaques are the cause of Alzheimer\'s disease or just a side effect....
...Tests showed no signs it had any effect on cognitive function.
....It strongly suggests that plaques are not sufficient on their own to account for disease progression," Holmes said.
....This study will undoubtedly evoke concern that anti-amyloid therapies will be ineffectual, and that two decades of experimental work supporting their development were spent barking up the wrong tree."
...A second showed that an experimental vaccine by Elan Corp (ELN.I: Quote, Profile, Research, Stock Buzz) and Wyeth (WYE.N: Quote, Profile, Research, Stock Buzz) known as AN1792 may have removed signature plaques from patients' brains, but they all developed severe dementia anyway.
.....dass es kritische Stimmen aus der Medizinerwelt zum Thema Beta-Amyloid-Plaque Theorie gibt ,ist wohl hinreichend bekannt...
......ich würde jetzt wirklich einfach mal die vollständigen Daten aus der PII abwarten.......und dann...
ja..... dann werden wir sehen, was " Bappy " so alles leisten kann......oder auch nicht...
..im übrigen, kann man aus o.g. Stellungnahmen ja auch Kapital schlagen....
...einfach auf fallende Kurse setzen...tick tack tick tack...
..im übrigen kann man aus Kellys Stellungnahmen ja auch Kapital schlagen....
...einfach auf steigende Kurse setzen...tick tack tick tack...
Grüße
bernie55 - the ticktack
..... But a new vaccine that did just that suggests the theory is wrong...
...Autopsies on seven patients who died of Alzheimer\'s during the study showed that nearly all of the sticky beta-amyloid protein thought to be dangerous had been removed. But all patients still had severe dementia....
...have never been sure whether the brain plaques are the cause of Alzheimer\'s disease or just a side effect....
...Tests showed no signs it had any effect on cognitive function.
....It strongly suggests that plaques are not sufficient on their own to account for disease progression," Holmes said.
....This study will undoubtedly evoke concern that anti-amyloid therapies will be ineffectual, and that two decades of experimental work supporting their development were spent barking up the wrong tree."
...A second showed that an experimental vaccine by Elan Corp (ELN.I: Quote, Profile, Research, Stock Buzz) and Wyeth (WYE.N: Quote, Profile, Research, Stock Buzz) known as AN1792 may have removed signature plaques from patients' brains, but they all developed severe dementia anyway.
.....dass es kritische Stimmen aus der Medizinerwelt zum Thema Beta-Amyloid-Plaque Theorie gibt ,ist wohl hinreichend bekannt...
......ich würde jetzt wirklich einfach mal die vollständigen Daten aus der PII abwarten.......und dann...

ja..... dann werden wir sehen, was " Bappy " so alles leisten kann......oder auch nicht...

..im übrigen, kann man aus o.g. Stellungnahmen ja auch Kapital schlagen....
...einfach auf fallende Kurse setzen...tick tack tick tack...

..im übrigen kann man aus Kellys Stellungnahmen ja auch Kapital schlagen....
...einfach auf steigende Kurse setzen...tick tack tick tack...

Grüße
bernie55 - the ticktack

Antwort auf Beitrag Nr.: 34.537.229 von ipollit am 18.07.08 13:39:30In a Research Report issued today by Credit Suisse on WYE:
"As we approach this month's ICAD Meeting, Holmes et al's publication in The Lancet reminds us that in AD we continue to have more questions than answers.
Holmes' study does not change our belief that effective anti-amyloid therapy, when given in the right patients at the right time, should be able to modify AD. The top-line results of the phase II bapineuzumab study suggest that in patients with mild to moderate AD who are not carriers of the Apo E4 allele, bapineuzumab could be a safe and effective disease modifying therapy.
The consistently positive results released to date acreoss clinical and MRI endpoints in this group of patients are especially encouaging. We look forward to the complete data presentation at ICAD (july 29th at 4:00 PM), to better understand the different responses seen in carriers vs non-carriers, and evaluate any imbalances between the treatment groups."
....und das sollten wir einfach tun...
I particularly liked the phrase "could be a safe and effective disease-modifying therapy!
Seven trading days til Xmas.
http://www.investorvillage.com/smbd.asp?mb=160&mn=253937&pt=…
"As we approach this month's ICAD Meeting, Holmes et al's publication in The Lancet reminds us that in AD we continue to have more questions than answers.
Holmes' study does not change our belief that effective anti-amyloid therapy, when given in the right patients at the right time, should be able to modify AD. The top-line results of the phase II bapineuzumab study suggest that in patients with mild to moderate AD who are not carriers of the Apo E4 allele, bapineuzumab could be a safe and effective disease modifying therapy.
The consistently positive results released to date acreoss clinical and MRI endpoints in this group of patients are especially encouaging. We look forward to the complete data presentation at ICAD (july 29th at 4:00 PM), to better understand the different responses seen in carriers vs non-carriers, and evaluate any imbalances between the treatment groups."
....und das sollten wir einfach tun...

I particularly liked the phrase "could be a safe and effective disease-modifying therapy!
Seven trading days til Xmas.
http://www.investorvillage.com/smbd.asp?mb=160&mn=253937&pt=…
Antwort auf Beitrag Nr.: 34.543.156 von moneyseeker am 19.07.08 11:13:32 !
!
 Elan ponders future of drugs unit
Elan ponders future of drugs unit
By Lina Saigol, M&A Editor
Published: July 18 2008 23:36 | Last updated: July 18 2008 23:36
Elan, Ireland’s biggest drug company, has hired investment bankers to carry out a strategic review of its development and manufacturing division, which could see the business floated or sold with a price tag of up to $1.5bn (£755m).
Lehman Brothers and Goldman Sachs are preparing Elan Drug Technology for a listing in London and Dublin, as well as a possible sale to private equity.
Elan will send an information memorandum to prospective bidders in the next few days and ask interested parties to submit first-round bids by the middle of next month. Interested bidders are likely to include Apax, Blackstone, Cinven, KKR and Warburg Pincus, all of which have experience in the healthcare business.
Financing an asset of this size has been possible during the credit crunch, as demonstrated by the $4.1bn buy-out of Bristol Myers’ ConvaTec business last month by Nordic Capital and US-based fund Avista Capital Partners.
The decision to float or sell Elan Drug Technology follows the results of clinical trials last month which could herald a breakthrough in treating Alzheimer’s disease after Elan said its drug slowed the onset of the disease in some patients.
Analysts at Goldman said the trials lowered the risk profile for other drugs in Elan’s research and development pipeline.
The move to separate EDT, which relies on manufacturing revenues and royalties, rather than product sales, will leave Elan focused on biopharmaceuticals – the division that markets Tysabri, its flagship multiple sclerosis drug.
EDT focuses on contract product development using an array of formulation and drug optimisation technologies, as well as scale-up and manufacturing services.
In May, Elan, which is recovering from a brush with bankruptcy in 2002, said the company was expected to return to profitability next year, before making a full-year profit in 2010, due to growing sales of Tysabri.
Copyright The Financial Times Limited 2008
 !
! Elan ponders future of drugs unit
Elan ponders future of drugs unitBy Lina Saigol, M&A Editor
Published: July 18 2008 23:36 | Last updated: July 18 2008 23:36
Elan, Ireland’s biggest drug company, has hired investment bankers to carry out a strategic review of its development and manufacturing division, which could see the business floated or sold with a price tag of up to $1.5bn (£755m).
Lehman Brothers and Goldman Sachs are preparing Elan Drug Technology for a listing in London and Dublin, as well as a possible sale to private equity.
Elan will send an information memorandum to prospective bidders in the next few days and ask interested parties to submit first-round bids by the middle of next month. Interested bidders are likely to include Apax, Blackstone, Cinven, KKR and Warburg Pincus, all of which have experience in the healthcare business.
Financing an asset of this size has been possible during the credit crunch, as demonstrated by the $4.1bn buy-out of Bristol Myers’ ConvaTec business last month by Nordic Capital and US-based fund Avista Capital Partners.
The decision to float or sell Elan Drug Technology follows the results of clinical trials last month which could herald a breakthrough in treating Alzheimer’s disease after Elan said its drug slowed the onset of the disease in some patients.
Analysts at Goldman said the trials lowered the risk profile for other drugs in Elan’s research and development pipeline.
The move to separate EDT, which relies on manufacturing revenues and royalties, rather than product sales, will leave Elan focused on biopharmaceuticals – the division that markets Tysabri, its flagship multiple sclerosis drug.
EDT focuses on contract product development using an array of formulation and drug optimisation technologies, as well as scale-up and manufacturing services.
In May, Elan, which is recovering from a brush with bankruptcy in 2002, said the company was expected to return to profitability next year, before making a full-year profit in 2010, due to growing sales of Tysabri.
Copyright The Financial Times Limited 2008

Study of Natalizumab in Relapsed/Refractory Multiple Myeloma
This study is currently recruiting participants.
Verified by Biogen Idec, July 2008
Preclinical data support the evaluation of natalizumab in oncology as a single agent or in combination with standard anti-cancer therapies. Therefore, natalizumab is being developed for the treatment of patients with relapsed or refractory multiple myeloma.
Multiple Myeloma
Drug: Natalizumab 300 mg - Phase I
Drug:Natalizumab 450 mg - Phase II
Study Type: Interventional
Study Design: Treatment, Randomized, Open Label, Single Group Assignment, Safety/Efficacy Study
Official Title: A Phase 1/2, Two-Arm, Dose-Finding Study of Natalizumab for the Treatment of Subjects With Relapsed or Refractory Multiple Myeloma.
Estimated Enrollment: 42
Study Start Date: May 2008
Estimated Study Completion Date: April 2012
Estimated Primary Completion Date: February 2012 (Final data collection date for primary outcome measure)
http://clinicaltrials.gov/ct2/show/NCT00675428?term=Tysabri&…
This study is currently recruiting participants.
Verified by Biogen Idec, July 2008
Preclinical data support the evaluation of natalizumab in oncology as a single agent or in combination with standard anti-cancer therapies. Therefore, natalizumab is being developed for the treatment of patients with relapsed or refractory multiple myeloma.
Multiple Myeloma
Drug: Natalizumab 300 mg - Phase I
Drug:Natalizumab 450 mg - Phase II
Study Type: Interventional
Study Design: Treatment, Randomized, Open Label, Single Group Assignment, Safety/Efficacy Study
Official Title: A Phase 1/2, Two-Arm, Dose-Finding Study of Natalizumab for the Treatment of Subjects With Relapsed or Refractory Multiple Myeloma.
Estimated Enrollment: 42
Study Start Date: May 2008
Estimated Study Completion Date: April 2012
Estimated Primary Completion Date: February 2012 (Final data collection date for primary outcome measure)
http://clinicaltrials.gov/ct2/show/NCT00675428?term=Tysabri&…
Elan and Wyeth Announce Webcast To Discuss Bapineuzumab Phase 2 Clinical Trial Results
Tuesday July 22, 2:00 am ET
DUBLIN, Ireland & MADISON, N.J.--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Wyeth (NYSE: WYE - News) today announced that the companies will hold a webcast at 6:00 p.m. Central Daylight Time (7:00 p.m. Eastern Daylight Time) on Tuesday, July 29, 2008 with the investment community to discuss the results of the Phase 2 clinical trial of bapineuzumab for Alzheimer’s disease.
Company participants who will discuss the trial results and field questions will include:
Ron Black, MD, Wyeth Research, Asst. Vice President, Neuroscience.
Allison Hulme, PhD, Elan, EVP and Head of Global Development.
Dale Schenk, PhD, Elan, EVP and Chief Scientific Officer.
Live audio of the conference call will be simultaneously broadcast over the Internet and will be available to investors, members of the news media and the general public.
This event can be accessed by visiting the companies’ web sites at www.elan.com or www.wyeth.com and clicking on the “Investor Relations” icon. Following the live webcast, an archived version of the call and slides will be available at the same URLs.
http://biz.yahoo.com/bw/080722/20080721006365.html?.v=1
Tuesday July 22, 2:00 am ET
DUBLIN, Ireland & MADISON, N.J.--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Wyeth (NYSE: WYE - News) today announced that the companies will hold a webcast at 6:00 p.m. Central Daylight Time (7:00 p.m. Eastern Daylight Time) on Tuesday, July 29, 2008 with the investment community to discuss the results of the Phase 2 clinical trial of bapineuzumab for Alzheimer’s disease.
Company participants who will discuss the trial results and field questions will include:
Ron Black, MD, Wyeth Research, Asst. Vice President, Neuroscience.
Allison Hulme, PhD, Elan, EVP and Head of Global Development.
Dale Schenk, PhD, Elan, EVP and Chief Scientific Officer.
Live audio of the conference call will be simultaneously broadcast over the Internet and will be available to investors, members of the news media and the general public.
This event can be accessed by visiting the companies’ web sites at www.elan.com or www.wyeth.com and clicking on the “Investor Relations” icon. Following the live webcast, an archived version of the call and slides will be available at the same URLs.
http://biz.yahoo.com/bw/080722/20080721006365.html?.v=1
mal auf deutsch:
22.07.2008 10:28
Biogen Idec und Elan feiern zweiten Jahrestag von TYSABRI(R) zur Behandlung von Multipler Sklerose
Biogen Idec (News) (NASDAQ: BIIB) und Elan Corporation, (News) plc (NYSE: ELN) verkündeten heute den zweiten Jahrestag von TYSABRI® (Natalizumab) als Behandlungsmittel für wiederkehrende Formen von Multipler Sklerose (MS), an dem sie die Wiedereinführung des Produkts in den USA und seine erste internationale Zulassung feiern. Die Unternehmen schätzen, dass mit Stand von Ende Juni 2008 über 31.800 Patienten aus aller Welt mit TYSABRI behandelt werden.
Im Einzelnen stellten die Unternehmen Ende Juni 2008 fest:
In den USA erhalten über 17.800 TYSABRI kommerziell und über 3.100 Ärzte haben das Arzneimittel verschrieben.
Außerhalb der USA erhalten über 13.400 Patienten TYSABRI kommerziell.
In weltweiten klinischen Studien werden ca. 600 Patienten mit TYSABRI behandelt.
Seit der Wiedereinführung in den USA und der ersten internationalen Zulassung im Juli 2006 sind keine bestätigten Fälle von progressiver multifokaler Leukoenzephalopathie (PML) aufgetreten.
Insgesamt wurden in den klinischen Studien und den Behandlungssituationen nach der Vermarktung zusammen:
-über 43.300 mit TYSABRI behandelt und
-von diesen Patienten wurden beinahe 13.900 mindestens ein Jahr lang mit TYSABRI behandelt und ca. 6.600 Patienten unterzogen sich der Therapie seit 18 Monaten oder länger.
„Seitdem ich die TYSABRI-Therapie vor über 18 Monaten begonnen habe, hat sich mein Leben verbessert, wie auch die Art, mit der ich mit meiner MS im Alltag umgehe“, sagte Patientin Patricia Substelny. „Im Hinblick auf die Anzahl von Krankheitsschüben, die ich erlebt habe, hat sich eine bedeutende Verbesserung eingestellt. Ich kann nun zuversichtlich in meinem Garten arbeiten, für meine Familie kochen und genießen, was das Leben zu bieten hat. Ich bin sehr dankbar, dass mir TYSABRI als eine Behandlungsmöglichkeit für meine MS zur Verfügung steht.“
In den zwei Jahren seit der Wiedereinführung in den USA und der ersten internationalen Zulassung untermauern die gesammelten Daten weiterhin die Vorteile von TYSABRI in der Behandlung von Patienten mit wiederkehrenden Formen von MS. Die Daten einer nachträglich durchgeführten Analyse der klinischen Phase-III-Studien, die auf der diesjährigen Jahreskonferenz der American Academy of Neurology vorgelegt wurden, zeigen, dass die Behandlung mit TYSABRI den Anteil der als krankheitsfrei eingestuften MS-Patienten deutlich erhöht. Außerdem belegen neue Daten aus einer Umfrage über von Patienten selbst gemeldete Ergebnisse, die auf der Jahreskonferenz des Consortium of Multiple Sclerosis Centers präsentiert wurden, dass einige Patienten nach bereits 3-monatiger TYSABRI-Behandlung von Verbesserungen ihrer allgemeinen Lebensqualität, ihres Krankheitsniveaus, ihres Funktionsstatus und ihrer MS-Symptome berichteten.
Zusammen mit TYSABRIs gut belegter klinischer Wirksamkeit wurde ein wachsender Bestand gesundheitswirtschaftlicher Daten aus aller Welt vorgelegt und veröffentlicht, der die pharmaökonomischen Vorteile der Behandlung von MS-Patienten mit TYSABRI verdeutlicht. Auf Grundlage dieser Daten haben die zuständigen Gesundheitsbehörden in Ländern wie Australien, Österreich, den Niederlanden, Großbritannien, Schweden, Frankreich und Deutschland alle TYSABRI zur Kostenerstattung durch staatliche Gesundheitsstellen empfohlen.
„Während der vergangenen zwei Jahre schienen meine mit TYSABRI behandelten Patienten sehr positive Vorteile durch das Arzneimittel zu erhalten“, sagte Dr. Thomas F. Scott, Professor für Neurologie am Drexel University College of Medicine und Direktor des Allegheny MS Treatment Center in Pittsburgh. „Viele meiner Patienten sagen mir, dass TYSABRI ihnen hilft, Kontrolle über ihr Leben zurückzuerhalten.“
Über TOUCH™, TYGRIS und CD INFORM
Vor Beginn einer Behandlung müssen alle in den USA befindlichen Patienten, Verschreiber und Infusionsstellen im TOUCH-Verschreibungsprogramm (TYSABRI Outreach: Unified Commitment to Health) registriert sein. TOUCH ist darauf ausgelegt, das Vorhandensein von und die Risikofaktoren für schwerwiegende opportunistische Infektionen (OIs) wie PML zu entdecken, die Patienten auf Anzeichen und Symptome von PML zu überwachen und eine informierte Diskussionen über die Vorteile und Risiken vor Beginn einer TYSABRI-Behandlung zu fördern. Ärzte erstatten laufend Bericht, falls PML, andere schwerwiegende OIs oder Todesfälle eintreten oder die Therapie abgesetzt wird.
TYGRIS (TYSABRI Global ObseRvation Program In Safety, globales Beobachtungsprogramm zur Sicherheit von TYSABRI) und CD INFORM (Crohn's Disease - Investigating Natalizumab through Further Observational Research and Monitoring, Untersuchung von Natalizumab bei Morbus Crohn durch weitere Beobachtungsstudien) sind Bestandteile eines globalen Risikomanagementplans für TYSABRI. Es wird erwartet, dass TYGRIS weltweit 5000 Patienten erfassen wird, darunter ca. 2.000 - 2.500 Patienten von TOUCH. CD INFORM wird den Erwartungen zufolge 2.000 Morbus-Crohn-Patienten in den USA erfassen. Die durch TYGRIS und CD INFORM registrierten Patienten werden zu Therapiebeginn und dann halbjährlich über fünf Jahre hinweg untersucht. Die Forscher werden die Daten und Anamnese untersuchen und mit Bezug auf den Befund vor der TYSABRI-Behandlung, den Befund vor der Verwendung von Immunomodulatoren, Antineoplastika oder Immunosuppressiva und mit Bezug auf alle schwerwiegenden Nebenwirkungen einschl. PML, andere schwerwiegende OIs und Malignitäten auswerten.
Die Berichterstattung unerwünschter Ereignisse, die außerhalb klinischer Studien nach der Marktzulassung eintreten, erfolgt freiwillig. Es besteht die Möglichkeit, dass nicht alle Reaktionen berichtet wurden oder dass manche Reaktionen verspätet an Biogen Idec oder Elan gemeldet werden.
Über TYSABRI
TYSABRI ist ein Arzneimittel, das in den USA für rezidivierende Formen von MS und in der EU für rezidivierende-remittierende MS zugelassen ist. Nach Angaben, die im New England Journal of Medicine veröffentlicht wurden, führte die Therapie mit TYSABRI nach zwei Jahren zu einer 68-prozentigen relativen Verringerung (p<0,001) der auf ein Jahr umgerechneten Schubrate, verglichen mit einem Placebo, und das relative Risiko der Behinderungsprogression reduzierte sich um 42-54 Prozent (p<0,001).
TYSABRI wurde kürzlich zur Induzierung und Erhaltung eines klinischen Ansprechens und einer klinischen Remission bei erwachsenen Patienten mit mäßig bis schwer aktivem Morbus Crohn (MC) zugelassen, bei denen Anzeichen für eine Entzündung vorliegen und die auf herkömmliche MC-Therapien und TNF-alpha-Hemmer nicht genügend ansprachen bzw. diese nicht vertragen.
TYSABRI erhöht das Risiko von progressiver multifokaler Leukoenzephalopathie (PML), einer opportunistischen Virusinfektion des Gehirns, die meistens tödlich verläuft oder schwere Behinderungen verursacht. Zu anderen ernsthaften Nebenwirkungen, die bei mit TYSABRI behandelten Patienten auftraten, gehören Überempfindlichkeitsreaktionen (z. B. Anaphylaxe) und Infektionen. Bei mit TYSABRI behandelten Patienten wurden schwerwiegende opportunistische und andere atypische Infektionen beobachtet, wobei manche dieser Patienten gleichzeitig mit Immunsuppressiva behandelt wurden. Herpesinfektionen traten bei mit TYSABRI behandelten Patienten etwas häufiger auf. In klinischen Studien über Multiple Sklerose und Morbus Crohn waren das Auftreten und die Quote anderer ernster unerwünschter Ereignisse einschließlich schwerwiegender Infektionen bei mit TYSABRI behandelten Patienten und den Patienten, die ein Placebo erhielten, einander ähnlich. Zu häufigen unerwünschten Ereignissen, die bei mit TYSABRI behandelten MS-Patienten beobachtet wurden, gehören Kopfschmerzen, Müdigkeit, Infusionsreaktionen, Harntraktinfektionen, Gelenk- und Gliederschmerzen und Ausschlag. Andere häufige unerwünschte Ereignisse, die bei mit TYSABRI behandelten Morbus-Crohn-Patienten berichtet wurden, gehören Infektionen der Atemwege und Übelkeit. Es liegen Berichte über klinisch bedeutende Leberschäden bei Patienten vor, die nach der Marktzulassung außerhalb klinischer Studien mit TYSABRI behandelt wurden.
TYSABRI ist in mehr als 35 Ländern zugelassen.
Für weitere Informationen über TYSABRI besuchen Sie bitte www.tysabri.com, www.biogenidec.com oder www.elan.com oder wählen Sie 1-800-456-2255 .
Über Biogen Idec
Biogen Idec setzt neue Maßstäbe in therapeutischen Bereichen, in denen erhebliche medizinische Versorgungslücken bestehen. Biogen Idec wurde 1978 gegründet und zählt in der Entdeckung, Entwicklung, Herstellung und Kommerzialisierung innovativer Therapieansätze zu den weltweit führenden Unternehmen. Biogen Idecs bedeutende Produkte zur Behandlung von Krankheiten wie Lymphomen, multipler Sklerose und rheumatoider Arthritis kommen heute Patienten in mehr als 90 Ländern zugute. Produktinformationen, Pressemitteilungen und zusätzliche Informationen über das Unternehmen finden Sie unter www.biogenidec.com.
Über Elan
Die Elan Corporation, plc ist ein Biotechnologie-Unternehmen mit neurowissenschaftlicher Ausrichtung und strebt danach, das Leben der Patienten und ihrer Familien zu verbessern. Das Unternehmen setzt sich dafür ein, wissenschaftliche Innovationen für ernste, nicht gelöste medizinische Probleme nutzbar zu machen, die nach wie vor weltweit anzutreffen sind. Die Aktien von Elan werden an den Börsen in New York, London und Dublin gehandelt. Weitere Informationen über das Unternehmen erhalten Sie unterwww.elan.com.
Safe-Harbor-Erklärung über zukunftsbezogene Aussagen
Diese Pressemitteilung enthält zukunftsbezogene Aussagen über TYSABRI. Diese Aussagen beruhen auf den derzeitigen Annahmen und Erwartungen der Unternehmen. Das kommerzielle Potenzial von TYSABRI unterliegt einer Anzahl von Risiken und Ungewissheiten. Zu den Faktoren, aufgrund derer die tatsächlichen Ergebnisse maßgeblich von den derzeitigen Erwartungen der Unternehmen abweichen könnten gehören das Risiko, dass wir nicht in der Lage sein könnten, angemessen auf von der US-amerikanischen Arzneimittelbehörde FDA oder anderen aufsichtsrechtlichen Stellen geäußerte Bedenken oder Anfragen zu reagieren, dass zusätzliche Daten neue Bedenken aufwerfen, dass das Auftreten und/oder das Risiko von PML oder anderen opportunistischen Infektionen bei mit TYSABRI behandelten Patienten höher als in klinischen Studien beobachtet ausfallen könnte, dass die Unternehmen auf andere unerwartete Hindernisse stoßen oder dass neue Therapien zur Behandlung von MS mit besseren Wirksamkeits- oder Sicherheitsprofilen oder einfacheren Verabreichungsmethoden auf den Markt kommen. Die Entwicklung und Kommerzialisierung von Arzneimitteln unterliegt einem hohen Risikograd.
Nähere Informationen über Risiken und Unwägbarkeiten, die mit den Aktivitäten der Unternehmen in der Arzneimittelentwicklung und anderen Bereichen verbunden sind, finden Sie in den periodisch verfassten und aktuellen Berichten, die Biogen Idec und Elan bei der US-amerikanischen Börsenaufsicht SEC eingereicht haben. Die Unternehmen verpflichten sich in keiner Weise zur öffentlichen Aktualisierung zukunftsbezogener Aussagen aufgrund von neuen Informationen, zukünftigen Ereignissen oder sonstigen Umständen.
Die Ausgangssprache, in der der Originaltext veröffentlicht wird, ist die offizielle und autorisierte Version. Übersetzungen werden zur besseren Verständigung mitgeliefert. Nur die Sprachversion, die im Original veröffentlicht wurde, ist rechtsgültig. Gleichen Sie deshalb Übersetzungen mit der originalen Sprachversion der Veröffentlichung ab.
http://www.finanznachrichten.de/nachrichten-2008-07/artikel-…
22.07.2008 10:28
Biogen Idec und Elan feiern zweiten Jahrestag von TYSABRI(R) zur Behandlung von Multipler Sklerose
Biogen Idec (News) (NASDAQ: BIIB) und Elan Corporation, (News) plc (NYSE: ELN) verkündeten heute den zweiten Jahrestag von TYSABRI® (Natalizumab) als Behandlungsmittel für wiederkehrende Formen von Multipler Sklerose (MS), an dem sie die Wiedereinführung des Produkts in den USA und seine erste internationale Zulassung feiern. Die Unternehmen schätzen, dass mit Stand von Ende Juni 2008 über 31.800 Patienten aus aller Welt mit TYSABRI behandelt werden.
Im Einzelnen stellten die Unternehmen Ende Juni 2008 fest:
In den USA erhalten über 17.800 TYSABRI kommerziell und über 3.100 Ärzte haben das Arzneimittel verschrieben.
Außerhalb der USA erhalten über 13.400 Patienten TYSABRI kommerziell.
In weltweiten klinischen Studien werden ca. 600 Patienten mit TYSABRI behandelt.
Seit der Wiedereinführung in den USA und der ersten internationalen Zulassung im Juli 2006 sind keine bestätigten Fälle von progressiver multifokaler Leukoenzephalopathie (PML) aufgetreten.
Insgesamt wurden in den klinischen Studien und den Behandlungssituationen nach der Vermarktung zusammen:
-über 43.300 mit TYSABRI behandelt und
-von diesen Patienten wurden beinahe 13.900 mindestens ein Jahr lang mit TYSABRI behandelt und ca. 6.600 Patienten unterzogen sich der Therapie seit 18 Monaten oder länger.
„Seitdem ich die TYSABRI-Therapie vor über 18 Monaten begonnen habe, hat sich mein Leben verbessert, wie auch die Art, mit der ich mit meiner MS im Alltag umgehe“, sagte Patientin Patricia Substelny. „Im Hinblick auf die Anzahl von Krankheitsschüben, die ich erlebt habe, hat sich eine bedeutende Verbesserung eingestellt. Ich kann nun zuversichtlich in meinem Garten arbeiten, für meine Familie kochen und genießen, was das Leben zu bieten hat. Ich bin sehr dankbar, dass mir TYSABRI als eine Behandlungsmöglichkeit für meine MS zur Verfügung steht.“
In den zwei Jahren seit der Wiedereinführung in den USA und der ersten internationalen Zulassung untermauern die gesammelten Daten weiterhin die Vorteile von TYSABRI in der Behandlung von Patienten mit wiederkehrenden Formen von MS. Die Daten einer nachträglich durchgeführten Analyse der klinischen Phase-III-Studien, die auf der diesjährigen Jahreskonferenz der American Academy of Neurology vorgelegt wurden, zeigen, dass die Behandlung mit TYSABRI den Anteil der als krankheitsfrei eingestuften MS-Patienten deutlich erhöht. Außerdem belegen neue Daten aus einer Umfrage über von Patienten selbst gemeldete Ergebnisse, die auf der Jahreskonferenz des Consortium of Multiple Sclerosis Centers präsentiert wurden, dass einige Patienten nach bereits 3-monatiger TYSABRI-Behandlung von Verbesserungen ihrer allgemeinen Lebensqualität, ihres Krankheitsniveaus, ihres Funktionsstatus und ihrer MS-Symptome berichteten.
Zusammen mit TYSABRIs gut belegter klinischer Wirksamkeit wurde ein wachsender Bestand gesundheitswirtschaftlicher Daten aus aller Welt vorgelegt und veröffentlicht, der die pharmaökonomischen Vorteile der Behandlung von MS-Patienten mit TYSABRI verdeutlicht. Auf Grundlage dieser Daten haben die zuständigen Gesundheitsbehörden in Ländern wie Australien, Österreich, den Niederlanden, Großbritannien, Schweden, Frankreich und Deutschland alle TYSABRI zur Kostenerstattung durch staatliche Gesundheitsstellen empfohlen.
„Während der vergangenen zwei Jahre schienen meine mit TYSABRI behandelten Patienten sehr positive Vorteile durch das Arzneimittel zu erhalten“, sagte Dr. Thomas F. Scott, Professor für Neurologie am Drexel University College of Medicine und Direktor des Allegheny MS Treatment Center in Pittsburgh. „Viele meiner Patienten sagen mir, dass TYSABRI ihnen hilft, Kontrolle über ihr Leben zurückzuerhalten.“
Über TOUCH™, TYGRIS und CD INFORM
Vor Beginn einer Behandlung müssen alle in den USA befindlichen Patienten, Verschreiber und Infusionsstellen im TOUCH-Verschreibungsprogramm (TYSABRI Outreach: Unified Commitment to Health) registriert sein. TOUCH ist darauf ausgelegt, das Vorhandensein von und die Risikofaktoren für schwerwiegende opportunistische Infektionen (OIs) wie PML zu entdecken, die Patienten auf Anzeichen und Symptome von PML zu überwachen und eine informierte Diskussionen über die Vorteile und Risiken vor Beginn einer TYSABRI-Behandlung zu fördern. Ärzte erstatten laufend Bericht, falls PML, andere schwerwiegende OIs oder Todesfälle eintreten oder die Therapie abgesetzt wird.
TYGRIS (TYSABRI Global ObseRvation Program In Safety, globales Beobachtungsprogramm zur Sicherheit von TYSABRI) und CD INFORM (Crohn's Disease - Investigating Natalizumab through Further Observational Research and Monitoring, Untersuchung von Natalizumab bei Morbus Crohn durch weitere Beobachtungsstudien) sind Bestandteile eines globalen Risikomanagementplans für TYSABRI. Es wird erwartet, dass TYGRIS weltweit 5000 Patienten erfassen wird, darunter ca. 2.000 - 2.500 Patienten von TOUCH. CD INFORM wird den Erwartungen zufolge 2.000 Morbus-Crohn-Patienten in den USA erfassen. Die durch TYGRIS und CD INFORM registrierten Patienten werden zu Therapiebeginn und dann halbjährlich über fünf Jahre hinweg untersucht. Die Forscher werden die Daten und Anamnese untersuchen und mit Bezug auf den Befund vor der TYSABRI-Behandlung, den Befund vor der Verwendung von Immunomodulatoren, Antineoplastika oder Immunosuppressiva und mit Bezug auf alle schwerwiegenden Nebenwirkungen einschl. PML, andere schwerwiegende OIs und Malignitäten auswerten.
Die Berichterstattung unerwünschter Ereignisse, die außerhalb klinischer Studien nach der Marktzulassung eintreten, erfolgt freiwillig. Es besteht die Möglichkeit, dass nicht alle Reaktionen berichtet wurden oder dass manche Reaktionen verspätet an Biogen Idec oder Elan gemeldet werden.
Über TYSABRI
TYSABRI ist ein Arzneimittel, das in den USA für rezidivierende Formen von MS und in der EU für rezidivierende-remittierende MS zugelassen ist. Nach Angaben, die im New England Journal of Medicine veröffentlicht wurden, führte die Therapie mit TYSABRI nach zwei Jahren zu einer 68-prozentigen relativen Verringerung (p<0,001) der auf ein Jahr umgerechneten Schubrate, verglichen mit einem Placebo, und das relative Risiko der Behinderungsprogression reduzierte sich um 42-54 Prozent (p<0,001).
TYSABRI wurde kürzlich zur Induzierung und Erhaltung eines klinischen Ansprechens und einer klinischen Remission bei erwachsenen Patienten mit mäßig bis schwer aktivem Morbus Crohn (MC) zugelassen, bei denen Anzeichen für eine Entzündung vorliegen und die auf herkömmliche MC-Therapien und TNF-alpha-Hemmer nicht genügend ansprachen bzw. diese nicht vertragen.
TYSABRI erhöht das Risiko von progressiver multifokaler Leukoenzephalopathie (PML), einer opportunistischen Virusinfektion des Gehirns, die meistens tödlich verläuft oder schwere Behinderungen verursacht. Zu anderen ernsthaften Nebenwirkungen, die bei mit TYSABRI behandelten Patienten auftraten, gehören Überempfindlichkeitsreaktionen (z. B. Anaphylaxe) und Infektionen. Bei mit TYSABRI behandelten Patienten wurden schwerwiegende opportunistische und andere atypische Infektionen beobachtet, wobei manche dieser Patienten gleichzeitig mit Immunsuppressiva behandelt wurden. Herpesinfektionen traten bei mit TYSABRI behandelten Patienten etwas häufiger auf. In klinischen Studien über Multiple Sklerose und Morbus Crohn waren das Auftreten und die Quote anderer ernster unerwünschter Ereignisse einschließlich schwerwiegender Infektionen bei mit TYSABRI behandelten Patienten und den Patienten, die ein Placebo erhielten, einander ähnlich. Zu häufigen unerwünschten Ereignissen, die bei mit TYSABRI behandelten MS-Patienten beobachtet wurden, gehören Kopfschmerzen, Müdigkeit, Infusionsreaktionen, Harntraktinfektionen, Gelenk- und Gliederschmerzen und Ausschlag. Andere häufige unerwünschte Ereignisse, die bei mit TYSABRI behandelten Morbus-Crohn-Patienten berichtet wurden, gehören Infektionen der Atemwege und Übelkeit. Es liegen Berichte über klinisch bedeutende Leberschäden bei Patienten vor, die nach der Marktzulassung außerhalb klinischer Studien mit TYSABRI behandelt wurden.
TYSABRI ist in mehr als 35 Ländern zugelassen.
Für weitere Informationen über TYSABRI besuchen Sie bitte www.tysabri.com, www.biogenidec.com oder www.elan.com oder wählen Sie 1-800-456-2255 .
Über Biogen Idec
Biogen Idec setzt neue Maßstäbe in therapeutischen Bereichen, in denen erhebliche medizinische Versorgungslücken bestehen. Biogen Idec wurde 1978 gegründet und zählt in der Entdeckung, Entwicklung, Herstellung und Kommerzialisierung innovativer Therapieansätze zu den weltweit führenden Unternehmen. Biogen Idecs bedeutende Produkte zur Behandlung von Krankheiten wie Lymphomen, multipler Sklerose und rheumatoider Arthritis kommen heute Patienten in mehr als 90 Ländern zugute. Produktinformationen, Pressemitteilungen und zusätzliche Informationen über das Unternehmen finden Sie unter www.biogenidec.com.
Über Elan
Die Elan Corporation, plc ist ein Biotechnologie-Unternehmen mit neurowissenschaftlicher Ausrichtung und strebt danach, das Leben der Patienten und ihrer Familien zu verbessern. Das Unternehmen setzt sich dafür ein, wissenschaftliche Innovationen für ernste, nicht gelöste medizinische Probleme nutzbar zu machen, die nach wie vor weltweit anzutreffen sind. Die Aktien von Elan werden an den Börsen in New York, London und Dublin gehandelt. Weitere Informationen über das Unternehmen erhalten Sie unterwww.elan.com.
Safe-Harbor-Erklärung über zukunftsbezogene Aussagen
Diese Pressemitteilung enthält zukunftsbezogene Aussagen über TYSABRI. Diese Aussagen beruhen auf den derzeitigen Annahmen und Erwartungen der Unternehmen. Das kommerzielle Potenzial von TYSABRI unterliegt einer Anzahl von Risiken und Ungewissheiten. Zu den Faktoren, aufgrund derer die tatsächlichen Ergebnisse maßgeblich von den derzeitigen Erwartungen der Unternehmen abweichen könnten gehören das Risiko, dass wir nicht in der Lage sein könnten, angemessen auf von der US-amerikanischen Arzneimittelbehörde FDA oder anderen aufsichtsrechtlichen Stellen geäußerte Bedenken oder Anfragen zu reagieren, dass zusätzliche Daten neue Bedenken aufwerfen, dass das Auftreten und/oder das Risiko von PML oder anderen opportunistischen Infektionen bei mit TYSABRI behandelten Patienten höher als in klinischen Studien beobachtet ausfallen könnte, dass die Unternehmen auf andere unerwartete Hindernisse stoßen oder dass neue Therapien zur Behandlung von MS mit besseren Wirksamkeits- oder Sicherheitsprofilen oder einfacheren Verabreichungsmethoden auf den Markt kommen. Die Entwicklung und Kommerzialisierung von Arzneimitteln unterliegt einem hohen Risikograd.
Nähere Informationen über Risiken und Unwägbarkeiten, die mit den Aktivitäten der Unternehmen in der Arzneimittelentwicklung und anderen Bereichen verbunden sind, finden Sie in den periodisch verfassten und aktuellen Berichten, die Biogen Idec und Elan bei der US-amerikanischen Börsenaufsicht SEC eingereicht haben. Die Unternehmen verpflichten sich in keiner Weise zur öffentlichen Aktualisierung zukunftsbezogener Aussagen aufgrund von neuen Informationen, zukünftigen Ereignissen oder sonstigen Umständen.
Die Ausgangssprache, in der der Originaltext veröffentlicht wird, ist die offizielle und autorisierte Version. Übersetzungen werden zur besseren Verständigung mitgeliefert. Nur die Sprachversion, die im Original veröffentlicht wurde, ist rechtsgültig. Gleichen Sie deshalb Übersetzungen mit der originalen Sprachversion der Veröffentlichung ab.
http://www.finanznachrichten.de/nachrichten-2008-07/artikel-…
Antwort auf Beitrag Nr.: 34.560.055 von Poppholz am 22.07.08 13:58:01mal auf deutsch:
..mal mit Bildchen..


http://www.tysabri.com/tysbProject/tysb.portal
..mal mit Bildchen..


http://www.tysabri.com/tysbProject/tysb.portal
Antwort auf Beitrag Nr.: 34.556.830 von bernie55 am 22.07.08 08:20:58Elan and Wyeth Announce Webcast To Discuss Bapineuzumab Phase 2 Clinical Trial Results
DUBLIN, Ireland & MADISON, N.J.--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Wyeth (NYSE: WYE - News) today announced that the companies will hold a webcast at 6:00 p.m. Central Daylight Time (7:00 p.m. Eastern Daylight Time) on Tuesday, July 29, 2008 with the investment community to discuss the results of the Phase 2 clinical trial of bapineuzumab for Alzheimer’s disease.
Company participants who will discuss the trial results and field questions will include:
Ron Black, MD, Wyeth Research, Asst. Vice President, Neuroscience.
Allison Hulme, PhD, Elan, EVP and Head of Global Development.
Dale Schenk, PhD, Elan, EVP and Chief Scientific Officer.
Live audio of the conference call will be simultaneously broadcast over the Internet and will be available to investors, members of the news media and the general public.
This event can be accessed by visiting the companies’ web sites at www.elan.com or www.wyeth.com and clicking on the “Investor Relations” icon. Following the live webcast, an archived version of the call and slides will be available at the same URLs.
Nun die Frage ..... warum eine CC ???
..hier die Antwort...
..sowohl für ipolits + cyberhexen ,als auch für ELANITES und ELANIACS
Why do they need a CC?
My guess is that the results are even better than most think. When you have what could be the most important drug of all time there will be questions. The company will attempt to tell the world in ordinary terms what to expect from BAP. There are some unique aspects to this trial. The results at the meeting won't include the drop outs. At this CC they can tell the world that the carrier group would have been stat sig if the VE patients were included. That the VE patients had an issue that resolved safely and were placed on the drug again. The VE patients were some of the best performers. VEs are not a safety issue. It is a signal that the drug is working. They can possibly talk about the path forward. The scientific conference is not the place for that discussion.
The CC should be viewed as a very positive development. If the data were not good the likelihood of a CC would be less. If this drug works, and it does, it could be the biggest event in medicine in the last 50 years. When this data is released and shows that patients have hope the whole game changes. At that point the FDA will be under extreme pressure. Imagine the number of patients that will call ELN, WYE, and the FDA begging to get their loved ones the drug. They won't want the placebo either. It the results are clear does it now become an ethical issue to allow patients in the trial to take a placebo when you know the drug will work. I would not be shocked if the P3 went open label at some point. Maybe after 6 months of data.
I know one thing I might not sleep well for the next week. It is not because I am nervous.
It is because I am excited. I can't wait for the day the dream is realized.
http://www.investorvillage.com/smbd.asp?mb=160&mn=255412&pt=…
DUBLIN, Ireland & MADISON, N.J.--(BUSINESS WIRE)--Elan Corporation, plc (NYSE: ELN - News) and Wyeth (NYSE: WYE - News) today announced that the companies will hold a webcast at 6:00 p.m. Central Daylight Time (7:00 p.m. Eastern Daylight Time) on Tuesday, July 29, 2008 with the investment community to discuss the results of the Phase 2 clinical trial of bapineuzumab for Alzheimer’s disease.
Company participants who will discuss the trial results and field questions will include:
Ron Black, MD, Wyeth Research, Asst. Vice President, Neuroscience.
Allison Hulme, PhD, Elan, EVP and Head of Global Development.
Dale Schenk, PhD, Elan, EVP and Chief Scientific Officer.
Live audio of the conference call will be simultaneously broadcast over the Internet and will be available to investors, members of the news media and the general public.
This event can be accessed by visiting the companies’ web sites at www.elan.com or www.wyeth.com and clicking on the “Investor Relations” icon. Following the live webcast, an archived version of the call and slides will be available at the same URLs.
Nun die Frage ..... warum eine CC ???
..hier die Antwort...
..sowohl für ipolits + cyberhexen ,als auch für ELANITES und ELANIACS

Why do they need a CC?
My guess is that the results are even better than most think. When you have what could be the most important drug of all time there will be questions. The company will attempt to tell the world in ordinary terms what to expect from BAP. There are some unique aspects to this trial. The results at the meeting won't include the drop outs. At this CC they can tell the world that the carrier group would have been stat sig if the VE patients were included. That the VE patients had an issue that resolved safely and were placed on the drug again. The VE patients were some of the best performers. VEs are not a safety issue. It is a signal that the drug is working. They can possibly talk about the path forward. The scientific conference is not the place for that discussion.
The CC should be viewed as a very positive development. If the data were not good the likelihood of a CC would be less. If this drug works, and it does, it could be the biggest event in medicine in the last 50 years. When this data is released and shows that patients have hope the whole game changes. At that point the FDA will be under extreme pressure. Imagine the number of patients that will call ELN, WYE, and the FDA begging to get their loved ones the drug. They won't want the placebo either. It the results are clear does it now become an ethical issue to allow patients in the trial to take a placebo when you know the drug will work. I would not be shocked if the P3 went open label at some point. Maybe after 6 months of data.
I know one thing I might not sleep well for the next week. It is not because I am nervous.
It is because I am excited. I can't wait for the day the dream is realized.

http://www.investorvillage.com/smbd.asp?mb=160&mn=255412&pt=…
..habe ein gutes statement zu AAB-001 und AN-1792 gefunden...
plaque v beta amyloid/nangasimon
Hi Simon,
I think there was a difference between the Holmes and Hock studies as far as clinical endpoints are concerned. Holmes study looked only at the overall survival measure and dementia severity before death of patients in AN-1792 trial. Hock and others looked at clinical endpoints at 12 months after immunization and the responders did better on both cognitive and functional scores compared to placebo . The 4.5 years follow up study showed that AN-1792 responders outperformed placebos on function and there was still a positive trend in cognition (mean MMSE 13 vs 10 for placebo).
The conclusions for me are :
1. AN-1792 and AAB-001 are related therapies, nevertheless there will most likely be differences in their efficacy
2. Plaque clearance itself is can provide clinical benefits only short term, but it is very unlikely to stop the disease progression
3. IMO AN-1792 must have produced also antibodies against soluble abeta, the question is in what amount
4. AAB-001 is very likely to be more efficient in neutralizing soluble abeta than AN-1792
5. We can assume that the neutralization of soluble abeta provides higher clinical benefits at least in APOE4 non-carriers than plaque removal (AAB-001 PII top line data)
6. It remains to be seen if these clinical benefits will be longer lasting than in the case of AN-1792
7. If patients on AAB-001 progress after some time to severe dementia (like Holmes established on a small sample of AN-1792 patients), it does not disprove amyloid as a valid AD target. It might be simply too late to interfere with abeta in mild to moderate stage AD in order to stop the whole pathological cascade that was triggered maybe a decade earlier.
http://www.investorvillage.com/smbd.asp?mb=160&mn=256075&pt=…
plaque v beta amyloid/nangasimon
Hi Simon,
I think there was a difference between the Holmes and Hock studies as far as clinical endpoints are concerned. Holmes study looked only at the overall survival measure and dementia severity before death of patients in AN-1792 trial. Hock and others looked at clinical endpoints at 12 months after immunization and the responders did better on both cognitive and functional scores compared to placebo . The 4.5 years follow up study showed that AN-1792 responders outperformed placebos on function and there was still a positive trend in cognition (mean MMSE 13 vs 10 for placebo).
The conclusions for me are :
1. AN-1792 and AAB-001 are related therapies, nevertheless there will most likely be differences in their efficacy
2. Plaque clearance itself is can provide clinical benefits only short term, but it is very unlikely to stop the disease progression
3. IMO AN-1792 must have produced also antibodies against soluble abeta, the question is in what amount
4. AAB-001 is very likely to be more efficient in neutralizing soluble abeta than AN-1792
5. We can assume that the neutralization of soluble abeta provides higher clinical benefits at least in APOE4 non-carriers than plaque removal (AAB-001 PII top line data)
6. It remains to be seen if these clinical benefits will be longer lasting than in the case of AN-1792
7. If patients on AAB-001 progress after some time to severe dementia (like Holmes established on a small sample of AN-1792 patients), it does not disprove amyloid as a valid AD target. It might be simply too late to interfere with abeta in mild to moderate stage AD in order to stop the whole pathological cascade that was triggered maybe a decade earlier.
http://www.investorvillage.com/smbd.asp?mb=160&mn=256075&pt=…
Prevention And Treatment of Synucleinopathic And Amyloidogenic Disease
Inventors: Schenk; Dale B.; (Burlingame, CA) et al.
The invention provides improved agents and methods for treatment of diseases associated with synucleinopathic diseases, including Lewy bodies of alpha-synuclein in the brain of a patient. Such methods entail administering agents that induce a beneficial immunogenic response against the Lewy body.
The methods are particularly useful for prophylactic and therapeutic treatment of Parkinson's disease.
http://appft1.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=…
finanzen.net
Elan kann Verlust im zweiten Quartal halbieren
Donnerstag 24. Juli 2008, 12:23 Uhr
Dublin (aktiencheck.de AG) - Der irische Pharmakonzern Elan Corp. plc (ISIN IE0003072950/ WKN 903801) meldete am Donnerstag, dass sich sein Verlust im zweiten Quartal halbiert hat, was mit einem Umsatzwachstum bei seinem MS-Medikament Tysabri zusammenhängt.
Demnach belief sich der Nettoverlust auf 71,5 Mio. Dollar bzw. 15 Cents pro Aktie, gegenüber einem Minus von 141,1 Mio. Dollar bzw. 30 Cents pro Aktie im Vorjahr. Der operative Verlust verringerte sich von 110,5 Mio. Dollar auf 36 Mio. Dollar. Der Umsatz nahm indes um 30 Prozent auf 245,6 Mio. Dollar zu.
Analysten waren im Vorfeld von einem Verlust von 13 Cents pro Aktie und einem Umsatz von 249,5 Mio. Dollar ausgegangen. Für das laufende Quartal sehen sie ein EPS-Ergebnis von -12 Cents bei Erlösen von 269 Mio. Dollar.
Für das Gesamtjahr rechnet der Konzern weiterhin mit einem Umsatz von rund 1 Mrd. Dollar. Analysten sehen hier Erlöse von 1,05 Mrd. Dollar.
Die Aktie von Elan (Dublin: DRX.IR - Nachrichten) verliert in London zurzeit 4,05 Prozent auf 21,11 Euro. (24.07.2008/ac/n/a)
Elan kann Verlust im zweiten Quartal halbieren
Donnerstag 24. Juli 2008, 12:23 Uhr
Dublin (aktiencheck.de AG) - Der irische Pharmakonzern Elan Corp. plc (ISIN IE0003072950/ WKN 903801) meldete am Donnerstag, dass sich sein Verlust im zweiten Quartal halbiert hat, was mit einem Umsatzwachstum bei seinem MS-Medikament Tysabri zusammenhängt.
Demnach belief sich der Nettoverlust auf 71,5 Mio. Dollar bzw. 15 Cents pro Aktie, gegenüber einem Minus von 141,1 Mio. Dollar bzw. 30 Cents pro Aktie im Vorjahr. Der operative Verlust verringerte sich von 110,5 Mio. Dollar auf 36 Mio. Dollar. Der Umsatz nahm indes um 30 Prozent auf 245,6 Mio. Dollar zu.
Analysten waren im Vorfeld von einem Verlust von 13 Cents pro Aktie und einem Umsatz von 249,5 Mio. Dollar ausgegangen. Für das laufende Quartal sehen sie ein EPS-Ergebnis von -12 Cents bei Erlösen von 269 Mio. Dollar.
Für das Gesamtjahr rechnet der Konzern weiterhin mit einem Umsatz von rund 1 Mrd. Dollar. Analysten sehen hier Erlöse von 1,05 Mrd. Dollar.
Die Aktie von Elan (Dublin: DRX.IR - Nachrichten) verliert in London zurzeit 4,05 Prozent auf 21,11 Euro. (24.07.2008/ac/n/a)
Dennis Selkoe July 27th recieves Award at ICAD
Dennis Selkoe, MD, codirector of the Center for Neurologic Diseases, received a Lifetime Achievement Award in Alzheimer's Disease Research at the 11th International Conference on Alzheimer's Disease (ICAD) in Chicago, Ill.
The Lifetime Achievement Award is given to outstanding scientists who have dedicated themselves to helping millions around the world through their research. Selkoe received the award July 27.
http://www.brighamandwomens.org/publicaffairs/Awardsand%20Ho…
Dennis Selkoe, MD, codirector of the Center for Neurologic Diseases, received a Lifetime Achievement Award in Alzheimer's Disease Research at the 11th International Conference on Alzheimer's Disease (ICAD) in Chicago, Ill.
The Lifetime Achievement Award is given to outstanding scientists who have dedicated themselves to helping millions around the world through their research. Selkoe received the award July 27.
http://www.brighamandwomens.org/publicaffairs/Awardsand%20Ho…
Zeitplan ( GMT= GreenwichMeanTime) der ELAN Aktivitäten in dieser Woche:

Alzheimer's Association International Conference on Alzheimer's Disease 2008
29.07.08
ICAD Konferenz in Chicago
Title:
Clinical trials of bapineuzumab a beta-amyloid-targeted immunotherapy in patients with mild-to-moderate Alzheimers disease
Category: Therapeutic strategies, amyloid-based
M. Grundman + R. Black
Presentation Time at 4:00 p.m.
29.07.07 > 23.00 GMT <
Elan and Wyeth Announce Webcast To Discuss Bapineuzumab Phase 2 Clinical Trial Results
Company participants who will discuss the trial results and field questions will include:
Ron Black, MD, Wyeth Research, Asst. Vice President, Neuroscience.
Allison Hulme, PhD, Elan, EVP and Head of Global Development.
Dale Schenk, PhD, Elan, EVP and Chief Scientific Officer.
webcast at 6:00 p.m. Central Daylight Time
30.07.08 > 1.00 GMT <
----------------------------------------------------------------------------------------------------------------------------------

30.07.08
Kelly Martin auf CNBC
live interview from the Alzheimer's conference in Chicago
around 9:40 a.m. Eastern Time
30.07.08 > 15.40 GMT <
....dann hoffe ich nur , dass ich die GreenwichMean Time richtig angegeben habe...

Alzheimer's Association International Conference on Alzheimer's Disease 2008
29.07.08
ICAD Konferenz in Chicago
Title:
Clinical trials of bapineuzumab a beta-amyloid-targeted immunotherapy in patients with mild-to-moderate Alzheimers disease
Category: Therapeutic strategies, amyloid-based
M. Grundman + R. Black
Presentation Time at 4:00 p.m.
29.07.07 > 23.00 GMT <
Elan and Wyeth Announce Webcast To Discuss Bapineuzumab Phase 2 Clinical Trial Results
Company participants who will discuss the trial results and field questions will include:
Ron Black, MD, Wyeth Research, Asst. Vice President, Neuroscience.
Allison Hulme, PhD, Elan, EVP and Head of Global Development.
Dale Schenk, PhD, Elan, EVP and Chief Scientific Officer.
webcast at 6:00 p.m. Central Daylight Time
30.07.08 > 1.00 GMT <
----------------------------------------------------------------------------------------------------------------------------------

30.07.08
Kelly Martin auf CNBC
live interview from the Alzheimer's conference in Chicago
around 9:40 a.m. Eastern Time
30.07.08 > 15.40 GMT <
....dann hoffe ich nur , dass ich die GreenwichMean Time richtig angegeben habe...

29 July 2008
Elan and Wyeth Present Encouraging Results from Phase 2 Clinical Trial of Bapineuzumab at International Conference on Alzheimer's Disease
Overall Assessment:
-- Safety and efficacy results support design of ongoing global
Phase 3 program
-- Vasogenic edema correlated with dose and ApoE4 carrier status
which influenced the Phase 3 program design
-- Pre-specified efficacy analysis did not reach significance in
the total population
In Post Hoc Analyses:
-- Trends were observed in the cognitive endpoints ADAS-cog and
NTB in the total population
-- Statistically significant and clinically meaningful effects
were observed in multiple endpoints in ApoE4 non-carriers
-- In ApoE4 carriers, favorable directional changes were seen in
some endpoints, warranting further study
CHICAGO--(BUSINESS WIRE)--July 29, 2008--Elan Corporation, plc (NYSE: ELN) and Wyeth (NYSE: WYE) today are presenting detailed results from the companies' 18-month Phase 2 study of bapineuzumab (AAB-001) in patients with mild to moderate Alzheimer's disease at the Alzheimer's Association's International Conference on Alzheimer's Disease 2008 in Chicago, Illinois. As previously announced, in the study, bapineuzumab appeared to have an acceptable safety profile and clinical activity in treating Alzheimer's disease. Potential efficacy signals were seen at a range of doses without a clear dose response. The study did not attain statistical significance on the pre-specified efficacy endpoints in the overall study population. Post-hoc analyses showed statistically significant and clinically meaningful benefits in important subgroups.
The data will be presented by Sid Gilman, M.D., William J. Herdman Distinguished University Professor of Neurology, Director of Michigan Alzheimer's Disease Research Center, University of Michigan, and Chair of the independent safety monitoring committee for bapineuzumab.
"This study was limited in its size, design and goals," said Dr. Gilman, "but if the findings seen in these post-hoc analyses are replicated in the global Phase 3 program, it would be a validation of the amyloid hypothesis and could change how physicians approach the treatment of Alzheimer's disease."
Elan and Wyeth believe that the safety and efficacy findings from this Phase 2 trial of bapineuzumab in patients with mild-to-moderate Alzheimer's disease support the design of the ongoing global Phase 3 program and plan to incorporate learnings from this study into the Phase 3 program. The companies will continue to work diligently to develop much needed new treatment options for patients and physicians.
About the Phase 2 Clinical Trial
The double-blind, placebo-controlled multiple ascending dose trial was designed to assess the safety and tolerability of bapineuzumab in mild-to-moderate Alzheimer's disease and to explore efficacy at a range of doses. Two-hundred-thirty-four (234) patients were randomized(1) to receive one of four doses of bapineuzumab (0.15 mg/kg (n=31), 0.5 mg/kg (n=33), 1.0 mg/kg (n=30) or 2.0 mg/kg (n=30)) or placebo (n=110) by intravenous infusion every 13 weeks. Findings were reported for 229 patients in a modified intent-to-treat (MITT) analysis. Patients were intended to receive up to six doses during the 18-month study.
The pre-specified primary efficacy endpoints were change from baseline in Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) and Disability Assessment Scale for Dementia (DAD) in the 0.5 mg/kg, 1.0 mg/kg and 2.0 mg/kg dose groups against their placebo cohorts. Other efficacy measures included change in concentrations of tau in cerebral spinal fluid (CSF), the Neuropsychological Test Battery (NTB), the Clinical Dementia Rating Sum of Boxes (CDR-SOB), the Mini Mental State Examination (MMSE) and brain volume as measured by MRI. Efficacy was assessed from baseline for 78 weeks.
Pre-Specified Efficacy Analysis:
In the total study population, statistical significance was not obtained on the pre-specified efficacy endpoints of ADAS-cog and DAD.
Post-Hoc Efficacy Analyses:
Modified Intent to Treat (MITT) included patients who received at least one infusion and one efficacy assessment. In analyzing the data, the following were taken into account: an assumption of non linearity of the data over time, ApoE4 carrier status, and baseline MMSE and test scores.
The clinical relevance of the results for patients receiving the full 18 months of therapy was analyzed in a completer analysis. The patients included in the completer analysis received six (6) infusions and a week 78 efficacy assessment.
Using these assumptions, trends in favor of bapineuzumab treated patients were observed in ADAS-cog and NTB in the total MITT population. Additional completer analyses reinforced these trends.
The study revealed important differences in the rate of vasogenic edema by carrier status and for this reason the total population was analyzed by ApoE4 carrier status(2).
ApoE4 Non-Carrier Population
In the ApoE4 non-carrier patients, statistically significant differences from baseline to week 78 were observed in favor of bapineuzumab treated patients on both cognitive and functional efficacy endpoints:
-- ADAS-cog treatment difference of 5.0; p=0.026
-- NTB treatment difference of 0.35; p=0.006
-- CDR-SB treatment difference of 1.5; p=0.040
A favorable directional change of 6.9, p>0.10 for DAD was observed.
The completer analysis for non-carrier patients was consistent with the above findings.
Additionally, in these non-carrier patients, MRI results showed significantly less brain volume reduction versus placebo, as measured by the Brain Boundary Shift Integral (BBSI), at 71 weeks(3), with a treatment difference of 10.7 cc; p=0.004. Smaller increases in ventricular volume (VBSI) in bapineuzumab treated patients compared to placebo were observed, which were not statistically significant. Progression of Alzheimer's disease is generally associated with loss in brain volume and increases in ventricular volume.
ApoE4 Carrier Population
In the ApoE4 carrier patients, no statistically significant changes were observed in any of the cognitive or functional efficacy endpoints. The completer analysis for the carrier population showed favorable directional changes on cognitive and functional endpoints. The ongoing Phase 3 studies in ApoE4 carriers will help clarify these findings.
MRI findings in the carrier patients showed no significant change in brain volume between bapineuzumab treated and placebo patients, while a significant increase in ventricular volume in treated patients was observed, mean 2.5cc; p=0.037. The clinical relevance of this finding is still unclear and will continue to be evaluated.
"The clinically significant benefit seen with bapineuzumab treatment in the ApoE4 non-carrier subgroup is encouraging," said Dale Schenk, Ph.D., Executive Vice President and Chief Scientific Officer of Elan. "These results across multiple endpoints are consistent with what we have seen for beta amyloid immunotherapy from animal studies through to the patients."
"These data represent scientific validation of our decision to move rapidly into Phase 3 last year," said Gary L. Stiles, M.D., Chief Medical Officer, Wyeth. "In our Phase 3 program, we will learn much more since we will be able to study bapineuzumab in larger patient populations and better assess the results in ApoE4 carriers and non-carriers in separate trials. We are encouraged by these results and we'll achieve greater insight as we move forward."
Safety Findings
Adverse Events (AE) were observed in 95% of bapineuzumab treated patients versus 90% of placebo treated patients. AEs were generally mild to moderate and transient. With the exception of vasogenic edema, AEs did not appear to be dose related.
Adverse events seen in greater than 5% of bapineuzumab treated patients and at twice the rate of placebo treated patients were: back pain; anxiety; vomiting; vasogenic edema; hypertension; weight loss; paranoia; skin laceration; gait disturbance; and muscle spasm.
Three deaths occurred in bapineuzumab-treated patients, though these were not considered by the investigators to be treatment related. No deaths were reported in the placebo group. Other adverse events of interest occurring in less than five percent of patients treated with bapineuzumab included cataract, deep vein thrombosis, syncope, seizures and pulmonary embolism.
Vasogenic Edema (VE)
Twelve (12) cases of vasogenic edema were reported, all in treated patients, and all resolved over time. Ten (10) of these cases were reported in ApoE4 carriers with 2 cases in ApoE4 non-carriers. Eight (8) of the 12 cases were reported in the highest dose group, including both cases seen in ApoE4 non-carriers. Six (6) of the 12 cases were not associated with clinical symptoms and were detected on routine MRI scan. One (1) patient was treated with steroids. Re-dosing was instituted in six (6) of the 12 patients and no recurrence of VE was observed.
Phase 3 Program Implications
The Phase 2 data reinforce the design of the ongoing Phase 3 studies by ApoE4 carrier and non-carrier populations and the selected dose groups. The companies plan to continue all four ongoing Phase 3 studies. The ApoE4 carrier dose in the Phase 3 trials was selected to seek to minimize the risk of VE observed in the Phase 2 trial. The companies intend to obtain feedback from regulatory authorities in the coming months to finalize parameters for the Phase 3 program and discuss and reach agreement on requirements for registration.
Investor Webcast
The Companies will host a webcast on July 29, 2008 from 6:00pm CDT (7:00pm EDT) to discuss the results of the Phase 2 clinical trial.
Participants who will discuss the trial results and field questions will include:
-- Ron Black, M.D., Wyeth Research, Assistant Vice President,
Neuroscience
-- Sid Gilman, M.D., F.R.C.P., University of Michigan, Chair of
Bapineuzumab Safety Monitoring Committee
-- Allison Hulme, Ph.D., Elan, Executive Vice President and Head
of Global Development
-- Dale Schenk, Ph.D., Elan, Executive Vice President and Chief
Scientific Officer
-- Gary L. Stiles, M.D., Wyeth, Chief Medical Officer
Live audio of the webcast will be simultaneously broadcast over the Internet. The webcast can be accessed by visiting the companies' web sites at www.elan.com or www.wyeth.com and clicking on the "Investor Relations" icon. Following the live webcast, an archived version, including the slides, will be available at the same URLs.
About Bapineuzumab
Bapineuzumab is the first humanized monoclonal antibody in late-stage investigation as a potential treatment for Alzheimer's disease. Bapineuzumab is designed to clear toxic beta amyloid from the brain. The beta amyloid protein is a key component of the neuritic plaques that are implicated in the pathology of Alzheimer's disease. A global, 4,100 patient Phase 3 clinical program was initiated in December 2007 and is intended to provide safety and efficacy data to support the filing and approval of licensing applications for bapineuzumab as a potential treatment for patients with mild to moderate Alzheimer's disease. To learn more about this enrollment, patients or caregivers should contact clinical sites directly. Participating clinical sites can be found by visiting www.icarastudy.com or, in the United States by calling 1 (888) 818-MEMORY. Study site details also can be found by visiting www.clinicaltrials.gov.
About Alzheimer's Disease
Alzheimer's disease is a progressive brain disorder that gradually destroys a person's memory and ability to learn, reason, make judgments, communicate and carry out daily activities, such as bathing and eating. As Alzheimer's disease progresses, individuals may also experience changes in personality and behavior, such as anxiety, suspiciousness or agitation, as well as delusions or hallucinations. As many as 5 million Americans are estimated to have Alzheimer's disease, and more than 26 million people worldwide. One in eight baby boomers, and half of all people over 85, will develop the disease.
About the Elan and Wyeth Collaboration
The Wyeth and Elan Alzheimer's Immunotherapy Program (AIP) includes investigational clinical programs for bapineuzumab. AIP is a collaboration between the two companies to research, develop and commercialize immunotherapeutic approaches that may be used to treat and possibly prevent the onset of Alzheimer's disease. AIP research focuses on the beta amyloid hypothesis, as the companies believe that enhancing the clearance of beta amyloid in the brain may provide a new treatment approach for Alzheimer's disease.
About Elan
Elan Corporation, plc is a neuroscience-based biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. Elan shares trade on the New York, London and Dublin Stock Exchanges. For additional information about the company, please visit http://www.elan.com.
About Wyeth
Wyeth Pharmaceuticals, a division of Wyeth, has leading products in the areas of women's health care, infectious disease, gastrointestinal health, central nervous system, inflammation, transplantation, hemophilia, oncology, vaccines and nutritional products.
Wyeth is one of the world's largest research-driven pharmaceutical and health care products companies. It is a leader in the discovery, development, manufacturing and marketing of pharmaceuticals, vaccines, biotechnology products, nutritionals and non-prescription medicines that improve the quality of life for people worldwide. The Company's major divisions include Wyeth Pharmaceuticals, Wyeth Consumer Healthcare and Fort Dodge Animal Health. For additional information about the company, please visit http://www.wyeth.com.
Safe Harbor/Forward-Looking Statements
The statements in this press release and on the related webcast regarding the companies' assessment of the Phase 2 data and its implications for the Phase 3 program and future development of bapineuzumab are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. In particular, these statements are subject to the risk that further analyses of the Phase 2 data may lead to different (including less favorable) interpretations of the data than the analyses conducted to date and/or may identify important implications of the Phase 2 data that are not reflected in these statements. Clinical trial data are subject to differing interpretations, and regulatory agencies, medical and scientific experts and others may not share the companies' views of the Phase 2 data or its implications for the Phase 3 program and future development of bapineuzumab. In addition, further analyses of the Phase 2 data and discussion with regulatory authorities may lead to important modifications to the Phase 3 program. There can be no assurance that the clinical program for bapineuzumab will be successful in demonstrating safety and/or efficacy, that we will not encounter problems or delays in clinical development, or that bapineuzumab will ever receive regulatory approval or be successfully commercialized. Other risks and uncertainties that could cause actual results to differ materially from those expressed or implied by these forward-looking statements include those detailed from time to time in the Companies' periodic reports filed with the Securities and Exchange Commission, including Wyeth's current reports on Form 8-K, quarterly reports on Form 10-Q and annual report on Form 10-K, particularly the discussion under the caption "Item 1A, Risk Factors" in Wyeth's Annual Report on Form 10-K for the year ended December 31, 2007, which was filed with the Securities and Exchange Commission on February 29, 2008, and Elan's Reports of Foreign Issuer on Form 6-K and Annual Report on Form 20-F, particularly the discussion under the caption "Item 3D, Risk Factors" in Elan's Annual Report on Form 20-F for the year ended December 31, 2007, which was filed with the Securities and Exchange Commission on February 28, 2008. The forward-looking statements in this press release are qualified by these risk factors. We assume no obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
(1) Randomization was on an 8:7 ratio, with more patients receiving bapineuzumab versus placebo.
(2) Literature estimates that 40-70 percent of Alzheimer's disease population are non-carriers of the Apolipoprotein E4 (ApoE4) allele
(3) MRI results were measured through week 71
http://www.elan.com/news/full.asp?ID=1180940
Elan and Wyeth Present Encouraging Results from Phase 2 Clinical Trial of Bapineuzumab at International Conference on Alzheimer's Disease
Overall Assessment:
-- Safety and efficacy results support design of ongoing global
Phase 3 program
-- Vasogenic edema correlated with dose and ApoE4 carrier status
which influenced the Phase 3 program design
-- Pre-specified efficacy analysis did not reach significance in
the total population
In Post Hoc Analyses:
-- Trends were observed in the cognitive endpoints ADAS-cog and
NTB in the total population
-- Statistically significant and clinically meaningful effects
were observed in multiple endpoints in ApoE4 non-carriers
-- In ApoE4 carriers, favorable directional changes were seen in
some endpoints, warranting further study
CHICAGO--(BUSINESS WIRE)--July 29, 2008--Elan Corporation, plc (NYSE: ELN) and Wyeth (NYSE: WYE) today are presenting detailed results from the companies' 18-month Phase 2 study of bapineuzumab (AAB-001) in patients with mild to moderate Alzheimer's disease at the Alzheimer's Association's International Conference on Alzheimer's Disease 2008 in Chicago, Illinois. As previously announced, in the study, bapineuzumab appeared to have an acceptable safety profile and clinical activity in treating Alzheimer's disease. Potential efficacy signals were seen at a range of doses without a clear dose response. The study did not attain statistical significance on the pre-specified efficacy endpoints in the overall study population. Post-hoc analyses showed statistically significant and clinically meaningful benefits in important subgroups.
The data will be presented by Sid Gilman, M.D., William J. Herdman Distinguished University Professor of Neurology, Director of Michigan Alzheimer's Disease Research Center, University of Michigan, and Chair of the independent safety monitoring committee for bapineuzumab.
"This study was limited in its size, design and goals," said Dr. Gilman, "but if the findings seen in these post-hoc analyses are replicated in the global Phase 3 program, it would be a validation of the amyloid hypothesis and could change how physicians approach the treatment of Alzheimer's disease."
Elan and Wyeth believe that the safety and efficacy findings from this Phase 2 trial of bapineuzumab in patients with mild-to-moderate Alzheimer's disease support the design of the ongoing global Phase 3 program and plan to incorporate learnings from this study into the Phase 3 program. The companies will continue to work diligently to develop much needed new treatment options for patients and physicians.
About the Phase 2 Clinical Trial
The double-blind, placebo-controlled multiple ascending dose trial was designed to assess the safety and tolerability of bapineuzumab in mild-to-moderate Alzheimer's disease and to explore efficacy at a range of doses. Two-hundred-thirty-four (234) patients were randomized(1) to receive one of four doses of bapineuzumab (0.15 mg/kg (n=31), 0.5 mg/kg (n=33), 1.0 mg/kg (n=30) or 2.0 mg/kg (n=30)) or placebo (n=110) by intravenous infusion every 13 weeks. Findings were reported for 229 patients in a modified intent-to-treat (MITT) analysis. Patients were intended to receive up to six doses during the 18-month study.
The pre-specified primary efficacy endpoints were change from baseline in Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog) and Disability Assessment Scale for Dementia (DAD) in the 0.5 mg/kg, 1.0 mg/kg and 2.0 mg/kg dose groups against their placebo cohorts. Other efficacy measures included change in concentrations of tau in cerebral spinal fluid (CSF), the Neuropsychological Test Battery (NTB), the Clinical Dementia Rating Sum of Boxes (CDR-SOB), the Mini Mental State Examination (MMSE) and brain volume as measured by MRI. Efficacy was assessed from baseline for 78 weeks.
Pre-Specified Efficacy Analysis:
In the total study population, statistical significance was not obtained on the pre-specified efficacy endpoints of ADAS-cog and DAD.
Post-Hoc Efficacy Analyses:
Modified Intent to Treat (MITT) included patients who received at least one infusion and one efficacy assessment. In analyzing the data, the following were taken into account: an assumption of non linearity of the data over time, ApoE4 carrier status, and baseline MMSE and test scores.
The clinical relevance of the results for patients receiving the full 18 months of therapy was analyzed in a completer analysis. The patients included in the completer analysis received six (6) infusions and a week 78 efficacy assessment.
Using these assumptions, trends in favor of bapineuzumab treated patients were observed in ADAS-cog and NTB in the total MITT population. Additional completer analyses reinforced these trends.
The study revealed important differences in the rate of vasogenic edema by carrier status and for this reason the total population was analyzed by ApoE4 carrier status(2).
ApoE4 Non-Carrier Population
In the ApoE4 non-carrier patients, statistically significant differences from baseline to week 78 were observed in favor of bapineuzumab treated patients on both cognitive and functional efficacy endpoints:
-- ADAS-cog treatment difference of 5.0; p=0.026
-- NTB treatment difference of 0.35; p=0.006
-- CDR-SB treatment difference of 1.5; p=0.040
A favorable directional change of 6.9, p>0.10 for DAD was observed.
The completer analysis for non-carrier patients was consistent with the above findings.
Additionally, in these non-carrier patients, MRI results showed significantly less brain volume reduction versus placebo, as measured by the Brain Boundary Shift Integral (BBSI), at 71 weeks(3), with a treatment difference of 10.7 cc; p=0.004. Smaller increases in ventricular volume (VBSI) in bapineuzumab treated patients compared to placebo were observed, which were not statistically significant. Progression of Alzheimer's disease is generally associated with loss in brain volume and increases in ventricular volume.
ApoE4 Carrier Population
In the ApoE4 carrier patients, no statistically significant changes were observed in any of the cognitive or functional efficacy endpoints. The completer analysis for the carrier population showed favorable directional changes on cognitive and functional endpoints. The ongoing Phase 3 studies in ApoE4 carriers will help clarify these findings.
MRI findings in the carrier patients showed no significant change in brain volume between bapineuzumab treated and placebo patients, while a significant increase in ventricular volume in treated patients was observed, mean 2.5cc; p=0.037. The clinical relevance of this finding is still unclear and will continue to be evaluated.
"The clinically significant benefit seen with bapineuzumab treatment in the ApoE4 non-carrier subgroup is encouraging," said Dale Schenk, Ph.D., Executive Vice President and Chief Scientific Officer of Elan. "These results across multiple endpoints are consistent with what we have seen for beta amyloid immunotherapy from animal studies through to the patients."
"These data represent scientific validation of our decision to move rapidly into Phase 3 last year," said Gary L. Stiles, M.D., Chief Medical Officer, Wyeth. "In our Phase 3 program, we will learn much more since we will be able to study bapineuzumab in larger patient populations and better assess the results in ApoE4 carriers and non-carriers in separate trials. We are encouraged by these results and we'll achieve greater insight as we move forward."
Safety Findings
Adverse Events (AE) were observed in 95% of bapineuzumab treated patients versus 90% of placebo treated patients. AEs were generally mild to moderate and transient. With the exception of vasogenic edema, AEs did not appear to be dose related.
Adverse events seen in greater than 5% of bapineuzumab treated patients and at twice the rate of placebo treated patients were: back pain; anxiety; vomiting; vasogenic edema; hypertension; weight loss; paranoia; skin laceration; gait disturbance; and muscle spasm.
Three deaths occurred in bapineuzumab-treated patients, though these were not considered by the investigators to be treatment related. No deaths were reported in the placebo group. Other adverse events of interest occurring in less than five percent of patients treated with bapineuzumab included cataract, deep vein thrombosis, syncope, seizures and pulmonary embolism.
Vasogenic Edema (VE)
Twelve (12) cases of vasogenic edema were reported, all in treated patients, and all resolved over time. Ten (10) of these cases were reported in ApoE4 carriers with 2 cases in ApoE4 non-carriers. Eight (8) of the 12 cases were reported in the highest dose group, including both cases seen in ApoE4 non-carriers. Six (6) of the 12 cases were not associated with clinical symptoms and were detected on routine MRI scan. One (1) patient was treated with steroids. Re-dosing was instituted in six (6) of the 12 patients and no recurrence of VE was observed.
Phase 3 Program Implications
The Phase 2 data reinforce the design of the ongoing Phase 3 studies by ApoE4 carrier and non-carrier populations and the selected dose groups. The companies plan to continue all four ongoing Phase 3 studies. The ApoE4 carrier dose in the Phase 3 trials was selected to seek to minimize the risk of VE observed in the Phase 2 trial. The companies intend to obtain feedback from regulatory authorities in the coming months to finalize parameters for the Phase 3 program and discuss and reach agreement on requirements for registration.
Investor Webcast
The Companies will host a webcast on July 29, 2008 from 6:00pm CDT (7:00pm EDT) to discuss the results of the Phase 2 clinical trial.
Participants who will discuss the trial results and field questions will include:
-- Ron Black, M.D., Wyeth Research, Assistant Vice President,
Neuroscience
-- Sid Gilman, M.D., F.R.C.P., University of Michigan, Chair of
Bapineuzumab Safety Monitoring Committee
-- Allison Hulme, Ph.D., Elan, Executive Vice President and Head
of Global Development
-- Dale Schenk, Ph.D., Elan, Executive Vice President and Chief
Scientific Officer
-- Gary L. Stiles, M.D., Wyeth, Chief Medical Officer
Live audio of the webcast will be simultaneously broadcast over the Internet. The webcast can be accessed by visiting the companies' web sites at www.elan.com or www.wyeth.com and clicking on the "Investor Relations" icon. Following the live webcast, an archived version, including the slides, will be available at the same URLs.
About Bapineuzumab
Bapineuzumab is the first humanized monoclonal antibody in late-stage investigation as a potential treatment for Alzheimer's disease. Bapineuzumab is designed to clear toxic beta amyloid from the brain. The beta amyloid protein is a key component of the neuritic plaques that are implicated in the pathology of Alzheimer's disease. A global, 4,100 patient Phase 3 clinical program was initiated in December 2007 and is intended to provide safety and efficacy data to support the filing and approval of licensing applications for bapineuzumab as a potential treatment for patients with mild to moderate Alzheimer's disease. To learn more about this enrollment, patients or caregivers should contact clinical sites directly. Participating clinical sites can be found by visiting www.icarastudy.com or, in the United States by calling 1 (888) 818-MEMORY. Study site details also can be found by visiting www.clinicaltrials.gov.
About Alzheimer's Disease
Alzheimer's disease is a progressive brain disorder that gradually destroys a person's memory and ability to learn, reason, make judgments, communicate and carry out daily activities, such as bathing and eating. As Alzheimer's disease progresses, individuals may also experience changes in personality and behavior, such as anxiety, suspiciousness or agitation, as well as delusions or hallucinations. As many as 5 million Americans are estimated to have Alzheimer's disease, and more than 26 million people worldwide. One in eight baby boomers, and half of all people over 85, will develop the disease.
About the Elan and Wyeth Collaboration
The Wyeth and Elan Alzheimer's Immunotherapy Program (AIP) includes investigational clinical programs for bapineuzumab. AIP is a collaboration between the two companies to research, develop and commercialize immunotherapeutic approaches that may be used to treat and possibly prevent the onset of Alzheimer's disease. AIP research focuses on the beta amyloid hypothesis, as the companies believe that enhancing the clearance of beta amyloid in the brain may provide a new treatment approach for Alzheimer's disease.
About Elan
Elan Corporation, plc is a neuroscience-based biotechnology company committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. Elan shares trade on the New York, London and Dublin Stock Exchanges. For additional information about the company, please visit http://www.elan.com.
About Wyeth
Wyeth Pharmaceuticals, a division of Wyeth, has leading products in the areas of women's health care, infectious disease, gastrointestinal health, central nervous system, inflammation, transplantation, hemophilia, oncology, vaccines and nutritional products.
Wyeth is one of the world's largest research-driven pharmaceutical and health care products companies. It is a leader in the discovery, development, manufacturing and marketing of pharmaceuticals, vaccines, biotechnology products, nutritionals and non-prescription medicines that improve the quality of life for people worldwide. The Company's major divisions include Wyeth Pharmaceuticals, Wyeth Consumer Healthcare and Fort Dodge Animal Health. For additional information about the company, please visit http://www.wyeth.com.
Safe Harbor/Forward-Looking Statements
The statements in this press release and on the related webcast regarding the companies' assessment of the Phase 2 data and its implications for the Phase 3 program and future development of bapineuzumab are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. In particular, these statements are subject to the risk that further analyses of the Phase 2 data may lead to different (including less favorable) interpretations of the data than the analyses conducted to date and/or may identify important implications of the Phase 2 data that are not reflected in these statements. Clinical trial data are subject to differing interpretations, and regulatory agencies, medical and scientific experts and others may not share the companies' views of the Phase 2 data or its implications for the Phase 3 program and future development of bapineuzumab. In addition, further analyses of the Phase 2 data and discussion with regulatory authorities may lead to important modifications to the Phase 3 program. There can be no assurance that the clinical program for bapineuzumab will be successful in demonstrating safety and/or efficacy, that we will not encounter problems or delays in clinical development, or that bapineuzumab will ever receive regulatory approval or be successfully commercialized. Other risks and uncertainties that could cause actual results to differ materially from those expressed or implied by these forward-looking statements include those detailed from time to time in the Companies' periodic reports filed with the Securities and Exchange Commission, including Wyeth's current reports on Form 8-K, quarterly reports on Form 10-Q and annual report on Form 10-K, particularly the discussion under the caption "Item 1A, Risk Factors" in Wyeth's Annual Report on Form 10-K for the year ended December 31, 2007, which was filed with the Securities and Exchange Commission on February 29, 2008, and Elan's Reports of Foreign Issuer on Form 6-K and Annual Report on Form 20-F, particularly the discussion under the caption "Item 3D, Risk Factors" in Elan's Annual Report on Form 20-F for the year ended December 31, 2007, which was filed with the Securities and Exchange Commission on February 28, 2008. The forward-looking statements in this press release are qualified by these risk factors. We assume no obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
(1) Randomization was on an 8:7 ratio, with more patients receiving bapineuzumab versus placebo.
(2) Literature estimates that 40-70 percent of Alzheimer's disease population are non-carriers of the Apolipoprotein E4 (ApoE4) allele
(3) MRI results were measured through week 71
http://www.elan.com/news/full.asp?ID=1180940
na, wo sind nun die tollen Ergebnisse? 
Dem Markt scheint da jetzt ein wenig anderer Meinung zu sein... langsam dämmert es einigen, was hier gespielt wird... AH -20% aktuell! :O
************
UPDATE 1-Elan, Wyeth Alzheimer drug results mixed, shrs fall
Tue Jul 29, 2008 6:16pm EDT
By Julie Steenhuysen
CHICAGO, July 29 (Reuters)- Keenly awaited details on Elan (ELN.I: Quote, Profile, Research, Stock Buzz) (ELN.N: Quote, Profile, Research, Stock Buzz) and Wyeth's (WYE.N: Quote, Profile, Research, Stock Buzz) new Alzheimer's drug bapineuzumab show it raised the risk of a potentially serious side effect, but may help people who do not have a common genetic risk of the disease, the companies said on Tuesday.
Shares of both companies plunged in after hours trade with Elan's New York shares falling more 18 percent and Wyeth's dropping more than 10 percent.
"I think the (drug's) side effects are going to be perhaps significant, but if they are temporary and tolerable and if the drug shows a benefit, the risk-benefit ratio will be worth the side effects," said Dr. Scott Turner, incoming director of the Memory Disorders Program at Georgetown University Medical Center in Washington.
"This is potentially the first disease-modifying therapy."
The update on the antibody medicine, also known as AAB-001, has been closely watched by investors. If proven to work, the drug could be the first to modify the course of Alzheimer's disease, rather than just offering symptom relief.
Some analysts have forecast eventual annual sales of $13 billion.
Twelve people with mild-to-moderate Alzheimer's who were treated with the drug, developed a build-up of fluid in the brain called vasogenic edema, researchers told the Alzheimer's Association International Conference on Alzheimer's Disease in Chicago.
Ten of those cases were in people who have the ApoE4 gene, which significantly raises their risk of developing Alzheimer's disease.
Preliminary findings released last month showed the drug failed to boost memory and functionality in most of 234 patients over 18 months. :O
GENETIC DIFFERENCES
Carriers of ApoE4, 60 percent of those who got the drug and 70 percent who got the placebo, did not show any improvement in their ability to think or function.
But among people who had different versions of the ApoE gene, the companies did find a statistically significant improvement in these measures.
When they looked at people do do not carry the gene and who completed the study, "we have absolutely dynamite data," said Dr. Sid Gilman of the University of Michigan, who helped work on the study.
"There is a very strong signal among non-carriers, suggesting a beneficial effect," Gilman told the meeting.
Other experts were cautious. "You can't conclude anything about the efficacy of the drug from this trial," said Dr Ronald Petersen of the Mayo Clinic in Rochester, Minnesota.
"It's a secondary analysis. You have to go with what they pre-specified and what their endpoints were, and they didn't make those," said Petersen, incoming chairman of the Alzheimer's Association's scientific board and chairman of a safety monitoring board for a therapeutic vaccine Wyeth and Elan are working on for Alzheimer's.
said Petersen, incoming chairman of the Alzheimer's Association's scientific board and chairman of a safety monitoring board for a therapeutic vaccine Wyeth and Elan are working on for Alzheimer's.
The results "give me pause that the statistical significance that we saw will bear out in the Phase 3 trials ," said David Moskowitz, an analyst at Caris & Co.
," said David Moskowitz, an analyst at Caris & Co.
"This is still a very risky endeavor," he said.
The companies decided to conduct larger studies aimed at proving bapineuzumab worked in ApoE4 non-carriers, who make up about half of all Alzheimer's patients, Dr. Ronald Black, assistant vice president in neuroscience research at Wyeth, said in an interview.
Black said the big difference in vasogenic edema between ApoE4 carriers and non-carriers prompted the companies to analyze the groups separately.
Shares of Wyeth fell 10.1 percent to $40.40 in extended trade on Tuesday, while shares of Elan dropped 18.5 percent to $27.50 in New York. (Additional reporting by Deena Beasley in Los Angeles; Editing by Maggie Fox, Leslie Gevirtz)
********
und die Bombe tickt munter weiter... unglaublich unverantwortlich von Weyth auf Basis dieser Daten eine zig 100 Mio USD PIII zu starten statt das Geld für etwas sinnvolles zu investieren!!!!!!!!!!
mfg ipollit

Dem Markt scheint da jetzt ein wenig anderer Meinung zu sein... langsam dämmert es einigen, was hier gespielt wird... AH -20% aktuell! :O
************
UPDATE 1-Elan, Wyeth Alzheimer drug results mixed, shrs fall
Tue Jul 29, 2008 6:16pm EDT
By Julie Steenhuysen
CHICAGO, July 29 (Reuters)- Keenly awaited details on Elan (ELN.I: Quote, Profile, Research, Stock Buzz) (ELN.N: Quote, Profile, Research, Stock Buzz) and Wyeth's (WYE.N: Quote, Profile, Research, Stock Buzz) new Alzheimer's drug bapineuzumab show it raised the risk of a potentially serious side effect, but may help people who do not have a common genetic risk of the disease, the companies said on Tuesday.
Shares of both companies plunged in after hours trade with Elan's New York shares falling more 18 percent and Wyeth's dropping more than 10 percent.
"I think the (drug's) side effects are going to be perhaps significant, but if they are temporary and tolerable and if the drug shows a benefit, the risk-benefit ratio will be worth the side effects," said Dr. Scott Turner, incoming director of the Memory Disorders Program at Georgetown University Medical Center in Washington.
"This is potentially the first disease-modifying therapy."
The update on the antibody medicine, also known as AAB-001, has been closely watched by investors. If proven to work, the drug could be the first to modify the course of Alzheimer's disease, rather than just offering symptom relief.
Some analysts have forecast eventual annual sales of $13 billion.
Twelve people with mild-to-moderate Alzheimer's who were treated with the drug, developed a build-up of fluid in the brain called vasogenic edema, researchers told the Alzheimer's Association International Conference on Alzheimer's Disease in Chicago.
Ten of those cases were in people who have the ApoE4 gene, which significantly raises their risk of developing Alzheimer's disease.
Preliminary findings released last month showed the drug failed to boost memory and functionality in most of 234 patients over 18 months. :O
GENETIC DIFFERENCES
Carriers of ApoE4, 60 percent of those who got the drug and 70 percent who got the placebo, did not show any improvement in their ability to think or function.
But among people who had different versions of the ApoE gene, the companies did find a statistically significant improvement in these measures.
When they looked at people do do not carry the gene and who completed the study, "we have absolutely dynamite data," said Dr. Sid Gilman of the University of Michigan, who helped work on the study.
"There is a very strong signal among non-carriers, suggesting a beneficial effect," Gilman told the meeting.
Other experts were cautious. "You can't conclude anything about the efficacy of the drug from this trial," said Dr Ronald Petersen of the Mayo Clinic in Rochester, Minnesota.
"It's a secondary analysis. You have to go with what they pre-specified and what their endpoints were, and they didn't make those,"
 said Petersen, incoming chairman of the Alzheimer's Association's scientific board and chairman of a safety monitoring board for a therapeutic vaccine Wyeth and Elan are working on for Alzheimer's.
said Petersen, incoming chairman of the Alzheimer's Association's scientific board and chairman of a safety monitoring board for a therapeutic vaccine Wyeth and Elan are working on for Alzheimer's. The results "give me pause that the statistical significance that we saw will bear out in the Phase 3 trials
 ," said David Moskowitz, an analyst at Caris & Co.
," said David Moskowitz, an analyst at Caris & Co."This is still a very risky endeavor," he said.
The companies decided to conduct larger studies aimed at proving bapineuzumab worked in ApoE4 non-carriers, who make up about half of all Alzheimer's patients, Dr. Ronald Black, assistant vice president in neuroscience research at Wyeth, said in an interview.
Black said the big difference in vasogenic edema between ApoE4 carriers and non-carriers prompted the companies to analyze the groups separately.
Shares of Wyeth fell 10.1 percent to $40.40 in extended trade on Tuesday, while shares of Elan dropped 18.5 percent to $27.50 in New York. (Additional reporting by Deena Beasley in Los Angeles; Editing by Maggie Fox, Leslie Gevirtz)
********
und die Bombe tickt munter weiter... unglaublich unverantwortlich von Weyth auf Basis dieser Daten eine zig 100 Mio USD PIII zu starten statt das Geld für etwas sinnvolles zu investieren!!!!!!!!!!

mfg ipollit
Antwort auf Beitrag Nr.: 34.610.989 von ipollit am 30.07.08 00:49:53ist die Verteilung der Non-Carrier in den einzelnen Dosierungsgruppen bereits bekannt?
Ohne Berücksichtigung der Dosierungen entnehme ich der Elan-AdHoc folgende Daten:
"Two-hundred-thirty-four (234) patients were randomized(1) to receive one of four doses of bapineuzumab (0.15 mg/kg (n=31), 0.5 mg/kg (n=33), 1.0 mg/kg (n=30) or 2.0 mg/kg (n=30)) or placebo (n=110) by intravenous infusion every 13 weeks. Findings were reported for 229 patients in a modified intent-to-treat (MITT) analysis. Patients were intended to receive up to six doses during the 18-month study."
"Modified Intent to Treat (MITT) included patients who received at least one infusion and one efficacy assessment.
The clinical relevance of the results for patients receiving the full 18 months of therapy was analyzed in a completer analysis. The patients included in the completer analysis received six (6) infusions and a week 78 efficacy assessment."
desweiteren...
"Carriers of ApoE4, 60 percent of those who got the drug and 70 percent who got the placebo, did not show any improvement in their ability to think or function."
************
jetzt wird es mathematisch...
1.) 234 Patienten wurden randomisiert... exakt zu 124 BAP und 110 Placebos, also mit einem BAP-Anteil von 53%
2.) 229 Patienten sind in der MITT... hochgerechnet 121 BAP und 108 Placebos
3.) die "completer analysis" enthält aber nur den Teil der MITT, der alle 6 Infusionen erhalten hat. Die Anzahl ist nicht angegeben... aber das drückt die Patienten der beiden Gruppen nochmal: <121 BAP und <108 Placebos
4.) die "Non-Carrier" der "completer analysis", die hier relevante Subgruppe, auf der der gesamte PIII-Start und die retrosprektive Analyse beruht ist hochgerechnet: 40% der BAP und 30% der Placebos... also:
weniger als 48 BAP und weniger als 32 Placebos bilden die Subgruppe!!!!! :O
Wenn nun zufällig unter den weniger als 32 Placebo-Patienten einige ziemlich hart von Alzheimer erwischt wurden, verzerrt das natürlich erheblich die gesamte Analyse und so werden plötzlich die Ergebnisse für die Non-Carrier auf wundersame Weise signifikant!
Wer es jetzt nicht glauben will, wird es in der PIII erleben...
mfg ipollit
Ohne Berücksichtigung der Dosierungen entnehme ich der Elan-AdHoc folgende Daten:
"Two-hundred-thirty-four (234) patients were randomized(1) to receive one of four doses of bapineuzumab (0.15 mg/kg (n=31), 0.5 mg/kg (n=33), 1.0 mg/kg (n=30) or 2.0 mg/kg (n=30)) or placebo (n=110) by intravenous infusion every 13 weeks. Findings were reported for 229 patients in a modified intent-to-treat (MITT) analysis. Patients were intended to receive up to six doses during the 18-month study."
"Modified Intent to Treat (MITT) included patients who received at least one infusion and one efficacy assessment.
The clinical relevance of the results for patients receiving the full 18 months of therapy was analyzed in a completer analysis. The patients included in the completer analysis received six (6) infusions and a week 78 efficacy assessment."
desweiteren...
"Carriers of ApoE4, 60 percent of those who got the drug and 70 percent who got the placebo, did not show any improvement in their ability to think or function."
************
jetzt wird es mathematisch...
1.) 234 Patienten wurden randomisiert... exakt zu 124 BAP und 110 Placebos, also mit einem BAP-Anteil von 53%
2.) 229 Patienten sind in der MITT... hochgerechnet 121 BAP und 108 Placebos
3.) die "completer analysis" enthält aber nur den Teil der MITT, der alle 6 Infusionen erhalten hat. Die Anzahl ist nicht angegeben... aber das drückt die Patienten der beiden Gruppen nochmal: <121 BAP und <108 Placebos
4.) die "Non-Carrier" der "completer analysis", die hier relevante Subgruppe, auf der der gesamte PIII-Start und die retrosprektive Analyse beruht ist hochgerechnet: 40% der BAP und 30% der Placebos... also:
weniger als 48 BAP und weniger als 32 Placebos bilden die Subgruppe!!!!! :O
Wenn nun zufällig unter den weniger als 32 Placebo-Patienten einige ziemlich hart von Alzheimer erwischt wurden, verzerrt das natürlich erheblich die gesamte Analyse und so werden plötzlich die Ergebnisse für die Non-Carrier auf wundersame Weise signifikant!
Wer es jetzt nicht glauben will, wird es in der PIII erleben...

mfg ipollit
Antwort auf Beitrag Nr.: 34.611.032 von ipollit am 30.07.08 01:25:10die Ergebnisse in den Dosierungen scheinen ebenfalls gegen BAP zu sprechen! Der Behandlungserfolg korreliert nicht mit den Dosierungen... zu erwarten wäre, dass es einen dosisabhängigen Effekt gibt. Zu höheren Dosierungen sollte die Wirkung zunehmen. Stattdessen nimmt anscheinend mit der Dosis nur das Risiko der sehr gefährlichen Nebenwirkungen der Gehirnschwellung zu!
The data showed that none of the patients, whether or not they had a genetic bias toward the disease, saw any more benefit from a higher dose of bapineuzumab than a lower dose.
Another concern is that the more statistical analyses that are conducted, the more likely it is that findings may show significance merely by chance. Also, patients didn't show a consistent pattern across doses.
With the exception of vasogenic edema, AEs did not appear to be dose related.
Eight (8) of the 12 cases were reported in the highest dose group, including both cases seen in ApoE4 non-carriers.
(das oben auf gut deutsch... egal ob mit oder ohne ApoE4, die Wirkung der höheren Dosierung ist nicht besser gewesen als die der niedrigen)
mfg ipollit
The data showed that none of the patients, whether or not they had a genetic bias toward the disease, saw any more benefit from a higher dose of bapineuzumab than a lower dose.
Another concern is that the more statistical analyses that are conducted, the more likely it is that findings may show significance merely by chance. Also, patients didn't show a consistent pattern across doses.
With the exception of vasogenic edema, AEs did not appear to be dose related.
Eight (8) of the 12 cases were reported in the highest dose group, including both cases seen in ApoE4 non-carriers.
(das oben auf gut deutsch... egal ob mit oder ohne ApoE4, die Wirkung der höheren Dosierung ist nicht besser gewesen als die der niedrigen)
mfg ipollit
Antwort auf Beitrag Nr.: 34.611.040 von ipollit am 30.07.08 01:38:23After Hours: 21.80 11.95 (-35.41%) 7:25PM ET


Antwort auf Beitrag Nr.: 34.611.032 von ipollit am 30.07.08 01:25:10hier nochmal die exakten Angaben aus der Präsentation (habe ich ja gar nicht schlecht geschätzt)...
Non-Carrier MITT-Subgruppe: 46-47 BAP, 30-32 Placebos
NonCarrier Completer-Subgruppe: 36 BAP, 21 Placebos
beste Dosis jeweils 0,5mg; schlechteste Dosis 1mg (bis auf NTB)
ADAS-cog, NTB und CDR-SB jeweils signifikant, DAD nicht-signifikant
mfg ipollit
Non-Carrier MITT-Subgruppe: 46-47 BAP, 30-32 Placebos
NonCarrier Completer-Subgruppe: 36 BAP, 21 Placebos
beste Dosis jeweils 0,5mg; schlechteste Dosis 1mg (bis auf NTB)
ADAS-cog, NTB und CDR-SB jeweils signifikant, DAD nicht-signifikant
mfg ipollit
weitere beachtenswerte Kommentare...
First impressions from ICAD
Just a quick note before I go out for dinner, will post more later. Lots of interesting stuff here.
As I expected, the Elan data were a mixed bag and will take some time for scientists and analysts to digest.
Here are some points that may have a negative influence on the pps:
1. No clear dose response. (actually the presenter refused to give further details when asked in Q&A session, very strange)
2. A relatively high decline rate in the placebo group (around 11 ADAS points). For illustration, other trials presented in the same session had decline rates of 7 to 8. Since the sample was small, some people could be worried that the 5 point benefit seen in the Bap arms could have been caused by higher than usual decline rate in placebos. This issue was very obvious as everybody in the room has previously seen the details of the failed Flurizan study.
This issue was very obvious as everybody in the room has previously seen the details of the failed Flurizan study.
3. The presentation itself. Mediocre at best. I think Elan should have done a better job in presenting the data.
4. Company TauRx had a presentation after Elan. Their PIIb phase of a drug that inhibits tau aggregation hit statistical significance on all endpoints, in a single dose (not aggregated across all doses like in the case of Elan) and regardless of APOE4 genotype. The ADAScog improvement over placebo was almost 7 points. The best part: since the placebos declined only a little more than 7 points, it means the drug virtually halted the progression of the disease. I would call this truly spectacular.
I would call this truly spectacular.
***********
Auch hier ein weiterer deutlicher Hinweis, dass es sich bei den BAP-Ergebnissen lediglich um Zufall handelt. Die Placebos schnitten erheblich schlechter ab, als dies zu erwarten war. Dies kann in einer kleiner Subgruppe leicht passieren... in einer großen PIII ist es dagegen eher ausgeschlossen. Außergewöhnlich schlechte Placebo-Patienten suggerieren natürlich ein positive Wirkung von BAP, die überhaupt nicht existiert! Die BAP-Subgruppe hat sich dagegen anscheinend so entwickelt wie es von unbehandelten Patienten zu erwarten wäre!
Ist TauRx eigentlich eine frei handelbare Aktiengesellschaft? Sie verfolgen ein anderes MOA als Anti-Beta-Amyloid... scheinbar bis jetzt erfolgreich (vielleicht allerdings auch nur Zahlenspielerei, ich kenne die Daten nicht)
mfg ipollit
First impressions from ICAD
Just a quick note before I go out for dinner, will post more later. Lots of interesting stuff here.
As I expected, the Elan data were a mixed bag and will take some time for scientists and analysts to digest.
Here are some points that may have a negative influence on the pps:
1. No clear dose response. (actually the presenter refused to give further details when asked in Q&A session, very strange)
2. A relatively high decline rate in the placebo group (around 11 ADAS points). For illustration, other trials presented in the same session had decline rates of 7 to 8. Since the sample was small, some people could be worried that the 5 point benefit seen in the Bap arms could have been caused by higher than usual decline rate in placebos.
 This issue was very obvious as everybody in the room has previously seen the details of the failed Flurizan study.
This issue was very obvious as everybody in the room has previously seen the details of the failed Flurizan study.3. The presentation itself. Mediocre at best. I think Elan should have done a better job in presenting the data.
4. Company TauRx had a presentation after Elan. Their PIIb phase of a drug that inhibits tau aggregation hit statistical significance on all endpoints, in a single dose (not aggregated across all doses like in the case of Elan) and regardless of APOE4 genotype. The ADAScog improvement over placebo was almost 7 points. The best part: since the placebos declined only a little more than 7 points, it means the drug virtually halted the progression of the disease.
 I would call this truly spectacular.
I would call this truly spectacular.***********
Auch hier ein weiterer deutlicher Hinweis, dass es sich bei den BAP-Ergebnissen lediglich um Zufall handelt. Die Placebos schnitten erheblich schlechter ab, als dies zu erwarten war. Dies kann in einer kleiner Subgruppe leicht passieren... in einer großen PIII ist es dagegen eher ausgeschlossen. Außergewöhnlich schlechte Placebo-Patienten suggerieren natürlich ein positive Wirkung von BAP, die überhaupt nicht existiert! Die BAP-Subgruppe hat sich dagegen anscheinend so entwickelt wie es von unbehandelten Patienten zu erwarten wäre!

Ist TauRx eigentlich eine frei handelbare Aktiengesellschaft? Sie verfolgen ein anderes MOA als Anti-Beta-Amyloid... scheinbar bis jetzt erfolgreich (vielleicht allerdings auch nur Zahlenspielerei, ich kenne die Daten nicht)
mfg ipollit
Antwort auf Beitrag Nr.: 34.611.040 von ipollit am 30.07.08 01:38:23Zu höheren Dosierungen sollte die Wirkung zunehmen. Stattdessen nimmt anscheinend mit der Dosis nur das Risiko der sehr gefährlichen Nebenwirkungen der Gehirnschwellung zu!
...Sinn und Zweck einer p2-Studie ist u.a. auch die Optimierung von Dosis und Wirkung. Da jedes Medikament einen maximalen therapeutischen Effekt hat, ist eine derartige Nutzen-Relation nichts Aussergewöhnliches.
Aber deine erneut pseudowissenschaftliche Argumentation sollte nicht wirklich überraschen!
...Sinn und Zweck einer p2-Studie ist u.a. auch die Optimierung von Dosis und Wirkung. Da jedes Medikament einen maximalen therapeutischen Effekt hat, ist eine derartige Nutzen-Relation nichts Aussergewöhnliches.
Aber deine erneut pseudowissenschaftliche Argumentation sollte nicht wirklich überraschen!
Antwort auf Beitrag Nr.: 34.614.596 von Cyberhexe am 30.07.08 14:13:56@ Cyberhexe
BM für Dich
BM für Dich
Cyberhexe, was sagst du zu diesem Artikel ????
The Big Picture
It would seem that the street missed the big picture, IMO.
The purpose of this phase 2 trial was to 1) establish safety 2) attempt to establish dosing 3) establish signs/signals of efficacy. The trial was not large and not powered to reach statistical significance, yet that appears to be what the street has expecting.
Did Elan acomplish its goals?
There would appear to be reasonable safety (at least thus far).
They have selected dosing
There are CLEAR signals of efficacy
To the last point, let me expound
The efficacy was designed on an intent to treat. All that was needed for a patient to be included in the final efficacy tally was ONE INFUSION (out of 6 planned) and ONE efficacy measurement beyond the initial baseline. So, if a patient dropped out after only 1-2 infusions at the 6 month point of the trial, they were still included in the results. If you look at the graphs that ELAN persented, you will see that the graphs START DIVERGING only AFTER approx 1 year. Therefore patients dropping out of the trial early had a negative effect on the final results......What's the big deal?? Well we only missed stat significance by mere fractions....... one or two patients could easily have tippped the results........... and the headline news would have been different.
Let me give you an anology. Let's say I want to prove that penicillin can succesfully treat pneumonia. Now normally, the drug is given for a full ten days...... But lets say I included in my final efficacy results patients that only took the antibiotic for 1-2 days. Depending on the number of total patients and the number that didn't take the full course of antibiotic, I might not be able to prove my case.
Look at the slide in the ELAN/WYE presentation labelled COMPLETER
These included ALL patients (carrier and non-carrier) who got the full 6 doses and MOST IMPORTANT got an efficacy measurement at week 78 (18 months). There were approx. 160 of thses patients.
AND THEY HIT STATISTICAL SIGNIFICANCE on
1. ADAS-cog p=0.003 (HIGH stat significance)
2. NTB p=0.045
3. DAD p=0.041
They hit statistical significance (in a trial not powered to hit statisical significance) in 2 cognitive endpoints and one functional endpoint. The FDA requires one cognitive and one functional.
http://www1.investorvillage.com/iv1/smbd.asp?mb=160&mn=26249…
The Big Picture
It would seem that the street missed the big picture, IMO.
The purpose of this phase 2 trial was to 1) establish safety 2) attempt to establish dosing 3) establish signs/signals of efficacy. The trial was not large and not powered to reach statistical significance, yet that appears to be what the street has expecting.
Did Elan acomplish its goals?
There would appear to be reasonable safety (at least thus far).
They have selected dosing
There are CLEAR signals of efficacy
To the last point, let me expound
The efficacy was designed on an intent to treat. All that was needed for a patient to be included in the final efficacy tally was ONE INFUSION (out of 6 planned) and ONE efficacy measurement beyond the initial baseline. So, if a patient dropped out after only 1-2 infusions at the 6 month point of the trial, they were still included in the results. If you look at the graphs that ELAN persented, you will see that the graphs START DIVERGING only AFTER approx 1 year. Therefore patients dropping out of the trial early had a negative effect on the final results......What's the big deal?? Well we only missed stat significance by mere fractions....... one or two patients could easily have tippped the results........... and the headline news would have been different.
Let me give you an anology. Let's say I want to prove that penicillin can succesfully treat pneumonia. Now normally, the drug is given for a full ten days...... But lets say I included in my final efficacy results patients that only took the antibiotic for 1-2 days. Depending on the number of total patients and the number that didn't take the full course of antibiotic, I might not be able to prove my case.
Look at the slide in the ELAN/WYE presentation labelled COMPLETER
These included ALL patients (carrier and non-carrier) who got the full 6 doses and MOST IMPORTANT got an efficacy measurement at week 78 (18 months). There were approx. 160 of thses patients.
AND THEY HIT STATISTICAL SIGNIFICANCE on
1. ADAS-cog p=0.003 (HIGH stat significance)
2. NTB p=0.045
3. DAD p=0.041
They hit statistical significance (in a trial not powered to hit statisical significance) in 2 cognitive endpoints and one functional endpoint. The FDA requires one cognitive and one functional.
http://www1.investorvillage.com/iv1/smbd.asp?mb=160&mn=26249…
Antwort auf Beitrag Nr.: 34.619.915 von bernie55 am 31.07.08 08:07:25...und zu diesem:
Elanians 10121 7/30/2008 8:21:57 PM
By: liposghost
the data
my concern foremost is the big drop in placebo patients. If those on aab were comparable then the stuff works great. If they were closer to average then it don't work so great.
If we assume the declines would have been the declines would have been unequal, then there is no doubt the stuff works. But it is weird how the dose relationships dance around. I haven't figured out what the NTB is supposed to show with its greater sensitivity.
I have now been 1st runner up for the Post Traumatic Stress Syndrome poster three times, it is painful.
Elanians 10121 7/30/2008 8:21:57 PM
By: liposghost
the data
my concern foremost is the big drop in placebo patients. If those on aab were comparable then the stuff works great. If they were closer to average then it don't work so great.
If we assume the declines would have been the declines would have been unequal, then there is no doubt the stuff works. But it is weird how the dose relationships dance around. I haven't figured out what the NTB is supposed to show with its greater sensitivity.
I have now been 1st runner up for the Post Traumatic Stress Syndrome poster three times, it is painful.
Antwort auf Beitrag Nr.: 34.619.915 von bernie55 am 31.07.08 08:07:25"There would appear to be reasonable safety (at least thus far)"
warum auch immer, bei den BAP-Patienten gab es immerhin 3 Todesfälle, bei den Placebos 0. Bei den BAP-Patienten gab es 12 Gehirnschwellungen (die sehr gefährlich werden können), bei den Placebos 0. Wenn BAP bei z.B. bei 10 Mio leicht bis mittelschweren Alzheimerfällen (darauf ist es ja ausgelegt), zum Einsatz kommen soll und dabei z.B. 1% gravierende lebensbedrohliche Nebenwirkungen auftreten (in der PII waren es sogar etwa 10% Gehirnschwellungen) und 0,1% Todesfälle als Folge, dann wären das 10.000 Tote durch BAP jährlich und 100.000 Fälle, bei denen es für die Patienten sehr kritisch wird.
Wenn die Nebenwirkungen so unproblematisch wären, warum testet Elan dann BAP in der PIII nicht mehr in der höheren Dosierung, die mit einem höheren Risiko von Gehirnschwellungen verbunden zu sein scheint.
Die anderen Nebenwirkungen waren bei Placebos und BAP etwa gleich. Seltenere Nebenwirkungen kann man aber ohnehin nur bei einer großen PIII oder PIV finden... siehe Vioxx.
****
"They have selected dosing"
ich dachte in der PIII werden wieder mehrere Dosierungen getestet, oder? - Zumindest gibt es nach den PII-Daten keine optimale Dosierung oder könntest du sie mir nennen? Die Ergebnisse lassen keinen Zusammenhang zwischen Dosis und Wirkung erkennen, ein Wahl wäre da mehr zufällig.
****
"There are CLEAR signals of efficacy"
ich finde, es gibt weitaus klarere Signale für eine Unwirksamkeit... letztlich ist die PII im primären Endpunkt gescheitert und die Subgruppenanalyse nach den vorliegenden Daten sehr wahrscheinlich verzerrt wurden (dafür gibt es klare Hinweise).
********
Wurde für die "completer"-Analyse das im Studienprotokoll vordefinierte statistische Verfahren angewendet oder wurde dieses nachträglich geändert bzw. "adjustiert"?
mfg ipollit
warum auch immer, bei den BAP-Patienten gab es immerhin 3 Todesfälle, bei den Placebos 0. Bei den BAP-Patienten gab es 12 Gehirnschwellungen (die sehr gefährlich werden können), bei den Placebos 0. Wenn BAP bei z.B. bei 10 Mio leicht bis mittelschweren Alzheimerfällen (darauf ist es ja ausgelegt), zum Einsatz kommen soll und dabei z.B. 1% gravierende lebensbedrohliche Nebenwirkungen auftreten (in der PII waren es sogar etwa 10% Gehirnschwellungen) und 0,1% Todesfälle als Folge, dann wären das 10.000 Tote durch BAP jährlich und 100.000 Fälle, bei denen es für die Patienten sehr kritisch wird.
Wenn die Nebenwirkungen so unproblematisch wären, warum testet Elan dann BAP in der PIII nicht mehr in der höheren Dosierung, die mit einem höheren Risiko von Gehirnschwellungen verbunden zu sein scheint.
Die anderen Nebenwirkungen waren bei Placebos und BAP etwa gleich. Seltenere Nebenwirkungen kann man aber ohnehin nur bei einer großen PIII oder PIV finden... siehe Vioxx.
****
"They have selected dosing"
ich dachte in der PIII werden wieder mehrere Dosierungen getestet, oder? - Zumindest gibt es nach den PII-Daten keine optimale Dosierung oder könntest du sie mir nennen? Die Ergebnisse lassen keinen Zusammenhang zwischen Dosis und Wirkung erkennen, ein Wahl wäre da mehr zufällig.
****
"There are CLEAR signals of efficacy"
ich finde, es gibt weitaus klarere Signale für eine Unwirksamkeit... letztlich ist die PII im primären Endpunkt gescheitert und die Subgruppenanalyse nach den vorliegenden Daten sehr wahrscheinlich verzerrt wurden (dafür gibt es klare Hinweise).
********
Wurde für die "completer"-Analyse das im Studienprotokoll vordefinierte statistische Verfahren angewendet oder wurde dieses nachträglich geändert bzw. "adjustiert"?
mfg ipollit
@ Cyberhexe
BM für Dich !!!
BM für Dich !!!
2 PML-Fälle in Europa!
Antwort auf Beitrag Nr.: 34.627.872 von Birgit.Tersteegen am 01.08.08 01:09:36Biogen, Elan Report Cases of Brain Disease
By Keith Winstein and John Hechinger
Two multiple-sclerosis patients treated with Biogen Idec Inc.'s drug Tysabri contracted a potentially deadly brain infection, casting a cloud over the revival of the medicine, already withdrawn once over safety concerns.
Biogen said it had no plans to recall the drug again or restrict its use. The company and its marketing partner, Ireland's Elan Corp., said that the two patients who contracted the ailment, progressive multifocal leukoencephalopathy, or PML, are alive. Both patients live in Europe. One is ambulatory and at home and the other has been hospitalized.
About 31,800 people take Tysabri, which is also used to treat Crohn's ...
http://online.wsj.com/article/SB121754121795502217.html
By Keith Winstein and John Hechinger
Two multiple-sclerosis patients treated with Biogen Idec Inc.'s drug Tysabri contracted a potentially deadly brain infection, casting a cloud over the revival of the medicine, already withdrawn once over safety concerns.
Biogen said it had no plans to recall the drug again or restrict its use. The company and its marketing partner, Ireland's Elan Corp., said that the two patients who contracted the ailment, progressive multifocal leukoencephalopathy, or PML, are alive. Both patients live in Europe. One is ambulatory and at home and the other has been hospitalized.
About 31,800 people take Tysabri, which is also used to treat Crohn's ...
http://online.wsj.com/article/SB121754121795502217.html
jo und ausserdem habe man gesagt man könne es nie ausschließen, dass so ein fall nie mehr wieder auftritt.
wenn man sich überlegt welchen nutzen tysabri hat und wie viele fatale ausgänge ist der kursverfall nicht berechtigt!
man kann tysabri trotz dieser fälle nicht ablehnen.
das risikoverfahren läuft gut und die patienten sind am leben.
wenn man die funktion des immunsystems wiederherstellen kann ist die krankheit durchaus behandelbar.
und dafür steht das riskmanagement von elan.
hoffe ihr habe ein starkes herz!
scheiß aktien!
wenn man sich überlegt welchen nutzen tysabri hat und wie viele fatale ausgänge ist der kursverfall nicht berechtigt!
man kann tysabri trotz dieser fälle nicht ablehnen.
das risikoverfahren läuft gut und die patienten sind am leben.
wenn man die funktion des immunsystems wiederherstellen kann ist die krankheit durchaus behandelbar.
und dafür steht das riskmanagement von elan.
hoffe ihr habe ein starkes herz!
scheiß aktien!
Antwort auf Beitrag Nr.: 34.628.593 von welke91 am 01.08.08 09:22:08Meine Frau hat Multiple Sklerose, bekam aber kein Tysabri, weil ihr Krankheitsverlauf angeblich nicht schwer genug (viele Schübe) war. Da sie aber meines Erachtens aber einen relativ schweren Verlauf hat, kann ich mir vorstellen, dass die meisten Tysabri-Patienten notwendig darauf angewiesen sind.
Und meiner Ansicht nach ist ein Absetzen praktisch unmöglich.
Jede Umstellung auf andere Medikamente führt bei MS-Patienten oft zu Rückschlägen.
So sehe ich das, ist mein persönlicher Eindruck, keine Auskunft eines Fachmannes.
Und meiner Ansicht nach ist ein Absetzen praktisch unmöglich.
Jede Umstellung auf andere Medikamente führt bei MS-Patienten oft zu Rückschlägen.
So sehe ich das, ist mein persönlicher Eindruck, keine Auskunft eines Fachmannes.
man war das ne harte woche. erlebt man auch nicht alle tage.
der kursrutsch auf das niveau ist meiner ansicht nach völlig übertrieben.
das nächste wichtigste ereignis werden die nächsten q-zahlen sein.
momentan berträgt das ty-wachstum ca 450 patienten pro woche. Denke das wird sich nach diesen neuen fällen etwas verringern. Schätze mal 350-400.
Was ich nicht verstehe, dass neue pml fälle mit so einem medien-hype veröffentlich werden. Bei anderen medis wird nicht so ein hype gemacht (zb. rituxan). dadurch werden vor allen dingen patienten verunsichert, die gleich wieder an killerdrogen denken. vor allen dingen ist pml inzwischen nicht mehr so schlimm, da heilbar.
wenn man sich mal die ms-foren durchliest, ist es echt erschreckend wie wenig ahnung ms-patienten von ihrer krankheit haben.
bei BAP hat KM den fehler gemacht die erwartungen zu hoch zu schrauben. ich fand die BAP phaseII-daten ziemlich mager und habe diese mit den phase II-daten von flurizan verglichen, welches in phase III eingestellt wurde.
die elan-daten sind vergleichbar mit den flurizan daten, insbesondere die effektivrate bei adas-cog. Hätte hier erwartet, dass BAP eine effektivität von >80% zeigt.
leider hat elan dazu keine zahlen angegeben, aber aufgrund der graphen lässt sich eine effektivität von 50 % abschätzen. Irgendwie hatte ich das gefühl, dass daten bewusst verschwiegen wurden.
Meine kritikpunkte an der darstellung der phaseII-daten sind folgende
1) ungewöhnlich starker abfall der plazebopatienten bei adas-cog um 11 punkte, dadurch lässt sich keine aussage über die wirksamkeit machen. durch die kleinen Subgruppen (35-45 personen) lässt sich ein, so stark schwankender Test wie ADAS-cog nicht beurteilen. dazu sind subgruppen von mindestens 100 personen notwendig.
http://www.alzforum.org/new/detail.asp?id=1884
der starke abfall in der plazebogruppe wurde dadurch erklärt, dass vor allem patienten mit moderatem alzheimer an der studie teilnahmen (normal ist mild bis moderat)
2) Interessant wäre die plazebo und BAP abfallsrate in der ApoE4 carrier gruppe gewesen. Diese wurde leider nicht gezeigt.
3) Verschleierungstaktik durch angabe der p-werte. Keine genaue angebe der rohdaten.
Nix desto trotz lässt sich ein positiver trend in der studie erkennen.
1) NTB zeigt hohe signifikanz bei den non carriern. NTB ist ein cognitiver test der bei weitem nicht so stark schwankt wie der standard-adas-test. Dadurch werden auch kleiner gruppen besser auswertbar. Vermute aber, dass dieser test nicht von der fda akzeptiert wird (im gegensatz zum adas-cog test).
2) Die studie lief über 78 wochen. Dadurch werden unterschiede besser erkennbar (auch bei adas-cog)
Denke BAP hat eine kleine Chance die Phase III zu überleben.
Habe leider nicht mehr den link zur präsentation. vielleicht kann ihn ja jemand reinstellen.
Über die hochgebauschten Nebenwirkungen würde ich mir keine sorgen machen. wenn die wirksamkeit in phase III gezeigt wird und gehirnschwellungen die einzigen ernstzunehmenden nebenwirkungen sind, wird das zeug zugelassen.
sehr interssant war das statement von einem schweizer mediziner (glaube christoph hock, er hatte mal bei 3sat ein interview gegeben), der die AN1792-Studie betreut hat (die studie wurde eingestellt da einige patienten gehirnhautentzündung entwickelt haben). er sagt, dass er noch nie so eine stabilisierung von alz- patienten gesehen hat. wenn er vor die wahl gestellt würde alzheimer oder gehirnhautentzündung, so würde er das risiko einer gehirnhautenzündung für sich in kauf nehmen.
wenn ich die wahl hätte, würde ich es auch tun!!!
da sind doch gehirnschwellungen ein witz dagegen.
Ipollit
ich denke schon das b-amyloid das richtige target ist. Es deutet immer mehr darauf hin, dass lösliches oligomeres b-amyloid der auslöser für alzheimer ist. Es gibt auch einige hinweise darauf, dass b-amyloid für die entstehung von tau verantwortlich ist.
wenn dich das thema wirklich interessiert, würde ich die die lektüre von nature medicine july 2006 empfehlen. Dort gibt es jede menge guter übersichtartikel.
der kursrutsch auf das niveau ist meiner ansicht nach völlig übertrieben.
das nächste wichtigste ereignis werden die nächsten q-zahlen sein.
momentan berträgt das ty-wachstum ca 450 patienten pro woche. Denke das wird sich nach diesen neuen fällen etwas verringern. Schätze mal 350-400.
Was ich nicht verstehe, dass neue pml fälle mit so einem medien-hype veröffentlich werden. Bei anderen medis wird nicht so ein hype gemacht (zb. rituxan). dadurch werden vor allen dingen patienten verunsichert, die gleich wieder an killerdrogen denken. vor allen dingen ist pml inzwischen nicht mehr so schlimm, da heilbar.
wenn man sich mal die ms-foren durchliest, ist es echt erschreckend wie wenig ahnung ms-patienten von ihrer krankheit haben.
bei BAP hat KM den fehler gemacht die erwartungen zu hoch zu schrauben. ich fand die BAP phaseII-daten ziemlich mager und habe diese mit den phase II-daten von flurizan verglichen, welches in phase III eingestellt wurde.
die elan-daten sind vergleichbar mit den flurizan daten, insbesondere die effektivrate bei adas-cog. Hätte hier erwartet, dass BAP eine effektivität von >80% zeigt.
leider hat elan dazu keine zahlen angegeben, aber aufgrund der graphen lässt sich eine effektivität von 50 % abschätzen. Irgendwie hatte ich das gefühl, dass daten bewusst verschwiegen wurden.
Meine kritikpunkte an der darstellung der phaseII-daten sind folgende
1) ungewöhnlich starker abfall der plazebopatienten bei adas-cog um 11 punkte, dadurch lässt sich keine aussage über die wirksamkeit machen. durch die kleinen Subgruppen (35-45 personen) lässt sich ein, so stark schwankender Test wie ADAS-cog nicht beurteilen. dazu sind subgruppen von mindestens 100 personen notwendig.
http://www.alzforum.org/new/detail.asp?id=1884
der starke abfall in der plazebogruppe wurde dadurch erklärt, dass vor allem patienten mit moderatem alzheimer an der studie teilnahmen (normal ist mild bis moderat)
2) Interessant wäre die plazebo und BAP abfallsrate in der ApoE4 carrier gruppe gewesen. Diese wurde leider nicht gezeigt.
3) Verschleierungstaktik durch angabe der p-werte. Keine genaue angebe der rohdaten.
Nix desto trotz lässt sich ein positiver trend in der studie erkennen.
1) NTB zeigt hohe signifikanz bei den non carriern. NTB ist ein cognitiver test der bei weitem nicht so stark schwankt wie der standard-adas-test. Dadurch werden auch kleiner gruppen besser auswertbar. Vermute aber, dass dieser test nicht von der fda akzeptiert wird (im gegensatz zum adas-cog test).
2) Die studie lief über 78 wochen. Dadurch werden unterschiede besser erkennbar (auch bei adas-cog)
Denke BAP hat eine kleine Chance die Phase III zu überleben.
Habe leider nicht mehr den link zur präsentation. vielleicht kann ihn ja jemand reinstellen.
Über die hochgebauschten Nebenwirkungen würde ich mir keine sorgen machen. wenn die wirksamkeit in phase III gezeigt wird und gehirnschwellungen die einzigen ernstzunehmenden nebenwirkungen sind, wird das zeug zugelassen.
sehr interssant war das statement von einem schweizer mediziner (glaube christoph hock, er hatte mal bei 3sat ein interview gegeben), der die AN1792-Studie betreut hat (die studie wurde eingestellt da einige patienten gehirnhautentzündung entwickelt haben). er sagt, dass er noch nie so eine stabilisierung von alz- patienten gesehen hat. wenn er vor die wahl gestellt würde alzheimer oder gehirnhautentzündung, so würde er das risiko einer gehirnhautenzündung für sich in kauf nehmen.
wenn ich die wahl hätte, würde ich es auch tun!!!
da sind doch gehirnschwellungen ein witz dagegen.
Ipollit
ich denke schon das b-amyloid das richtige target ist. Es deutet immer mehr darauf hin, dass lösliches oligomeres b-amyloid der auslöser für alzheimer ist. Es gibt auch einige hinweise darauf, dass b-amyloid für die entstehung von tau verantwortlich ist.
wenn dich das thema wirklich interessiert, würde ich die die lektüre von nature medicine july 2006 empfehlen. Dort gibt es jede menge guter übersichtartikel.
...was ich noch sehr interessant finde ist die absicht von elan die forschung in eine eigene firma auszugliedern.
dadurch würde elan auf dem papier schnell aus den roten zahlen kommen und sich für eine übernahme attraktiv machen.
das übertrieben schön reden der pipeline, würde damit auch zusammenpassen.
vielleicht ist ja diesbezüglich schon etwas im busch ...
dadurch würde elan auf dem papier schnell aus den roten zahlen kommen und sich für eine übernahme attraktiv machen.
das übertrieben schön reden der pipeline, würde damit auch zusammenpassen.
vielleicht ist ja diesbezüglich schon etwas im busch ...
Antwort auf Beitrag Nr.: 34.638.413 von 2CB_06 am 03.08.08 15:16:20schön, einige gute Punkte... ich sehe es nicht soviel anders. Hinzu kommt allerdings noch die retrospektive Analyse und das data mining: Elan hatte die ApoE4-Gruppe vorher nicht spezifiziert, sie ist nicht randomisiert, unausgewogen (z.B. sind bei ohne-ApoE4 die Placebo-Patienten im Schnitt ein halbes Jahr länger krank und haben einen 1,5 Punkte niedrigeren MMSE zu Beginn der Studie) und klein. Zudem wurden die statistischen Methoden verändert, soweit ich das verstanden habe.
"sehr interssant war das statement von einem schweizer mediziner (glaube christoph hock, er hatte mal bei 3sat ein interview gegeben), der die AN1792-Studie betreut hat (die studie wurde eingestellt da einige patienten gehirnhautentzündung entwickelt haben). er sagt, dass er noch nie so eine stabilisierung von alz- patienten gesehen hat."
gut, es gibt aber auch Aussagen von mindestens 2 Spezialisten, die bei der Durchführung und Auswertung der PII von AN1792 maßgeblich beteiligt waren. Sie haben nach Auswertung der Langzeitdaten und durch Autopsie festgestellt, dass bei den Respondern zwar das Plaque teilweise vollständig entfernt werden konnte, aber dies keinen Einfluß auf den Verlauf der Krankheit hatte. Müsste man nicht wenigstens einen gewissen Hinweis auf eine Wirksamkeit oder Verbesserung der Krankheit finden, wenn ein Medikament erfolgreich den Plaque auflöst? Obwohl viele hundert Mio aufgrund dieser Theorie bis heute investiert worden sind, gibt es trotzdem keinen einigermaßen sicheren Beleg dafür, dass sie überhaupt richtig ist. Ist das nicht seltsam? Soweit ich das sehe, mehren sich immer stärker die Zweifel an dieser Theorie.
von einer Stabilisierung der Patienten kann in der PII ja auch keine Rede sein, oder? Hier geht es höchstens um ein langsameres Fortschreiten von Alzheimer.
mfg ipollit
"sehr interssant war das statement von einem schweizer mediziner (glaube christoph hock, er hatte mal bei 3sat ein interview gegeben), der die AN1792-Studie betreut hat (die studie wurde eingestellt da einige patienten gehirnhautentzündung entwickelt haben). er sagt, dass er noch nie so eine stabilisierung von alz- patienten gesehen hat."
gut, es gibt aber auch Aussagen von mindestens 2 Spezialisten, die bei der Durchführung und Auswertung der PII von AN1792 maßgeblich beteiligt waren. Sie haben nach Auswertung der Langzeitdaten und durch Autopsie festgestellt, dass bei den Respondern zwar das Plaque teilweise vollständig entfernt werden konnte, aber dies keinen Einfluß auf den Verlauf der Krankheit hatte. Müsste man nicht wenigstens einen gewissen Hinweis auf eine Wirksamkeit oder Verbesserung der Krankheit finden, wenn ein Medikament erfolgreich den Plaque auflöst? Obwohl viele hundert Mio aufgrund dieser Theorie bis heute investiert worden sind, gibt es trotzdem keinen einigermaßen sicheren Beleg dafür, dass sie überhaupt richtig ist. Ist das nicht seltsam? Soweit ich das sehe, mehren sich immer stärker die Zweifel an dieser Theorie.
von einer Stabilisierung der Patienten kann in der PII ja auch keine Rede sein, oder? Hier geht es höchstens um ein langsameres Fortschreiten von Alzheimer.
mfg ipollit
... die wirksamkeit von AN1792 kann ich nicht beurteilen. dadurch das bei einer impfung polyklonale ak entstehen, kann die wirkung von patient zu patient verschieden sein (bei dem einen sind die ak's mehr plaque gerichtet und bei dem andern mehr gegen das oligomer). denke er hat die wirksamkeit auch nicht bei allen patienten gesehen. manche haben eben gut auf den impfstoff reagiert.
egal, was ich damit sagen wollte war, daß man bei alzheimer bereit ist ein höheres nebenwirkungsrisiko zu tragen.
würdest du nicht auch ein höheres risiko eingehen, wenn du von alzheimer betroffen wärst????
egal, was ich damit sagen wollte war, daß man bei alzheimer bereit ist ein höheres nebenwirkungsrisiko zu tragen.
würdest du nicht auch ein höheres risiko eingehen, wenn du von alzheimer betroffen wärst????
Antwort auf Beitrag Nr.: 34.638.560 von 2CB_06 am 03.08.08 16:16:28prinzipiell hast du recht bezügl der Nebenwirkungen. Ähnlich sehe ich das auch bei Tysabri und PML (wobei PML sicherlich keine Kleinigkeit ist, die sich gut behandeln lässt, wie andere hier schon meinten). Allerdings traten in der PII die Hirnschwellungen wohl im Schnitt bei ca. 10% BAP auf, wenn ich die Zahl noch richtig im Kopf habe. Zudem gabe es bei den ca. 124 BAP-Patienten auch 3 Todesfälle, die allerdings nicht mit BAP in Zusammenhang gebracht wurden. Naja wer weiß, wie sich das weiter entwickelt. Nur als Beispiel: ob ich mich bei einer leichten Alzheimererkrankung bereits dem Risiko aussetzen würde, mit 1:10 eine Gehirnschwellung zu erleiden, die mein Gehirn ebenfalls irreversibel schädigen oder mich gar töten kann bzw. mit 1:100 daran zu sterben, um die Chance auf einen etwas langsameren Krankheitsverlauf zu erhalten, weiß ich nicht. Natürlich ist es immer ein Abwägen.
Das Entscheidende bei BAP ist allerdings die Wirkung. Die sollte erstmal klar belegt werden!
mfg ipollit
Das Entscheidende bei BAP ist allerdings die Wirkung. Die sollte erstmal klar belegt werden!
mfg ipollit
Etwas zu den AN1792-Studien
The paper by Holmes et al. examines pathology and cognition of eight patients from the AN1792 Aβ vaccination trial. Despite the suspension of this trial in 2002, the patients continued to be followed clinically. Two patients showed almost complete removal of amyloid in the brain. The important finding of the current report is that cognitive decline was identical to placebo-treated patients despite the pronounced removal of amyloid. While these data contrast with the many mouse studies showing cognitive improvement and indeed suggest a more limited role for Aβ in the progression of Alzheimer disease, extensive speculation from such a small cohort should be avoided. In contrast to the current report, the 2003 report from Hock et al. showed slowed cognitive decline in a group of 30 patients over a year following treatment; however, this was correlated with a modified antibody titer; the TAPIR assay (tissue amyloid plaque immunoreactivity; the ability of circulating antibodies to bind to amyloid plaques on tissue) (Hock et al., 2003). In the current study the authors [...continued] suggest several scenarios for the lack of clinical efficacy: 1) amyloid plaques initiate but do not maintain progressive neurodegeneration, 2) very slow plaque removal, 3) inability to remove oligomeric Aβ, and 4) overactivation of the innate immune system.
An important effect of immunization that has not been reported on in the current study is cerebral amyloid angiopathy (CAA) and microhemorrhage. It has been shown that passive immunotherapy increases CAA in transgenic mice (Wilcock et al., 2004) and causes increased incidence of microhemorrhage (Pfeifer et al., 2001, Wilcock et al., 2004, Racke et al., 2005). We also reported that these adverse events occurred with active vaccination (Wilcock et al., 2007). Indeed, the authors of the current report use CAA and Aβ accumulation around capillaries as histopathological factors used to determine the degree of amyloid clearance. It also seems the microhemorrhage occurrence will be difficult to overcome. The recent report from Schroeter et al. showed that even low doses of antibody, which were associated with essentially no amyloid removal, resulted in an apparent subtle increase in microhemorrhage (Schroeter et al., 2008; control mice had no animals with microhemorrhage rated 2 or 3 while the lowest dose of 3D6 had three mice rated 2 or 3). Accumulation of CAA and associated microhemorrhage likely contributes significantly to the clinical progression of disease. Additionally, as the authors suggest, a change in inflammatory state could certainly contribute to further cognitive decline. Recent data show that the inflammatory profile of Alzheimer’s and transgenic mouse brain is highly complex (Colton et al., 2006). It is likely that Fcγ receptor activation affects the inflammatory state.
These data highlight the significant differences between human and mouse studies. Since neurodegeneration is not abundant in the majority of mouse models, it has not been possible, to date, to study this. It is likely that while amyloid may initiate the cascade, neurodegeneration may be self-perpetuating and neuroprotection may also be critical for successful anti-amyloid therapeutics. It has been suggested that passive immunization will overcome some of the limitations of active vaccination, and we certainly eagerly anticipate the data from Elan’s passive immunization trial of bapineuzumab.
References:
Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006 Sep 27;3:27. Abstract
Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron 2003 May 22;38(4):547-554. Abstract
Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science 2002 Nov 15;298 (5597):299. Abstract
Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci 2005 Jan 19;25(3):629-636. Abstract
Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, Games D. Immunotherapy reduces vascular amyloid-β in PDAPP mice. J Neurosci 2008 Jul 2; 28(27): 6787-6793. Abstract
Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation 2004 Dec 8;1(1):24. Abstract
Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience. 2007 Feb 9;144(3):950-60. Abstrac
The paper by Holmes et al. examines pathology and cognition of eight patients from the AN1792 Aβ vaccination trial. Despite the suspension of this trial in 2002, the patients continued to be followed clinically. Two patients showed almost complete removal of amyloid in the brain. The important finding of the current report is that cognitive decline was identical to placebo-treated patients despite the pronounced removal of amyloid. While these data contrast with the many mouse studies showing cognitive improvement and indeed suggest a more limited role for Aβ in the progression of Alzheimer disease, extensive speculation from such a small cohort should be avoided. In contrast to the current report, the 2003 report from Hock et al. showed slowed cognitive decline in a group of 30 patients over a year following treatment; however, this was correlated with a modified antibody titer; the TAPIR assay (tissue amyloid plaque immunoreactivity; the ability of circulating antibodies to bind to amyloid plaques on tissue) (Hock et al., 2003). In the current study the authors [...continued] suggest several scenarios for the lack of clinical efficacy: 1) amyloid plaques initiate but do not maintain progressive neurodegeneration, 2) very slow plaque removal, 3) inability to remove oligomeric Aβ, and 4) overactivation of the innate immune system.
An important effect of immunization that has not been reported on in the current study is cerebral amyloid angiopathy (CAA) and microhemorrhage. It has been shown that passive immunotherapy increases CAA in transgenic mice (Wilcock et al., 2004) and causes increased incidence of microhemorrhage (Pfeifer et al., 2001, Wilcock et al., 2004, Racke et al., 2005). We also reported that these adverse events occurred with active vaccination (Wilcock et al., 2007). Indeed, the authors of the current report use CAA and Aβ accumulation around capillaries as histopathological factors used to determine the degree of amyloid clearance. It also seems the microhemorrhage occurrence will be difficult to overcome. The recent report from Schroeter et al. showed that even low doses of antibody, which were associated with essentially no amyloid removal, resulted in an apparent subtle increase in microhemorrhage (Schroeter et al., 2008; control mice had no animals with microhemorrhage rated 2 or 3 while the lowest dose of 3D6 had three mice rated 2 or 3). Accumulation of CAA and associated microhemorrhage likely contributes significantly to the clinical progression of disease. Additionally, as the authors suggest, a change in inflammatory state could certainly contribute to further cognitive decline. Recent data show that the inflammatory profile of Alzheimer’s and transgenic mouse brain is highly complex (Colton et al., 2006). It is likely that Fcγ receptor activation affects the inflammatory state.
These data highlight the significant differences between human and mouse studies. Since neurodegeneration is not abundant in the majority of mouse models, it has not been possible, to date, to study this. It is likely that while amyloid may initiate the cascade, neurodegeneration may be self-perpetuating and neuroprotection may also be critical for successful anti-amyloid therapeutics. It has been suggested that passive immunization will overcome some of the limitations of active vaccination, and we certainly eagerly anticipate the data from Elan’s passive immunization trial of bapineuzumab.
References:
Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006 Sep 27;3:27. Abstract
Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Müller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron 2003 May 22;38(4):547-554. Abstract
Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science 2002 Nov 15;298 (5597):299. Abstract
Racke MM, Boone LI, Hepburn DL, Parsadainian M, Bryan MT, Ness DK, Piroozi KS, Jordan WH, Brown DD, Hoffman WP, Holtzman DM, Bales KR, Gitter BD, May PC, Paul SM, DeMattos RB. Exacerbation of cerebral amyloid angiopathy-associated microhemorrhage in amyloid precursor protein transgenic mice by immunotherapy is dependent on antibody recognition of deposited forms of amyloid beta. J Neurosci 2005 Jan 19;25(3):629-636. Abstract
Schroeter S, Khan K, Barbour R, Doan M, Chen M, Guido T, Gill D, Basi G, Schenk D, Seubert P, Games D. Immunotherapy reduces vascular amyloid-β in PDAPP mice. J Neurosci 2008 Jul 2; 28(27): 6787-6793. Abstract
Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation 2004 Dec 8;1(1):24. Abstract
Wilcock DM, Jantzen PT, Li Q, Morgan D, Gordon MN. Amyloid-beta vaccination, but not nitro-nonsteroidal anti-inflammatory drug treatment, increases vascular amyloid and microhemorrhage while both reduce parenchymal amyloid. Neuroscience. 2007 Feb 9;144(3):950-60. Abstrac
Bericht zu BAP-Phase II Daten
http://www.alzforum.org/new/detail.asp?id=1894
Chicago: Bapineuzumab’s Phase 2—Was the Data Better Than the Spin?
11 August 2008. It is tempting to say that the story of how Elan/Wyeth’s antibody therapy fared at the 11th International Conference on Alzheimer’s Disease, held last month in Chicago, is one of the stranger tales in present-day AD drug development. (Then, again, weren’t all the recent ones a little strange? Take Alzhemed’s inglorious demise and subsequent resurrection as a nutraceutical, or Flurizan’s sudden death just weeks after its sponsor had announced a European commercialization agreement.)
Bapineuzumab’s story is different, of course. This humanized monoclonal anti-Aβ antibody is very much alive as an experimental treatment. Four separate Phase 3 trials in the U.S., Canada, and Europe are presently enrolling up to 4,100 Alzheimer disease patients. Elan/Wyeth are running an open-label extension of the present Phase 2 trial and are gearing up to test subcutaneous bapineuzumab delivery in a Phase 2 trial. But like the previous two anti-amyloid drugs, bapineuzumab rode to ICAD on high expectations, and its luster has dimmed somewhat after the company’s presentation there. This is not only because investors promptly dumped Elan stock but also because scientists felt that the presentation could have been more straightforward.
What happened? The company presented an overall negative result balanced against a positive effect on a subgroup of patients who don’t carry the AD risk allele ApoE4. Many listeners did not buy it but interpreted the presentation as having massaged the data. In conversations with scientists, most of whom spoke privately, reactions ranged from “they stratified until they got the result they wanted” to “the effect on the ApoE4 negatives makes sense; it’s reassuring,” to “told you so, anti-amyloid drugs lead nowhere” to “I am cautiously optimistic.” Several agreed, however, that the way in which Elan/Wyeth sought to portray the Phase 2 data in the best possible light backfired. In essence, emphasizing a post-hoc pharmacogenomic interpretation shortchanged the trial’s overall decent results, they said. Here’s the lowdown:
At ICAD, Sid Gilman of the University of Michigan, Ann Arbor, presented a more detailed version of the previous top-line data Elan had released on June 16 (see ARF related news story). In this talk, Gilman said that the trial had randomized 234 patients to receive six infusions, one every 13 weeks, of one of four different treatment doses or placebo. He reported that 26 percent of patients dropped out of the treatment groups versus 21 percent in the placebo group.
As a Phase 2, this was first and foremost a safety trial. The safety data Gilman showed received less attention than the efficacy data summarized below, but several scientists noted afterward that some of the reported side effects might bear watching. The most widely discussed side effect is vasogenic edema. Gilman showed that 12 patients developed this, all in the treatment group. Ten occurred in ApoE4 carriers and two in non-carriers, though the trial had twice as many people with ApoE4 than without, meaning that this pharmacogenomic distinction works out to roughly 10/4 rather than 10/2 in this trial. The edemas showed up on MRI and all resolved. Most caused few clinical symptoms, but one patient needed steroid treatment. Six people dropped out of the study; the other six resumed treatment. It is unclear at present how troublesome these edemas really are, and whether they may eventually be deemed an acceptable side effect for treating a deadly disease such as Alzheimer’s. This question generated animated discussion at ICAD and is the subject of ongoing research. Some scientists felt that vasogenic edemas may reflect the underlying mechanism of the drug, as Aβ peptide gets released from plaques, protein concentrations in the interstitial space and in capillary beds rise, osmotic pressure rises along with that, and clearance mechanisms, perhaps some involving ApoE, gradually dispose of the surplus protein. Others doubted whether such a therapy would get the FDA’s blessing.
Concern over vasogenic edema overshadowed the fact that Gilman’s presentation included additional side effects that were more common in the treated patients. The list of unwanted effects that occurred twice as often as in placebo, and in more than 5 percent of patients on active drug, included back pain, anxiety, vomiting, hypertension, weight loss, paranoia, skin lacerations, gait disturbance, and muscle spasms. Syncope and cataracts, among some other side effects, occurred in fewer than 5 percent of patients on active drug. Adverse events were generally mild or moderate and transient, Gilman said. Three men in treatment groups died. According to Gilman, one died of an aortic aneurysm, one succumbed to a fall and Alzheimer’s, and one to infection and Alzheimer’s. None of these deaths was attributed to study drug.
Moving on to efficacy signals, Gilman then briefly noted that the companies had decided to change the originally planned intent-to-treat analysis away from a slope analysis, which shows how the patients do at every time point, to a different analysis he called “MITT repeated measures model without assumption of linearity.” This mouthful essentially compares baseline to the final assessment at 78 weeks/19.5 months and incorporates the time points in between in ways that are not directly shown. That left many in the audience puzzled. To some, the decision to show the data this way reflects a biological argument that a plaque-clearing antibody, unlike a conventional drug, cannot be expected to show a linear effect over time as it might make a patient temporarily worse before it makes him/her better in the long run. To others, this change in the statistical model to a complex one they don’t fully understand implied that Elan/Wyeth might not want to show outcome data over all seven time points because those data looked bad. These scientists would have welcomed trend lines, i.e., graphs plotting the slope of outcome measures along the time axis, because that is how they have come to expect clinical trial data to be shown.
Gilman then showed efficacy data according to the modified analysis. In the total population, the trial posted a 2.3-point improvement on the ADAS-Cog, with a p value of 0.078. On the NTB battery, the improvement was 0.13 with a p value of 0.068. Thus, the trial failed to achieve statistical significance on pre-specified endpoints. Gilman stated this clearly and then moved right on to present a pharmacogenomic stratification of the data. But wait, not so fast! Commentators noted that this overall finding is important. “This is a respectable efficacy signal for a Phase 2 trial. It is a consistent effect, the trial is powered to detect it, and you cannot expect a larger effect from a trial of 230 patients,” Lon Schneider of the University of Southern California told this reporter. Other scientists agreed with this assessment.
In post-hoc analysis, Elan/Wyeth split the patients into those who received all six injections, i.e., the “completers,” versus those who missed one or more doses. Analyzed in this way, the patients who completed the trial (N = 78, ApoE4 carriers and non-carriers combined), showed a 4.3-point improvement over placebo on the ADAS-Cog with a p value of 0.003. “My gut feeling is that [bapineuzumab] is probably modestly effective. But my other gut feeling is that they are chasing their tail by emphasizing efficacy in the E4 non-carriers,” said Schneider.
What, then, is this pharmacogenomic result? Prior research suggests biologic differences between ApoE4 carriers and non-carriers, which might be expected to affect the course of an amyloid-removing therapeutic approach. ApoE4 carriers tend to get the disease earlier than people with ApoE3 or the rare allele ApoE2, and they develop more amyloid in their brain (Rowe et al., 2007). Numerous studies have implicated ApoE in amyloid deposition and removal, and a recent one showed that ApoE4 is less effective at clearing Aβ than the other two alleles (Jiang et al., 2008). Hence, Elan/Wyeth split trial participants into ApoE4 carriers and non-carriers, and then analyzed these groups separately. This created some imbalance between the groups because two-thirds of the patients in the trial happened to have an ApoE4 allele. At 78 weeks, the ApoE4 carriers showed a respectable 4.5-point improvement in ADAS-Cog on the lowest dose but a worsening at the highest dose. Results were inconsistent across the other outcome measures (DAD, NTB, CDR-SB). Some 70 ApoE carriers were in the four treatment groups combined. Of these ApoE4 carriers, a total of 42 across all treatment groups completed the study, i.e., about 10 per group on average. They showed a larger and more consistent benefit. ApoE non-carriers (N = 47) showed a benefit primarily with the second-lowest dose. The completers among this group numbered only 36 patients, i.e., on average less than 10 per dose comparison. But they had larger effects, some reaching a whopping 20 points on ADAS-Cog. (This was relative to an 11-point decline in the placebo group.)
Looking at the best result of all those different groupings, it seemed that a small subset of patients, i.e., the ApoE non-carriers who received the second-lowest of the four doses six times, responded truly well by 78 weeks. The presentation did not state how many people were in this group; it could have been 30, it could have been one. At the same time, the overall appearance of the various bar graphs of these subgroups was one of considerable variation across doses and across the different cognitive/clinical assessments. Subgroup analysis gets statistically weaker the smaller the group sizes become, opening it to criticism. “It could be noise,” Schneider cautioned.
Gilman did show slope curves for one subgroup, i.e., the ApoE4 non-carriers. They suggest that the curves diverged for ADAS-Cog after 36 weeks, on the NTB and CDR-SB right after baseline, but not until 63 weeks on the DAD.
Furthermore, the trial featured three kinds of biomarker, two imaging, and one fluid. Scanned at 71 weeks, neither brain volume nor ventricular volume (both measured by the boundary shift integral) changed in the total population, but again, stratification by ApoE appeared to yield a signal. ApoE carriers on bapineuzumab showed an increase in ventricular brain volume over placebo. Whether this is good or bad is unclear, Gilman said. It could reflect ongoing protein clearance or something else. ApoE4 non-carriers on bapineuzumab suffered less brain atrophy than those on placebo, and here, too, a slope diagram of diverging curves was shown. The CSF biomarker measurements, gleaned from spinal taps at baseline and 52 weeks in 20 antibody- and 15 placebo-treated patients, showed a trend toward lower phospho-tau in treated versus placebo with a p value of 0.056. CSF Aβ and total tau were the same between treated and placebo groups.
“This was a relatively small Phase 2 trial, and it was not realistic that a trial of this size with multiple doses would yield a definitive proof of efficacy. The trial was clearly a success, however, in demonstrating the feasibility of completing multiple IV infusions with intensive safety monitoring in AD patients,” Reisa Sperling of Brigham and Women’s Hospital in Boston, commented by e-mail. “There are some potential safety issues that will need to be followed closely in the larger trials. There may be some encouraging signals that we are on the right track with immunotherapy, but it is important not to over-interpret post-hoc analyses with small sample sizes. We will just have to wait and see."
It is notoriously difficult to extract a solid efficacy signal from Phase 2 trials for AD drugs (see ARF ICAD story). Interestingly, Elan/Wyeth’s immunotherapy competitor Eli Lilly and Company dealt with this dilemma in the opposite way. Rather than slice the data to find efficacy in Phase 2, Lilly placed its bets on biomarker characterization alone and decided to move to Phase 3 just based on that and safety (see upcoming ICAD story). It’s fitting, perhaps, to end with a philosophical remark from a leading basic researcher in the field. “We are at a turning point with regard to developing therapeutics. Everybody is very nervous about the outcome of current anti-amyloid trials because so much is at stake,” said Bart de Strooper at K.U. Leuven and the Flanders Institute for Biotechnology (VIB) in Belgium. “The basis for this test of the amyloid hypothesis is strong, but clearly amyloid is not everything that is going wrong in AD. We should interpret any negative results in this context and try to learn from it. We also need to think about prevention. We need to develop safer medicines that we can test for prevention.”—Gabrielle Strobel.
http://www.alzforum.org/new/detail.asp?id=1894
Chicago: Bapineuzumab’s Phase 2—Was the Data Better Than the Spin?
11 August 2008. It is tempting to say that the story of how Elan/Wyeth’s antibody therapy fared at the 11th International Conference on Alzheimer’s Disease, held last month in Chicago, is one of the stranger tales in present-day AD drug development. (Then, again, weren’t all the recent ones a little strange? Take Alzhemed’s inglorious demise and subsequent resurrection as a nutraceutical, or Flurizan’s sudden death just weeks after its sponsor had announced a European commercialization agreement.)
Bapineuzumab’s story is different, of course. This humanized monoclonal anti-Aβ antibody is very much alive as an experimental treatment. Four separate Phase 3 trials in the U.S., Canada, and Europe are presently enrolling up to 4,100 Alzheimer disease patients. Elan/Wyeth are running an open-label extension of the present Phase 2 trial and are gearing up to test subcutaneous bapineuzumab delivery in a Phase 2 trial. But like the previous two anti-amyloid drugs, bapineuzumab rode to ICAD on high expectations, and its luster has dimmed somewhat after the company’s presentation there. This is not only because investors promptly dumped Elan stock but also because scientists felt that the presentation could have been more straightforward.
What happened? The company presented an overall negative result balanced against a positive effect on a subgroup of patients who don’t carry the AD risk allele ApoE4. Many listeners did not buy it but interpreted the presentation as having massaged the data. In conversations with scientists, most of whom spoke privately, reactions ranged from “they stratified until they got the result they wanted” to “the effect on the ApoE4 negatives makes sense; it’s reassuring,” to “told you so, anti-amyloid drugs lead nowhere” to “I am cautiously optimistic.” Several agreed, however, that the way in which Elan/Wyeth sought to portray the Phase 2 data in the best possible light backfired. In essence, emphasizing a post-hoc pharmacogenomic interpretation shortchanged the trial’s overall decent results, they said. Here’s the lowdown:
At ICAD, Sid Gilman of the University of Michigan, Ann Arbor, presented a more detailed version of the previous top-line data Elan had released on June 16 (see ARF related news story). In this talk, Gilman said that the trial had randomized 234 patients to receive six infusions, one every 13 weeks, of one of four different treatment doses or placebo. He reported that 26 percent of patients dropped out of the treatment groups versus 21 percent in the placebo group.
As a Phase 2, this was first and foremost a safety trial. The safety data Gilman showed received less attention than the efficacy data summarized below, but several scientists noted afterward that some of the reported side effects might bear watching. The most widely discussed side effect is vasogenic edema. Gilman showed that 12 patients developed this, all in the treatment group. Ten occurred in ApoE4 carriers and two in non-carriers, though the trial had twice as many people with ApoE4 than without, meaning that this pharmacogenomic distinction works out to roughly 10/4 rather than 10/2 in this trial. The edemas showed up on MRI and all resolved. Most caused few clinical symptoms, but one patient needed steroid treatment. Six people dropped out of the study; the other six resumed treatment. It is unclear at present how troublesome these edemas really are, and whether they may eventually be deemed an acceptable side effect for treating a deadly disease such as Alzheimer’s. This question generated animated discussion at ICAD and is the subject of ongoing research. Some scientists felt that vasogenic edemas may reflect the underlying mechanism of the drug, as Aβ peptide gets released from plaques, protein concentrations in the interstitial space and in capillary beds rise, osmotic pressure rises along with that, and clearance mechanisms, perhaps some involving ApoE, gradually dispose of the surplus protein. Others doubted whether such a therapy would get the FDA’s blessing.
Concern over vasogenic edema overshadowed the fact that Gilman’s presentation included additional side effects that were more common in the treated patients. The list of unwanted effects that occurred twice as often as in placebo, and in more than 5 percent of patients on active drug, included back pain, anxiety, vomiting, hypertension, weight loss, paranoia, skin lacerations, gait disturbance, and muscle spasms. Syncope and cataracts, among some other side effects, occurred in fewer than 5 percent of patients on active drug. Adverse events were generally mild or moderate and transient, Gilman said. Three men in treatment groups died. According to Gilman, one died of an aortic aneurysm, one succumbed to a fall and Alzheimer’s, and one to infection and Alzheimer’s. None of these deaths was attributed to study drug.
Moving on to efficacy signals, Gilman then briefly noted that the companies had decided to change the originally planned intent-to-treat analysis away from a slope analysis, which shows how the patients do at every time point, to a different analysis he called “MITT repeated measures model without assumption of linearity.” This mouthful essentially compares baseline to the final assessment at 78 weeks/19.5 months and incorporates the time points in between in ways that are not directly shown. That left many in the audience puzzled. To some, the decision to show the data this way reflects a biological argument that a plaque-clearing antibody, unlike a conventional drug, cannot be expected to show a linear effect over time as it might make a patient temporarily worse before it makes him/her better in the long run. To others, this change in the statistical model to a complex one they don’t fully understand implied that Elan/Wyeth might not want to show outcome data over all seven time points because those data looked bad. These scientists would have welcomed trend lines, i.e., graphs plotting the slope of outcome measures along the time axis, because that is how they have come to expect clinical trial data to be shown.
Gilman then showed efficacy data according to the modified analysis. In the total population, the trial posted a 2.3-point improvement on the ADAS-Cog, with a p value of 0.078. On the NTB battery, the improvement was 0.13 with a p value of 0.068. Thus, the trial failed to achieve statistical significance on pre-specified endpoints. Gilman stated this clearly and then moved right on to present a pharmacogenomic stratification of the data. But wait, not so fast! Commentators noted that this overall finding is important. “This is a respectable efficacy signal for a Phase 2 trial. It is a consistent effect, the trial is powered to detect it, and you cannot expect a larger effect from a trial of 230 patients,” Lon Schneider of the University of Southern California told this reporter. Other scientists agreed with this assessment.
In post-hoc analysis, Elan/Wyeth split the patients into those who received all six injections, i.e., the “completers,” versus those who missed one or more doses. Analyzed in this way, the patients who completed the trial (N = 78, ApoE4 carriers and non-carriers combined), showed a 4.3-point improvement over placebo on the ADAS-Cog with a p value of 0.003. “My gut feeling is that [bapineuzumab] is probably modestly effective. But my other gut feeling is that they are chasing their tail by emphasizing efficacy in the E4 non-carriers,” said Schneider.
What, then, is this pharmacogenomic result? Prior research suggests biologic differences between ApoE4 carriers and non-carriers, which might be expected to affect the course of an amyloid-removing therapeutic approach. ApoE4 carriers tend to get the disease earlier than people with ApoE3 or the rare allele ApoE2, and they develop more amyloid in their brain (Rowe et al., 2007). Numerous studies have implicated ApoE in amyloid deposition and removal, and a recent one showed that ApoE4 is less effective at clearing Aβ than the other two alleles (Jiang et al., 2008). Hence, Elan/Wyeth split trial participants into ApoE4 carriers and non-carriers, and then analyzed these groups separately. This created some imbalance between the groups because two-thirds of the patients in the trial happened to have an ApoE4 allele. At 78 weeks, the ApoE4 carriers showed a respectable 4.5-point improvement in ADAS-Cog on the lowest dose but a worsening at the highest dose. Results were inconsistent across the other outcome measures (DAD, NTB, CDR-SB). Some 70 ApoE carriers were in the four treatment groups combined. Of these ApoE4 carriers, a total of 42 across all treatment groups completed the study, i.e., about 10 per group on average. They showed a larger and more consistent benefit. ApoE non-carriers (N = 47) showed a benefit primarily with the second-lowest dose. The completers among this group numbered only 36 patients, i.e., on average less than 10 per dose comparison. But they had larger effects, some reaching a whopping 20 points on ADAS-Cog. (This was relative to an 11-point decline in the placebo group.)
Looking at the best result of all those different groupings, it seemed that a small subset of patients, i.e., the ApoE non-carriers who received the second-lowest of the four doses six times, responded truly well by 78 weeks. The presentation did not state how many people were in this group; it could have been 30, it could have been one. At the same time, the overall appearance of the various bar graphs of these subgroups was one of considerable variation across doses and across the different cognitive/clinical assessments. Subgroup analysis gets statistically weaker the smaller the group sizes become, opening it to criticism. “It could be noise,” Schneider cautioned.
Gilman did show slope curves for one subgroup, i.e., the ApoE4 non-carriers. They suggest that the curves diverged for ADAS-Cog after 36 weeks, on the NTB and CDR-SB right after baseline, but not until 63 weeks on the DAD.
Furthermore, the trial featured three kinds of biomarker, two imaging, and one fluid. Scanned at 71 weeks, neither brain volume nor ventricular volume (both measured by the boundary shift integral) changed in the total population, but again, stratification by ApoE appeared to yield a signal. ApoE carriers on bapineuzumab showed an increase in ventricular brain volume over placebo. Whether this is good or bad is unclear, Gilman said. It could reflect ongoing protein clearance or something else. ApoE4 non-carriers on bapineuzumab suffered less brain atrophy than those on placebo, and here, too, a slope diagram of diverging curves was shown. The CSF biomarker measurements, gleaned from spinal taps at baseline and 52 weeks in 20 antibody- and 15 placebo-treated patients, showed a trend toward lower phospho-tau in treated versus placebo with a p value of 0.056. CSF Aβ and total tau were the same between treated and placebo groups.
“This was a relatively small Phase 2 trial, and it was not realistic that a trial of this size with multiple doses would yield a definitive proof of efficacy. The trial was clearly a success, however, in demonstrating the feasibility of completing multiple IV infusions with intensive safety monitoring in AD patients,” Reisa Sperling of Brigham and Women’s Hospital in Boston, commented by e-mail. “There are some potential safety issues that will need to be followed closely in the larger trials. There may be some encouraging signals that we are on the right track with immunotherapy, but it is important not to over-interpret post-hoc analyses with small sample sizes. We will just have to wait and see."
It is notoriously difficult to extract a solid efficacy signal from Phase 2 trials for AD drugs (see ARF ICAD story). Interestingly, Elan/Wyeth’s immunotherapy competitor Eli Lilly and Company dealt with this dilemma in the opposite way. Rather than slice the data to find efficacy in Phase 2, Lilly placed its bets on biomarker characterization alone and decided to move to Phase 3 just based on that and safety (see upcoming ICAD story). It’s fitting, perhaps, to end with a philosophical remark from a leading basic researcher in the field. “We are at a turning point with regard to developing therapeutics. Everybody is very nervous about the outcome of current anti-amyloid trials because so much is at stake,” said Bart de Strooper at K.U. Leuven and the Flanders Institute for Biotechnology (VIB) in Belgium. “The basis for this test of the amyloid hypothesis is strong, but clearly amyloid is not everything that is going wrong in AD. We should interpret any negative results in this context and try to learn from it. We also need to think about prevention. We need to develop safer medicines that we can test for prevention.”—Gabrielle Strobel.
hier mal wieder etwas aktuelles aus der nature zum lesen.
wie es scheint legt sich die FDA nicht auf den stark schwankenden ADAS-cog als endpunkt fest und ist durchaus flexibel was endpunkte betrifft.
memantine wurde 2003 sogar ohne ADAS-cog zugelassen!!!
nature biotechnology vol. 26,843,2008
FDA and surrogate endpoints in Alzheimer’s disease
Not only is the attrition rate for Alzheimer’s disease therapeutics
notoriously high, but also any company hoping to bring a drug to
market faces a further layer of uncertainty: how open will the FDA
be to new ways of proving efficacy for drug candidates? In March,
Russell Katz, director of the agency’s neurology products division
told a meeting of the coalition to Accelerate Cure/Treatments for
Alzheimer’s Disease that Alzheimer’s disease Assessment Scale
cognitive subscale (ADAS-cog), long the standard in Alzheimer’s
disease measures, might not be required to prove a therapy works.
He said the same about the Clinician’s Interview-Based Impression
of Change plus caregiver interview, another common gauge. Some
had worried that the FDA might look less fondly on such tests as
the Neuropsychological Test Battery (NTB), but Katz’s remarks
seemed to indicate the bar may be lowered, or criteria at least
potentially relaxed.
Much talk at the meeting—which brought Alzheimer’s disease
researchers “one of our few documented insights” from the
FDA, analyst Brian McCarthy with Merriman Curhan Ford in New
York says—had to do with the bottom-line demand for showing
improvements in global functions, such as those covered by the
Disability Assessment for Dementia scale and Clinical Dementia
Rating Sum of Boxes (CDR-SB), as well as boosts in cognitive
abilities, such as those measured by NTB and ADAS-cog. “You can
make somebody an Einstein, but if you haven’t improved the globalfunction
score, the FDA isn’t going to be interested,” he says.
The agency’s willingness to accept new endpoints could make
or break some up-and-coming drugs, Marwan Sabbagh, staff
physician at Boswell Memorial Hospital in Sun City, Arizona, says.
He cites Indianapolis-based Eli Lilly’s LY2062430 currently in
phase 2 trials. The compound is designed to inhibit β-secretase.
But according to Sabbagh, the uncertainty surrounding the disease
mechanism is what complicates surrogate markers in Alzheimer’s
disease compared with other indications. “The ultimate study is
hypertension—you give [patients] a drug, their blood pressure goes
down, and this means the drug works,” he says. Ideally, the Lilly
compound would have a similarly straightforward effect on amyloid
plaque in the brains of Alzheimer’s patients. “Lilly could show they
give the drug and amyloid goes down, but we [wouldn’t] know what
that means,” he says. “What if amyloid goes down and ADAS-cog
doesn’t move? Is that a negative? Does the drug work?”
Scott Turner, director of the memory disorders program at
Georgetown University Medical Center, says ADAS-cog and CDR-SB,
as cognitive and functional tests respectively, “were never set in
stone.” For example, New York–based Forest Laboratories’ Namenda
(memantine), an N-methyl-d-aspartate antagonist for moderate to
severe Alzheimer’s disease, was approved in October 2003 on the
basis of trials that did not use ADAS-cog. Turner—who is involved
in the phase 3 bapineuzumab trial, among others—says further
flexibility of regulators in considering measures with real-world
value can only help patients, as well as drug developers. Wider use
of biomarkers, such as Pittsburgh Compound-B positron emission
tomography imaging, could prove beneficial, too, he says.
The question of what determines efficacy for the FDA is “in flux,
and it’s good that it is,” Turner says. No matter what the endpoints,
“there are going to be a lot of negative trials—that’s the nature of
this business—but I think we’re getting closer” to a viable drug, he
adds. Sabbagh agrees and suggests that more about the disease
could be learned by detecting and treating patients earlier, when
they show mild cognitive impairment. Spinal-fluid proteins might be
useful as another potential marker, along with imaging. “That would
be a paradigm shift,” Sabbagh says. “I think we need to go down
that road.” Randy Osborne Mill Valley, California
wie es scheint legt sich die FDA nicht auf den stark schwankenden ADAS-cog als endpunkt fest und ist durchaus flexibel was endpunkte betrifft.
memantine wurde 2003 sogar ohne ADAS-cog zugelassen!!!
nature biotechnology vol. 26,843,2008
FDA and surrogate endpoints in Alzheimer’s disease
Not only is the attrition rate for Alzheimer’s disease therapeutics
notoriously high, but also any company hoping to bring a drug to
market faces a further layer of uncertainty: how open will the FDA
be to new ways of proving efficacy for drug candidates? In March,
Russell Katz, director of the agency’s neurology products division
told a meeting of the coalition to Accelerate Cure/Treatments for
Alzheimer’s Disease that Alzheimer’s disease Assessment Scale
cognitive subscale (ADAS-cog), long the standard in Alzheimer’s
disease measures, might not be required to prove a therapy works.
He said the same about the Clinician’s Interview-Based Impression
of Change plus caregiver interview, another common gauge. Some
had worried that the FDA might look less fondly on such tests as
the Neuropsychological Test Battery (NTB), but Katz’s remarks
seemed to indicate the bar may be lowered, or criteria at least
potentially relaxed.
Much talk at the meeting—which brought Alzheimer’s disease
researchers “one of our few documented insights” from the
FDA, analyst Brian McCarthy with Merriman Curhan Ford in New
York says—had to do with the bottom-line demand for showing
improvements in global functions, such as those covered by the
Disability Assessment for Dementia scale and Clinical Dementia
Rating Sum of Boxes (CDR-SB), as well as boosts in cognitive
abilities, such as those measured by NTB and ADAS-cog. “You can
make somebody an Einstein, but if you haven’t improved the globalfunction
score, the FDA isn’t going to be interested,” he says.
The agency’s willingness to accept new endpoints could make
or break some up-and-coming drugs, Marwan Sabbagh, staff
physician at Boswell Memorial Hospital in Sun City, Arizona, says.
He cites Indianapolis-based Eli Lilly’s LY2062430 currently in
phase 2 trials. The compound is designed to inhibit β-secretase.
But according to Sabbagh, the uncertainty surrounding the disease
mechanism is what complicates surrogate markers in Alzheimer’s
disease compared with other indications. “The ultimate study is
hypertension—you give [patients] a drug, their blood pressure goes
down, and this means the drug works,” he says. Ideally, the Lilly
compound would have a similarly straightforward effect on amyloid
plaque in the brains of Alzheimer’s patients. “Lilly could show they
give the drug and amyloid goes down, but we [wouldn’t] know what
that means,” he says. “What if amyloid goes down and ADAS-cog
doesn’t move? Is that a negative? Does the drug work?”
Scott Turner, director of the memory disorders program at
Georgetown University Medical Center, says ADAS-cog and CDR-SB,
as cognitive and functional tests respectively, “were never set in
stone.” For example, New York–based Forest Laboratories’ Namenda
(memantine), an N-methyl-d-aspartate antagonist for moderate to
severe Alzheimer’s disease, was approved in October 2003 on the
basis of trials that did not use ADAS-cog. Turner—who is involved
in the phase 3 bapineuzumab trial, among others—says further
flexibility of regulators in considering measures with real-world
value can only help patients, as well as drug developers. Wider use
of biomarkers, such as Pittsburgh Compound-B positron emission
tomography imaging, could prove beneficial, too, he says.
The question of what determines efficacy for the FDA is “in flux,
and it’s good that it is,” Turner says. No matter what the endpoints,
“there are going to be a lot of negative trials—that’s the nature of
this business—but I think we’re getting closer” to a viable drug, he
adds. Sabbagh agrees and suggests that more about the disease
could be learned by detecting and treating patients earlier, when
they show mild cognitive impairment. Spinal-fluid proteins might be
useful as another potential marker, along with imaging. “That would
be a paradigm shift,” Sabbagh says. “I think we need to go down
that road.” Randy Osborne Mill Valley, California
....und weiter gehts. hier ein aktueller artikel über das Ty-konkurrenzprodukt von norvatis (FTY720) mit ähnlicher wirkung wie ty. vorteil von FTY720, es kann oral eingenommen werden!
FTY720 sollte ursprünglich ende 2009 zugelassen werden. durch todesfälle während der klinischen studien, kommt es zu verzögerungen.
außerdem stehen weitere MS-medikamente in den startlöchern.
das wirksamste ms-medikament scheint campath zu sein. (wirksamer als ty)
campath hat allerdings auch mit nebenwirkungen zu kämpfen.
nature biotechnology, 26,844, 2008
infections cast cloud over novartis’ MS therapy
Novartis’ small molecule FTY720 (fingolimod),
now in phase 3, is a potential threat
to Biogen Idec’s lucrative multiple sclerosis
(MS) franchise. But in June Novartis reported
that two phase 3 patients had suffered devastating
viral infections, one fatal. Nevertheless,
as one of several compounds aiming to
become the first orally available drug for MS,
FTY720 may still be on track to gain a major
slice of the ~$6 billion worldwide market for
this disease.
FTY720’s phase 2 extension data released
in April were impressive: more than twothirds
of MS patients remained relapse-free
after three years—the same efficacy range
as Tysabri (natalizumab), marketed by
Cambridge, Massachusetts–based Biogen
Idec and Dublin-based Elan. But this news
has been tempered by phase 3 data showing
that one patient on FTY720 died from
varicella zoster (chicken pox) and the other
fell into a coma due to herpes encephalitis.
Shreeram Aradhye, Novartis’ global medical
director for the FTY720 program, says the
dead patient was also on high-dose steroids
for an MS attack (potentially contributing to
the outcome); indeed, the data safety monitoring
board unanimously recommended
that the trials continue with increased vigilance
for infections.
But the announcement has cast a pall on
FTY720. “Any time a patient dies on a drug,
one has to question mechanism,” says Brett
Kaplan, an analyst at SG Cowen in Boston.
“The more we see [of FTY720], the more
safety becomes a concern.”
The future of FTY720, which Yoshitomi
Pharmaceutical Industries of Saitama, Japan
out-licensed to Novartis in September 1997,
now hinges on the remaining patients in the
three phase 3 trials avoiding serious infections.
“There have been two very serious
outcomes at this point,” says Daniel Mikol,
a neurologist at the University of Michigan
in Ann Arbor who is participating in one of
the trials. “If there were any other cases like
this, it might make it harder to even continue
the trial.”
Others outside the MS community are also
watching FTY720’s fate closely. The drug is a
first-in-class immunosuppressant that binds
to sphingosine-1 phosphate (S1P) receptors,
which have become popular targets in biotech
and big pharma. The FTY720 molecule itself
pointed the way to these targets and helped
open the field. In 1992 scientists in Japan
synthesized the drug by structurally modifying
myriocin, a fungal metabolite isolated from
a vegetative wasp. The compound was later
found to sequester naive and central memory
T cells in the lymph nodes and thymus,
whereas effector memory T cells continued to
circulate, maintaining some cellular immunity.
S1P receptors—expressed on immune
cells, including T cells, B cells, dendritic
cells, natural killer cells and monocytes—
are important for directed cell movement,
though exactly how they control lymphocyte
trafficking has yet to be fully elucidated.
As FTY720 sequesters T cells in lymph
nodes—as opposed to wiping them out—it
is thought to preserve the host’s ability to
respond to infectious agents. “Unlike many
of the immunosuppressants, it really doesn’t
destroy lymphocytes, it doesn’t seem to
have major effects on their ability to be activated,
to proliferate,” says Novartis’ Aradhye.
“Humoral responses seem relatively intact.”
Five pharmaceutical and two biotech companies
are pursuing candidates that either
neutralize the sphingolipid S1P or block its
receptors. At least one of these compounds
is indicated not for MS, but for cancer. For
example, Lpath in San Diego is currently
testing Asonep (sonepcizumab/LT1009), a
humanized monoclonal antibody (mAb)
targeting the S1P lipid, in phase 1 trials in
patients with solid tumors. Asonep’s antibody
acts mainly through an antiangiogenic effect,
and may also promote apoptosis. Swiss companies
Actelion of Allschwill and Roche of
Basel are also developing an oral S1P1 receptor
agonist—R-3477—currently in phase 1
trials for undisclosed autoimmune disorders.
Novartis has another oral S1P receptor modulator,
BAF-312, which recently completed
phase 1 trials.
FTY720 has impressively opened the S1P
field but is clearly no miracle drug. The
drug’s kidney transplantation anti-rejection
trials were discontinued in 2004, partly
due to unexpected side effects. FTY720 “is
relatively nonselective,” notes Jonathan
Bromberg, a transplantation researcher at
the Mount Sinai Medical Center in New
York. “It binds to and interacts with four of
the five S1P receptors. And that’s probably
one of the reasons why it failed in clinical
trials in transplantation.”
Until the June infections, FTY720’s MS
prospects were almost uniformly positive,
but there were some hints of potential problems.
Cases of skin cancer were seen in phase 2,
although it’s not clear whether FTY720 was
the cause. In the phase 3 trial, the patient with
herpes encephalitis was taking FTY720 monotherapy,
whereas the two Tysabri patients who
died several years ago from progressive multifocal
leukoencephalopathy (PML) were on
combination therapy. If FTY720 alone leads
to infections, that would be a serious setback.
“I wouldn’t be surprised if fingolimod is going
to be associated with PML,” says Bibiana
Bielekova, an MS researcher at the National
Institutes of Health. Novartis plans to file for
US Food and Drug Administration registration
in late 2009, although Kaplan thinks that
date may be pushed back about six months,
due to the new safety concerns. Timing might
be crucial, because three other oral agents for
MS are in phase 3 (Table 1).
Even if most or all of these drugs win regulatory
approval, that won’t render obsolete
current injectable treatments, such as Biogen
Idec’s Tysabri and Avonex (interferon (IFN)-
beta), Darmstadt, Germany–based Merck
Serono’s Rebif (IFN-beta) or Jerusalem-based
Teva Pharmaceutical’s Copaxone (glatiramer
acetate). “If you’re on an injection therapy,
doing well and tolerating it fairly well, you
have to ask yourself whether you want to
make a switch to a drug where…the longterm
safety isn’t known,” says Mikol. But
oral delivery is where the MS field is going.
“People hate interferons,” says Bielekova. “A
really large proportion of patients get tired
and achy, and they hate giving themselves a
shot. So there is absolutely a huge demand
for oral therapies.”
Several injectable immunomodulating
mAbs are also in advanced development for
MS (Table 1). But by far the best-performing
antibody for MS is Campath IH (alemtuzumab)
from Genzyme in Cambridge,
Massachusetts. Campath, already approved
for treating B-cell chronic lymphocytic leukemia,
binds the glycoprotein CD52, which
is expressed on the surface of T and B cells,
and indirectly kills them. Phase 2 results in
MS were dramatic: patients taking Campath
had a 73% reduction in the risk of relapse
compared to IFN treatment. “That’s something
that could potentially impact the [MS]
landscape,” says Aaron Reames, an analyst
at Wachovia Capital Markets in Boston.
Unfortunately, Campath can cause immune
thrombocytopenia purpura, a condition that
results in abnormal bleeding from low platelet
counts. Campath “is by far the most effective
drug for MS,” says Caroline Stewart, an
analyst at Piper Jaffray in New York. “It’s also
the most dangerous.”
In the end, market dominance will
come down to safety, for FTY720 and for
its competitors. But none of them is the
final answer for MS. “The biggest question
is [whether MS is] only immune system
driven or is the immune system responding
to something that is happening in the
brain?” says Bielekova. “If we can completely
tackle the immune system part, can
we cure the disease? And I think we don’t
know that.”
table 1
Agent Company likely mechanisms Status
Small molecules
FTY720 (fingolimod) Novartis (Basel) S1P receptor modulation with
immunosuppression
Phase 3 (three
trials at various
stages)
Leustatin (cladribine) EMD Serono
(Rockland,
Massachusetts)
Purine nucleoside analog,
depletes lymphocytes
Phase 3
BG-12 (fumarate
derivative)
Biogen Idec
(Cambridge,
Massachusetts)
Activates Nrf2 pathway,
anti-inflammatory, neuroprotective
Phase 3
Teriflunomide Sanofi-Aventis (Paris) Unknown, pyrimidine synthesis
inhibitor
Phase 3
Laquinimod
(oral Copaxone)
Teva Pharmaceutical
Industries (Jerusalem)
T-helper 2 regulatory cell
induction, others possible
Phase 3
Monoclonal antibodies
Campath 1H
(alemtuzumab)
Genzyme
(Cambridge,
Massachusetts)
Targets CD52, depletes variety of
immune cells
Phase 3
Ocrelizumab Genentech
(S. San Francisco,
California)
Targets CD20 receptor, depletes
mature B cells
Phase 2
Lymphostat B
(belimumab)
Human Genome Sciences
(Rockville, Maryland)
Targets BLyS, reduces
autoantibody levels
Phase 2
Zenapax (daclizumab) Biogen Idec Anti-CD25, modulates T cells Phase 2
FTY720 sollte ursprünglich ende 2009 zugelassen werden. durch todesfälle während der klinischen studien, kommt es zu verzögerungen.
außerdem stehen weitere MS-medikamente in den startlöchern.
das wirksamste ms-medikament scheint campath zu sein. (wirksamer als ty)
campath hat allerdings auch mit nebenwirkungen zu kämpfen.
nature biotechnology, 26,844, 2008
infections cast cloud over novartis’ MS therapy
Novartis’ small molecule FTY720 (fingolimod),
now in phase 3, is a potential threat
to Biogen Idec’s lucrative multiple sclerosis
(MS) franchise. But in June Novartis reported
that two phase 3 patients had suffered devastating
viral infections, one fatal. Nevertheless,
as one of several compounds aiming to
become the first orally available drug for MS,
FTY720 may still be on track to gain a major
slice of the ~$6 billion worldwide market for
this disease.
FTY720’s phase 2 extension data released
in April were impressive: more than twothirds
of MS patients remained relapse-free
after three years—the same efficacy range
as Tysabri (natalizumab), marketed by
Cambridge, Massachusetts–based Biogen
Idec and Dublin-based Elan. But this news
has been tempered by phase 3 data showing
that one patient on FTY720 died from
varicella zoster (chicken pox) and the other
fell into a coma due to herpes encephalitis.
Shreeram Aradhye, Novartis’ global medical
director for the FTY720 program, says the
dead patient was also on high-dose steroids
for an MS attack (potentially contributing to
the outcome); indeed, the data safety monitoring
board unanimously recommended
that the trials continue with increased vigilance
for infections.
But the announcement has cast a pall on
FTY720. “Any time a patient dies on a drug,
one has to question mechanism,” says Brett
Kaplan, an analyst at SG Cowen in Boston.
“The more we see [of FTY720], the more
safety becomes a concern.”
The future of FTY720, which Yoshitomi
Pharmaceutical Industries of Saitama, Japan
out-licensed to Novartis in September 1997,
now hinges on the remaining patients in the
three phase 3 trials avoiding serious infections.
“There have been two very serious
outcomes at this point,” says Daniel Mikol,
a neurologist at the University of Michigan
in Ann Arbor who is participating in one of
the trials. “If there were any other cases like
this, it might make it harder to even continue
the trial.”
Others outside the MS community are also
watching FTY720’s fate closely. The drug is a
first-in-class immunosuppressant that binds
to sphingosine-1 phosphate (S1P) receptors,
which have become popular targets in biotech
and big pharma. The FTY720 molecule itself
pointed the way to these targets and helped
open the field. In 1992 scientists in Japan
synthesized the drug by structurally modifying
myriocin, a fungal metabolite isolated from
a vegetative wasp. The compound was later
found to sequester naive and central memory
T cells in the lymph nodes and thymus,
whereas effector memory T cells continued to
circulate, maintaining some cellular immunity.
S1P receptors—expressed on immune
cells, including T cells, B cells, dendritic
cells, natural killer cells and monocytes—
are important for directed cell movement,
though exactly how they control lymphocyte
trafficking has yet to be fully elucidated.
As FTY720 sequesters T cells in lymph
nodes—as opposed to wiping them out—it
is thought to preserve the host’s ability to
respond to infectious agents. “Unlike many
of the immunosuppressants, it really doesn’t
destroy lymphocytes, it doesn’t seem to
have major effects on their ability to be activated,
to proliferate,” says Novartis’ Aradhye.
“Humoral responses seem relatively intact.”
Five pharmaceutical and two biotech companies
are pursuing candidates that either
neutralize the sphingolipid S1P or block its
receptors. At least one of these compounds
is indicated not for MS, but for cancer. For
example, Lpath in San Diego is currently
testing Asonep (sonepcizumab/LT1009), a
humanized monoclonal antibody (mAb)
targeting the S1P lipid, in phase 1 trials in
patients with solid tumors. Asonep’s antibody
acts mainly through an antiangiogenic effect,
and may also promote apoptosis. Swiss companies
Actelion of Allschwill and Roche of
Basel are also developing an oral S1P1 receptor
agonist—R-3477—currently in phase 1
trials for undisclosed autoimmune disorders.
Novartis has another oral S1P receptor modulator,
BAF-312, which recently completed
phase 1 trials.
FTY720 has impressively opened the S1P
field but is clearly no miracle drug. The
drug’s kidney transplantation anti-rejection
trials were discontinued in 2004, partly
due to unexpected side effects. FTY720 “is
relatively nonselective,” notes Jonathan
Bromberg, a transplantation researcher at
the Mount Sinai Medical Center in New
York. “It binds to and interacts with four of
the five S1P receptors. And that’s probably
one of the reasons why it failed in clinical
trials in transplantation.”
Until the June infections, FTY720’s MS
prospects were almost uniformly positive,
but there were some hints of potential problems.
Cases of skin cancer were seen in phase 2,
although it’s not clear whether FTY720 was
the cause. In the phase 3 trial, the patient with
herpes encephalitis was taking FTY720 monotherapy,
whereas the two Tysabri patients who
died several years ago from progressive multifocal
leukoencephalopathy (PML) were on
combination therapy. If FTY720 alone leads
to infections, that would be a serious setback.
“I wouldn’t be surprised if fingolimod is going
to be associated with PML,” says Bibiana
Bielekova, an MS researcher at the National
Institutes of Health. Novartis plans to file for
US Food and Drug Administration registration
in late 2009, although Kaplan thinks that
date may be pushed back about six months,
due to the new safety concerns. Timing might
be crucial, because three other oral agents for
MS are in phase 3 (Table 1).
Even if most or all of these drugs win regulatory
approval, that won’t render obsolete
current injectable treatments, such as Biogen
Idec’s Tysabri and Avonex (interferon (IFN)-
beta), Darmstadt, Germany–based Merck
Serono’s Rebif (IFN-beta) or Jerusalem-based
Teva Pharmaceutical’s Copaxone (glatiramer
acetate). “If you’re on an injection therapy,
doing well and tolerating it fairly well, you
have to ask yourself whether you want to
make a switch to a drug where…the longterm
safety isn’t known,” says Mikol. But
oral delivery is where the MS field is going.
“People hate interferons,” says Bielekova. “A
really large proportion of patients get tired
and achy, and they hate giving themselves a
shot. So there is absolutely a huge demand
for oral therapies.”
Several injectable immunomodulating
mAbs are also in advanced development for
MS (Table 1). But by far the best-performing
antibody for MS is Campath IH (alemtuzumab)
from Genzyme in Cambridge,
Massachusetts. Campath, already approved
for treating B-cell chronic lymphocytic leukemia,
binds the glycoprotein CD52, which
is expressed on the surface of T and B cells,
and indirectly kills them. Phase 2 results in
MS were dramatic: patients taking Campath
had a 73% reduction in the risk of relapse
compared to IFN treatment. “That’s something
that could potentially impact the [MS]
landscape,” says Aaron Reames, an analyst
at Wachovia Capital Markets in Boston.
Unfortunately, Campath can cause immune
thrombocytopenia purpura, a condition that
results in abnormal bleeding from low platelet
counts. Campath “is by far the most effective
drug for MS,” says Caroline Stewart, an
analyst at Piper Jaffray in New York. “It’s also
the most dangerous.”
In the end, market dominance will
come down to safety, for FTY720 and for
its competitors. But none of them is the
final answer for MS. “The biggest question
is [whether MS is] only immune system
driven or is the immune system responding
to something that is happening in the
brain?” says Bielekova. “If we can completely
tackle the immune system part, can
we cure the disease? And I think we don’t
know that.”
table 1
Agent Company likely mechanisms Status
Small molecules
FTY720 (fingolimod) Novartis (Basel) S1P receptor modulation with
immunosuppression
Phase 3 (three
trials at various
stages)
Leustatin (cladribine) EMD Serono
(Rockland,
Massachusetts)
Purine nucleoside analog,
depletes lymphocytes
Phase 3
BG-12 (fumarate
derivative)
Biogen Idec
(Cambridge,
Massachusetts)
Activates Nrf2 pathway,
anti-inflammatory, neuroprotective
Phase 3
Teriflunomide Sanofi-Aventis (Paris) Unknown, pyrimidine synthesis
inhibitor
Phase 3
Laquinimod
(oral Copaxone)
Teva Pharmaceutical
Industries (Jerusalem)
T-helper 2 regulatory cell
induction, others possible
Phase 3
Monoclonal antibodies
Campath 1H
(alemtuzumab)
Genzyme
(Cambridge,
Massachusetts)
Targets CD52, depletes variety of
immune cells
Phase 3
Ocrelizumab Genentech
(S. San Francisco,
California)
Targets CD20 receptor, depletes
mature B cells
Phase 2
Lymphostat B
(belimumab)
Human Genome Sciences
(Rockville, Maryland)
Targets BLyS, reduces
autoantibody levels
Phase 2
Zenapax (daclizumab) Biogen Idec Anti-CD25, modulates T cells Phase 2
Elan and Transition Therapeutics Achieve Patient Enrollment Target in Phase 2 Clinical Study of ELND005 (AZD-103) in Alzheimer's Disease
Elan and Transition Therapeutics Achieve Patient Enrollment Target in Phase 2 Clinical Study of ELND005 (AZD-103) in Alzheimer's Disease
DUBLIN, Ireland & TORONTO--(BUSINESS WIRE)--
Elan Corporation, plc (NYSE: ELN) and Transition Therapeutics Inc. (NASDAQ: TTHI, TSX: TTH) today announced the achievement of the patient enrollment target for a Phase 2 clinical study of ELND005 (AZD-103) in patients with Alzheimer's disease. The study is a randomized, double-blind, placebo-controlled, dose-ranging, safety and efficacy study in patients with mild to moderate Alzheimer's disease. Each patient's planned treatment period is approximately 18 months.
About ELND005 (AZD-103)
ELND005 is an orally-administered therapeutic agent that has received fast track designation from the U.S. Food and Drug Administration (FDA) for treatment of mild to moderate Alzheimer's disease. Fast track designation facilitates development and may expedite regulatory review of drugs that the FDA recognizes as potentially addressing an unmet medical need for serious or life-threatening conditions.
About Alzheimer's Disease
Alzheimer's disease, a leading cause of dementia, is a progressive brain disorder that gradually destroys a person's memory and ability to learn, reason, make judgments, communicate and carry out daily activities. Alzheimer's disease may result from the build-up of toxic beta-amyloid peptides in the brain. As Alzheimer's disease progresses, individuals may also experience changes in personality and behavior, such as anxiety, suspiciousness or agitation, as well as delusions or hallucinations. It is currently estimated that more than 5 million Americans have Alzheimer's disease and more than 24 million people worldwide over the age of 60 have some form of dementia (Source: Alzheimer's Association and Alzheimer's Disease International).
http://www1.investorvillage.com/iv1/smbd.asp?mb=160&pt=qn
Elan and Transition Therapeutics Achieve Patient Enrollment Target in Phase 2 Clinical Study of ELND005 (AZD-103) in Alzheimer's Disease
DUBLIN, Ireland & TORONTO--(BUSINESS WIRE)--
Elan Corporation, plc (NYSE: ELN) and Transition Therapeutics Inc. (NASDAQ: TTHI, TSX: TTH) today announced the achievement of the patient enrollment target for a Phase 2 clinical study of ELND005 (AZD-103) in patients with Alzheimer's disease. The study is a randomized, double-blind, placebo-controlled, dose-ranging, safety and efficacy study in patients with mild to moderate Alzheimer's disease. Each patient's planned treatment period is approximately 18 months.
About ELND005 (AZD-103)
ELND005 is an orally-administered therapeutic agent that has received fast track designation from the U.S. Food and Drug Administration (FDA) for treatment of mild to moderate Alzheimer's disease. Fast track designation facilitates development and may expedite regulatory review of drugs that the FDA recognizes as potentially addressing an unmet medical need for serious or life-threatening conditions.
About Alzheimer's Disease
Alzheimer's disease, a leading cause of dementia, is a progressive brain disorder that gradually destroys a person's memory and ability to learn, reason, make judgments, communicate and carry out daily activities. Alzheimer's disease may result from the build-up of toxic beta-amyloid peptides in the brain. As Alzheimer's disease progresses, individuals may also experience changes in personality and behavior, such as anxiety, suspiciousness or agitation, as well as delusions or hallucinations. It is currently estimated that more than 5 million Americans have Alzheimer's disease and more than 24 million people worldwide over the age of 60 have some form of dementia (Source: Alzheimer's Association and Alzheimer's Disease International).
http://www1.investorvillage.com/iv1/smbd.asp?mb=160&pt=qn
Author: splaylaywahtheepi
why are institutions buying more now?
Because Tysabri is the best and safest med for MS.
Because beta amyloid is indeed at the root of AD and Elan's approaches are the best for addressing this cause.
Because the debt problem is not a problem - all can see the company goes cash flow positive shortly - and that is all you need to refinance, especially when you have revenues growing at more than 50% annually.
If you thought Elan was a slam dunk at $35 then you have to love the same story at 20% of $35.
why are institutions buying more now?
Because Tysabri is the best and safest med for MS.
Because beta amyloid is indeed at the root of AD and Elan's approaches are the best for addressing this cause.
Because the debt problem is not a problem - all can see the company goes cash flow positive shortly - and that is all you need to refinance, especially when you have revenues growing at more than 50% annually.
If you thought Elan was a slam dunk at $35 then you have to love the same story at 20% of $35.
Hallo
Weiss jemand wie die Aufgaben zwischen Elan Corporation, und Transition Therapeutics verteilt sind in der Entwicklung vom Alzheimer Medikament. ?
Wer von beiden würde bei einer Zulassung am Meisten profitieren ?
Danke
Weiss jemand wie die Aufgaben zwischen Elan Corporation, und Transition Therapeutics verteilt sind in der Entwicklung vom Alzheimer Medikament. ?
Wer von beiden würde bei einer Zulassung am Meisten profitieren ?
Danke
kracht es hier bald genauso wie bei dndn?

Antwort auf Beitrag Nr.: 36.965.094 von [KERN]Codex am 14.04.09 15:08:59...spätestens bei der Zulassung von Bapi bestimmt...

Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 237 | ||
| 90 | ||
| 76 | ||
| 68 | ||
| 52 | ||
| 48 | ||
| 37 | ||
| 35 | ||
| 32 | ||
| 31 |
| Wertpapier | Beiträge | |
|---|---|---|
| 28 | ||
| 27 | ||
| 24 | ||
| 23 | ||
| 23 | ||
| 20 | ||
| 20 | ||
| 20 | ||
| 15 | ||
| 13 |





















