Nachfrage zur Hautkrebsfrüherkennung explodiert ! - 500 Beiträge pro Seite
eröffnet am 28.06.13 08:06:26 von
neuester Beitrag 12.07.13 13:28:55 von
neuester Beitrag 12.07.13 13:28:55 von
Beiträge: 13
ID: 1.183.335
ID: 1.183.335
Aufrufe heute: 0
Gesamt: 1.041
Gesamt: 1.041
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| 08.05.24, 11:56 | 543 | |
| vor 1 Stunde | 305 | |
| vor 1 Stunde | 229 | |
| gestern 18:51 | 220 | |
| 11.05.24, 11:52 | 175 | |
| gestern 20:31 | 171 | |
| heute 02:17 | 157 | |
| gestern 23:22 | 149 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 1. | 0,2170 | +3,33 | 48 | |||
| 2. | 2. | 18.763,00 | -0,01 | 44 | |||
| 3. | 3. | 168,47 | -2,04 | 27 | |||
| 4. | 4. | 0,1640 | 0,00 | 22 | |||
| 5. | 6. | 0,2980 | -3,87 | 17 | |||
| 6. | 5. | 2,5600 | -6,91 | 16 | |||
| 7. | 8. | 10,320 | 0,00 | 15 | |||
| 8. | 7. | 898,78 | +1,27 | 13 |

http://simsys-molemate.com/
http://medxhealth.com/
Hautkrebs die neue Volkskrankheit.
 Das Unternehmen MedX Health Corp. (WKN: A0YJHF) ist eines der wenigen Anbieter / Herstellet von Geräten für den Einsatz bei Hautkrebsfrüherkennung.
Das Unternehmen MedX Health Corp. (WKN: A0YJHF) ist eines der wenigen Anbieter / Herstellet von Geräten für den Einsatz bei Hautkrebsfrüherkennung. Guys, it's time to take skin cancer seriously !

http://www.turnto23.com/lifestyle/health/guys-its-time-to-ta…
Die Nachfrage nach sogenannten "skin cancer screenings" - also Test´s zur Früherkennung von Hautkrebs - steigt massiv - für 2017 rechnet man mit einem ca. 4 Mrd. Dollar Markt im Bereich Hautkrebs Diagnostic. !
Die Diagnose Hautkrebs wird leider immer häufiger gestellt. Tendenz stark steigend. Allein in den USA kommen jährlich fast 4 Millionen neue Hautkrebsfälle dazu.
http://www.wwlp.com/dpp/health/cancer/skin-cancer-the-most-c…
Aufgrund der signifikanten Zuwachsrate an Hautkrebserkrankungen explodiert die Nachfrage nach "full body skin cancer screenings" - also Früherkennung.
Die canadische MedX Health Corp. (V.MDX) ist eines von wenigen Unternehmen das auf Hautkrebsfrüherkennung spezialisiert ist. Mit SIMSYS+MoleMate & SiasCopy verfügt man über eine revolutionäre Produkte die im Bereich Hautkrebsfrüherkennung einzigartig sind.
About MoleMate
http://simsys-molemate.com/simsys-molemate/about/
The FDA approved MoleMate Skin Imaging System is a significant advance in the early detection of potentially life threatening moles and lesions.
Physicians have also found the hand-held device easy to learn and use, and that it rapidly provides accurate images of the pigment, blood, and collagen below the mole or lesion.
Now, for the first time, physicians can more accurately evaluate suspicious moles and lesions in a non-invasive, pain-free way. Experts also believe it may reduce the need for time consuming and expensive biopsies
About Siascopy
http://simsys-molemate.com/simsys-molemate/siascopy/
MedX produces the Siascope which is a handheld siascopy medical device used in conjunction with the SIMSYS™ and MoleMate™ range of software.
What is Siascopy?
Spectrophotometric Intracutaneous Analysis (SIA) is a fast, non-invasive and completely safe method of pigmented skin lesions differentiation with distinct advantages over clinical and dermatoscopic diagnosis of melanoma. It is easy to perform and allows examination of skin lesions with great accuracy and in a highly objective manner. The sensitivity of the method reaches the value of 96%. It has also been shown that Siascopy is the most reliable and reproducible of melanoma non-invasive diagnostic techniques.
http://www.uhhc.co.nz/services/molemate/
SIMSYS-MoleMate hat die Zulassung der Health Canada sowie die Zulassung der amerikanischen FDA. MoleMate™, has approval from Health Canada, and is immediately available and in stock for all Canadian physicians, joining their colleagues in Europe, the UK, Australia, and the US
About MoleMate & SIMSYS
MoleMate and SIMSYS Skin Imaging Systems is a significant advance in the early detection of potentially life threatening moles and lesions. MoleMate and SIMSYS rapidly provide accurate images of the pigment, blood, and collagen below the mole or lesion. MoleMate and SIMSYS are both FDA approved, and have been sold in the European Community, the UK, Australia, and theUS.
“Among the sophisticated equipment used by University of California Irvine Melanoma Center physicians is a SIMSYS-MoleMate SIAscope, one of the most advanced melanoma imaging systems in the world.
 For patients with many moles, this computer imaging can make a big difference, it decreases the number of biopsies needed, so they won’t look like a pincushion.” - Dr. James G. Jakowatz, surgical oncologist and UC Irvine Melanoma Center director – Dr. Janellen Smith, UC Irvine dermatologist and co-director of the Pigmented Lesion Program, UC Irvine
For patients with many moles, this computer imaging can make a big difference, it decreases the number of biopsies needed, so they won’t look like a pincushion.” - Dr. James G. Jakowatz, surgical oncologist and UC Irvine Melanoma Center director – Dr. Janellen Smith, UC Irvine dermatologist and co-director of the Pigmented Lesion Program, UC IrvineIn den kommenden Jahren wird der Bereich Skin cancer prevention signifikant und überdurchschnittlich wachsen. Die Nachfrage nach geeigneten Diagnosegeräten, Scanner ect. wird ungebremst steigen. Für das Unternehmen MedX Health Corp. sind kräftige Zuwächse bei Umsatz- und Gewinn zu erwarten. Das Absatzpotential für MoleMate ect. dürfte sich vervielfachen.
Der im Verhältnis stehende niedrige Börsenwert von gerade einmal 1,4 Mio € wirkt sich nun zusätzlich positiv auf die Kursprognose aus. Die Aktie sollte sich in den kommenden 12 Monaten mind. verdreifachen. Das entspreche einem Börsenwert von gerade einmal 4,5 Mio € was in Anbetracht der massiven Wachstumsstory lächerlich erscheint. Wird MedX Health richtig entdeckt dürften Kurse zwischen 0,50 - 0,80 $ realistisch werden.
MoleMate aktuell in Australien ect.
http://www.uhhc.co.nz/services/molemate/
http://www.nzms.co.nz/site/cms/point-of-care/skin-cancer-ima…
http://www.moorhousemedical.co.nz/Services/Skin-Clinic/
http://www.willandramedical.com.au/industrialhealthcare.html
Das britische Gesundheitssystem erlaubt den Einsatz aufgrund der Kosten/Nutzen Struktur.
Das UK System ist für die strenge Kosten-Nutzen-Kontrolle bekannt.
1. Value Health. 2013 Mar-Apr;16(2):356-66. doi: 10.1016/j.jval.2012.12.008.
The cost-effectiveness of a novel SIAscopic diagnostic aid for the management of
pigmented skin lesions in primary care: a decision-analytic model.
Wilson EC, Emery JD, Kinmonth AL, Prevost AT, Morris HC, Humphrys E, Hall PN,
Burrows N, Bradshaw L, Walls J, Norris P, Johnson M, Walter FM.
Health Economics Group, University of East Anglia, Norwich, UK.
ed.wilson@uea.ac.uk
OBJECTIVES: Pigmented skin lesions are commonly presented in primary care.
Appropriate diagnosis and management is challenging because the vast majority are
benign. The MoleMate system is a handheld SIAscopy scanner integrated with a
primary care diagnostic algorithm aimed at improving the management of pigmented
skin lesions in primary care.
METHODS: This decision-model-based economic evaluation draws on the results of a
randomized controlled trial of the MoleMate system versus best practice
(ISRCTN79932379) to estimate the expected long-term cost and health gain of
diagnosis with the MoleMate system versus best practice in an English primary
care setting. The model combines trial results with data from the wider
literature to inform long-term prognosis, health state utilities, and cost.
RESULTS:
Results are reported as mean and incremental cost and quality-adjusted
life-years (QALYs) gained, incremental cost-effectiveness ratio with
probabilistic sensitivity analysis, and value of information analysis. Over a lifetime horizon, the MoleMate system is expected to cost an extra £18 over best practice alone, and yield an extra 0.01 QALYs per patient examined. The incremental cost-effectiveness ratio is £1,896 per QALY gained, with a 66.1% probability of being below £30,000 per QALY gained. The expected value of perfect information is £43.1 million.
CONCLUSIONS: Given typical thresholds in the United Kingdom (£20,000-£30,000 per
QALY), the MoleMate system may be cost-effective compared with best practice
diagnosis alone in a primary care setting.
Ein Researchbericht zu MedX Health aus dem letzten Jahr aber aufgrund der aktuellen Tendenz aktueller denn je !
Diese Empfehlung ist zwar aus Mitte 2012 aber aktueller den je .........
http://www.munknee.com/2012/05/...ment-in-your-health-and-yo…
This Skin Cancer Detection Device Could Be a Great Investment in Your Health – and Your Wealth I attended an investment presentation last week by a company that is introducing an innovative skin cancer detection device into the Canadian/American markets that was so enlightening, so potentially financially rewarding and so potentially life-saving, that I just have to share it with you. So says Lorimer Wilson, editor of www.munKNEE.com. Most of us enjoy getting a tan, even if it entails the occasional sunburn but, unfortunately, too much sun can eventually have an adverse affect on your health in the form of skin cancer which is growing at a rate of 10% per year. Indeed, the Canadian and US Cancer societies confirm that skin cancer is the most common form of cancer. Each year there are more new cases of skin cancer than the combined incidence of cancers of the breast, prostrate, lung and colon. Nearly 800,000 Americans are living with a history of melanoma and 13 million Americans are living with a history of non-melanoma skin cancer, typically diagnosed as basal cell carcinoma (BCC) or squamous cell carcinoma (SCC). When melanoma is detected early, the survival rate is about 99% and falls to 15% as the disease advances. If you have experienced too many “too much sun” events over the years, however, (or you have some unchecked moles, or have a family history of malignant melanoma,) you may well be making regular visits to your dermatologist to have the unattractive – and potentially deadly – effects of too much sun (lesions, etc.) frozen off with the application of liquid nitrogen (called cryosurgery or cryotherapy) or otherwise treated ( the application of Efudex cream comes to mind). Some of you may have even: had an invasive and sometimes painful biopsy of a suspicious looking growth (mole, lesion or some other change in skin appearance), had to wait for weeks for the answer as to whether it was benign, skin cancer or malignant melanoma, had to schedule and wait for a follow-up appointment and then had to schedule, wait and undergo plastic surgery to repair the damage done by the biopsy. Unfortunately, I know of what I speak as I have been making quarterly visits over the past 20 years to my friendly and accomplished dermatologist undergoing the whole range of treatments. Well, no more! Future biopsies, and the need to wait for the results (an immediate diagnosis is made during your appointment) will be a thing of the past in large measure (see video here – 4:56 minutes) once family physicians and dermatologists begin practising a new non-invasive skin cancer screening procedure that has been in use in Europe, the UK, Australia for a number of years and has recently been introduced to the medical communities in Canada and the U.S. by MedX Health Corpoation (TSX-V:MDX). The system is the FDA approved and Health Canada cleared SIMSYS-MoleMate Skin Imaging System ( see a video here - 2:48 minutes – and here – 2:08 minutes) which provides a significant advance in the early detection of potentially life threatening moles and lesions. MoleMate uses a patented device and technology (MedX has been granted 17 international patents to date and has another 23 patents pending) that features a hand-held scanner designed for office use that utilizes light to view beneath suspicious moles or lesions in a pain free, non-invasive manner, creating images for physicians to evaluate all types of moles and lesions within seconds, providing images that can reveal if a mole is benign, or something more serious, often eliminating the need for skin biopsies, resulting in less pain, scarring, and expense. [See this video - 5:29 minutes - by Dr. Ronald H. Falcon, MD, FAAD on the merits of the SIMSYS-MoleMate.] It is just a matter of time before this system is owned by every family physician, dermatologist, clinic and hospital in North America so you can just imagine the upcoming growth and increased profits and share price
So says Lorimer Wilson, editor of www.munKNEE.com. Most of us enjoy getting a tan, even if it entails the occasional sunburn but, unfortunately, too much sun can eventually have an adverse affect on your health in the form of skin cancer which is growing at a rate of 10% per year. Indeed, the Canadian and US Cancer societies confirm that skin cancer is the most common form of cancer. Each year there are more new cases of skin cancer than the combined incidence of cancers of the breast, prostrate, lung and colon. Nearly 800,000 Americans are living with a history of melanoma and 13 million Americans are living with a history of non-melanoma skin cancer, typically diagnosed as basal cell carcinoma (BCC) or squamous cell carcinoma (SCC). When melanoma is detected early, the survival rate is about 99% and falls to 15% as the disease advances. If you have experienced too many “too much sun” events over the years, however, (or you have some unchecked moles, or have a family history of malignant melanoma,) you may well be making regular visits to your dermatologist to have the unattractive – and potentially deadly – effects of too much sun (lesions, etc.) frozen off with the application of liquid nitrogen (called cryosurgery or cryotherapy) or otherwise treated ( the application of Efudex cream comes to mind). Some of you may have even: had an invasive and sometimes painful biopsy of a suspicious looking growth (mole, lesion or some other change in skin appearance), had to wait for weeks for the answer as to whether it was benign, skin cancer or malignant melanoma, had to schedule and wait for a follow-up appointment and then had to schedule, wait and undergo plastic surgery to repair the damage done by the biopsy. Unfortunately, I know of what I speak as I have been making quarterly visits over the past 20 years to my friendly and accomplished dermatologist undergoing the whole range of treatments. Well, no more! Future biopsies, and the need to wait for the results (an immediate diagnosis is made during your appointment) will be a thing of the past in large measure (see video here – 4:56 minutes) once family physicians and dermatologists begin practising a new non-invasive skin cancer screening procedure that has been in use in Europe, the UK, Australia for a number of years and has recently been introduced to the medical communities in Canada and the U.S. by MedX Health Corpoation (TSX-V:MDX). The system is the FDA approved and Health Canada cleared SIMSYS-MoleMate Skin Imaging System ( see a video here - 2:48 minutes – and here – 2:08 minutes) which provides a significant advance in the early detection of potentially life threatening moles and lesions. MoleMate uses a patented device and technology (MedX has been granted 17 international patents to date and has another 23 patents pending) that features a hand-held scanner designed for office use that utilizes light to view beneath suspicious moles or lesions in a pain free, non-invasive manner, creating images for physicians to evaluate all types of moles and lesions within seconds, providing images that can reveal if a mole is benign, or something more serious, often eliminating the need for skin biopsies, resulting in less pain, scarring, and expense. [See this video - 5:29 minutes - by Dr. Ronald H. Falcon, MD, FAAD on the merits of the SIMSYS-MoleMate.] It is just a matter of time before this system is owned by every family physician, dermatologist, clinic and hospital in North America so you can just imagine the upcoming growth and increased profits and share price  of MedX Health Corp. MedX is a 12 year old Canadian company, headquartered in Mississauga, Ontario (Toronto). MDX trades on the TSX Venture Exchange under the symbol TSXV:MDX
of MedX Health Corp. MedX is a 12 year old Canadian company, headquartered in Mississauga, Ontario (Toronto). MDX trades on the TSX Venture Exchange under the symbol TSXV:MDX
Diese Empfehlung ist zwar aus Mitte 2012 aber aktueller den je .........
http://www.munknee.com/2012/05/...ment-in-your-health-and-yo…
This Skin Cancer Detection Device Could Be a Great Investment in Your Health – and Your Wealth I attended an investment presentation last week by a company that is introducing an innovative skin cancer detection device into the Canadian/American markets that was so enlightening, so potentially financially rewarding and so potentially life-saving, that I just have to share it with you.
 So says Lorimer Wilson, editor of www.munKNEE.com. Most of us enjoy getting a tan, even if it entails the occasional sunburn but, unfortunately, too much sun can eventually have an adverse affect on your health in the form of skin cancer which is growing at a rate of 10% per year. Indeed, the Canadian and US Cancer societies confirm that skin cancer is the most common form of cancer. Each year there are more new cases of skin cancer than the combined incidence of cancers of the breast, prostrate, lung and colon. Nearly 800,000 Americans are living with a history of melanoma and 13 million Americans are living with a history of non-melanoma skin cancer, typically diagnosed as basal cell carcinoma (BCC) or squamous cell carcinoma (SCC). When melanoma is detected early, the survival rate is about 99% and falls to 15% as the disease advances. If you have experienced too many “too much sun” events over the years, however, (or you have some unchecked moles, or have a family history of malignant melanoma,) you may well be making regular visits to your dermatologist to have the unattractive – and potentially deadly – effects of too much sun (lesions, etc.) frozen off with the application of liquid nitrogen (called cryosurgery or cryotherapy) or otherwise treated ( the application of Efudex cream comes to mind). Some of you may have even: had an invasive and sometimes painful biopsy of a suspicious looking growth (mole, lesion or some other change in skin appearance), had to wait for weeks for the answer as to whether it was benign, skin cancer or malignant melanoma, had to schedule and wait for a follow-up appointment and then had to schedule, wait and undergo plastic surgery to repair the damage done by the biopsy. Unfortunately, I know of what I speak as I have been making quarterly visits over the past 20 years to my friendly and accomplished dermatologist undergoing the whole range of treatments. Well, no more! Future biopsies, and the need to wait for the results (an immediate diagnosis is made during your appointment) will be a thing of the past in large measure (see video here – 4:56 minutes) once family physicians and dermatologists begin practising a new non-invasive skin cancer screening procedure that has been in use in Europe, the UK, Australia for a number of years and has recently been introduced to the medical communities in Canada and the U.S. by MedX Health Corpoation (TSX-V:MDX). The system is the FDA approved and Health Canada cleared SIMSYS-MoleMate Skin Imaging System ( see a video here - 2:48 minutes – and here – 2:08 minutes) which provides a significant advance in the early detection of potentially life threatening moles and lesions. MoleMate uses a patented device and technology (MedX has been granted 17 international patents to date and has another 23 patents pending) that features a hand-held scanner designed for office use that utilizes light to view beneath suspicious moles or lesions in a pain free, non-invasive manner, creating images for physicians to evaluate all types of moles and lesions within seconds, providing images that can reveal if a mole is benign, or something more serious, often eliminating the need for skin biopsies, resulting in less pain, scarring, and expense. [See this video - 5:29 minutes - by Dr. Ronald H. Falcon, MD, FAAD on the merits of the SIMSYS-MoleMate.] It is just a matter of time before this system is owned by every family physician, dermatologist, clinic and hospital in North America so you can just imagine the upcoming growth and increased profits and share price
So says Lorimer Wilson, editor of www.munKNEE.com. Most of us enjoy getting a tan, even if it entails the occasional sunburn but, unfortunately, too much sun can eventually have an adverse affect on your health in the form of skin cancer which is growing at a rate of 10% per year. Indeed, the Canadian and US Cancer societies confirm that skin cancer is the most common form of cancer. Each year there are more new cases of skin cancer than the combined incidence of cancers of the breast, prostrate, lung and colon. Nearly 800,000 Americans are living with a history of melanoma and 13 million Americans are living with a history of non-melanoma skin cancer, typically diagnosed as basal cell carcinoma (BCC) or squamous cell carcinoma (SCC). When melanoma is detected early, the survival rate is about 99% and falls to 15% as the disease advances. If you have experienced too many “too much sun” events over the years, however, (or you have some unchecked moles, or have a family history of malignant melanoma,) you may well be making regular visits to your dermatologist to have the unattractive – and potentially deadly – effects of too much sun (lesions, etc.) frozen off with the application of liquid nitrogen (called cryosurgery or cryotherapy) or otherwise treated ( the application of Efudex cream comes to mind). Some of you may have even: had an invasive and sometimes painful biopsy of a suspicious looking growth (mole, lesion or some other change in skin appearance), had to wait for weeks for the answer as to whether it was benign, skin cancer or malignant melanoma, had to schedule and wait for a follow-up appointment and then had to schedule, wait and undergo plastic surgery to repair the damage done by the biopsy. Unfortunately, I know of what I speak as I have been making quarterly visits over the past 20 years to my friendly and accomplished dermatologist undergoing the whole range of treatments. Well, no more! Future biopsies, and the need to wait for the results (an immediate diagnosis is made during your appointment) will be a thing of the past in large measure (see video here – 4:56 minutes) once family physicians and dermatologists begin practising a new non-invasive skin cancer screening procedure that has been in use in Europe, the UK, Australia for a number of years and has recently been introduced to the medical communities in Canada and the U.S. by MedX Health Corpoation (TSX-V:MDX). The system is the FDA approved and Health Canada cleared SIMSYS-MoleMate Skin Imaging System ( see a video here - 2:48 minutes – and here – 2:08 minutes) which provides a significant advance in the early detection of potentially life threatening moles and lesions. MoleMate uses a patented device and technology (MedX has been granted 17 international patents to date and has another 23 patents pending) that features a hand-held scanner designed for office use that utilizes light to view beneath suspicious moles or lesions in a pain free, non-invasive manner, creating images for physicians to evaluate all types of moles and lesions within seconds, providing images that can reveal if a mole is benign, or something more serious, often eliminating the need for skin biopsies, resulting in less pain, scarring, and expense. [See this video - 5:29 minutes - by Dr. Ronald H. Falcon, MD, FAAD on the merits of the SIMSYS-MoleMate.] It is just a matter of time before this system is owned by every family physician, dermatologist, clinic and hospital in North America so you can just imagine the upcoming growth and increased profits and share price  of MedX Health Corp. MedX is a 12 year old Canadian company, headquartered in Mississauga, Ontario (Toronto). MDX trades on the TSX Venture Exchange under the symbol TSXV:MDX
of MedX Health Corp. MedX is a 12 year old Canadian company, headquartered in Mississauga, Ontario (Toronto). MDX trades on the TSX Venture Exchange under the symbol TSXV:MDX
kurzfristiges Kursziel 0,22 $ 
mittelfristiges Kursziel 0,58 $
langfristiges Kursziel 1,38 $

mittelfristiges Kursziel 0,58 $
langfristiges Kursziel 1,38 $
MCAP aktuell sogar nur 1,1 Mio € - diese niedrige Bewertung rechtfertigt intraday also innerhalb eines Handelstag zweistellige Kurszuwächse. MedX ist bekannt für derartige Kurssprünge von bis zu 300 % intraday.
CRV sehr gut!
CRV sehr gut!
Vorsicht PUSHERALARM !!!!!!!!!!!!!!!!! 

Antwort auf Beitrag Nr.: 44.942.231 von Keilfleckbarbe am 28.06.13 10:12:52Wegen dem Nutzernamen? 

Antwort auf Beitrag Nr.: 44.941.355 von Gesprengt am 28.06.13 08:06:26Ist die ISIN US45767W1071 ?
40% kursplus mit 900, 000 shares an der kanadischen heimatbörse medx health wird von investoren entdeckt.
Da werden die kommenden Wochen interessant.
Da werden die kommenden Wochen interessant.
Zitat von Datteljongleur:Zitat von Gesprengt: kurzfristiges Kursziel 0,22 $
mittelfristiges Kursziel 0,58 $
langfristiges Kursziel 1,38 $
Sehr langlangfristiges Kursziel Dausend $
In der Medx Aktie ist Bewegung drin. In Kürze sollte der Kurs kräftig nach oben steigen. Die Aktie dürfte Richtung 1 $ explodieren. In den letzten Tagen konnte man einen ersten Anstieg beobachten. Investoren steigen ebenfalls ein. Der niedrige einstellige Börsenwert ist wie Dynamit. Die Medx Aktie dürfte sobald neue hochs erreichen.
177 Millionen Deal
Medx Screening Product in Boots Pharmacy
BTG bought Biocapatibles in November of 2010 they divested their Cancer Screening Technology.
http://www.standard.co.uk/business/btgs-177m-deal-to-buy-can…
MedX successfully purchased the technology and at the time was in competition with other potential suitors. MedX won the bid partly because they had a fully compliant ISO 14385 Manufacturing and Testing Facility.ScreenCancer is a Private Company signed on as a non-exclusive distribution partner.
http://screencancer.com/about-screencancer-europe/
Screen Cancer seems to have had success in launching MedX’s screening technology into the Boots Drug Store Chain in Norway where they are based.ScreenCancer is an extremely private company however their key investor is a Norwegian Seed Capital Fund called Sarsia Seed Fund. This fund has released news about the distribution of our product in the Boots Pharmacy Chain. It appears from their press release that they are in 88 Boots Pharmacies in Norway and looking to expand up to 100 stores. They have preformed over 3000 scans in Quarter 2/2013 and since the inception of this program have identified over 100 cases of cancer.
http://www.sarsiaseed.com/blog-template-1-2/?lang=en
MedX receives 5 pounds per scan from ScreenCancer. If ScreenCancer is able to increase this penetration rate it would be very beneficial to MedX.
177 Millionen Deal
Medx Screening Product in Boots Pharmacy
BTG bought Biocapatibles in November of 2010 they divested their Cancer Screening Technology.
http://www.standard.co.uk/business/btgs-177m-deal-to-buy-can…
MedX successfully purchased the technology and at the time was in competition with other potential suitors. MedX won the bid partly because they had a fully compliant ISO 14385 Manufacturing and Testing Facility.ScreenCancer is a Private Company signed on as a non-exclusive distribution partner.
http://screencancer.com/about-screencancer-europe/
Screen Cancer seems to have had success in launching MedX’s screening technology into the Boots Drug Store Chain in Norway where they are based.ScreenCancer is an extremely private company however their key investor is a Norwegian Seed Capital Fund called Sarsia Seed Fund. This fund has released news about the distribution of our product in the Boots Pharmacy Chain. It appears from their press release that they are in 88 Boots Pharmacies in Norway and looking to expand up to 100 stores. They have preformed over 3000 scans in Quarter 2/2013 and since the inception of this program have identified over 100 cases of cancer.
http://www.sarsiaseed.com/blog-template-1-2/?lang=en
MedX receives 5 pounds per scan from ScreenCancer. If ScreenCancer is able to increase this penetration rate it would be very beneficial to MedX.
Nachfrage nach HautkrebsScreening explodiert
Boots Comments on Melanoma Screening
1July 08, 2013
I found this on the Boots Sustainability Report
http://www.allianceboots.com/CorporateSocialResponsibilityRe…
Walgreens has announced it is buying Boots. If this was in all the Walgreens and Boots Pharmacies that would certainly make MedX a cash flow company. The apparently make 5 lbs per scan in Europe todate they have according to press releases conducted over 8700 scans and detected 100 potential melanomas.
Die gegenwärtige Börsen Bewertung beträgt ca. 2, 5 Mill. EUR. Dieser extrem niedrigen MCAP stehen mehrere positive Faktoren und TOP Medx Produkte gegenüber die für einen sehr kräftigen und unerwarteten Kursanstieg sorgen werden.Der rasante Anstieg der Hautkrebs Erkrankungen löst eine regelrechte Screening Panik aus. Die Nachfrage nach Hautkrebs Erkennungs Diagnostik Produkten explodiert. Medx ist als ein Markführer in diesem Bereich ein direkter Profiteur.In den kommenden Monaten dürfte sich die Aktie zum Highflyer entwickeln.
Boots Comments on Melanoma Screening
1July 08, 2013
I found this on the Boots Sustainability Report
http://www.allianceboots.com/CorporateSocialResponsibilityRe…
Walgreens has announced it is buying Boots. If this was in all the Walgreens and Boots Pharmacies that would certainly make MedX a cash flow company. The apparently make 5 lbs per scan in Europe todate they have according to press releases conducted over 8700 scans and detected 100 potential melanomas.
Die gegenwärtige Börsen Bewertung beträgt ca. 2, 5 Mill. EUR. Dieser extrem niedrigen MCAP stehen mehrere positive Faktoren und TOP Medx Produkte gegenüber die für einen sehr kräftigen und unerwarteten Kursanstieg sorgen werden.Der rasante Anstieg der Hautkrebs Erkrankungen löst eine regelrechte Screening Panik aus. Die Nachfrage nach Hautkrebs Erkennungs Diagnostik Produkten explodiert. Medx ist als ein Markführer in diesem Bereich ein direkter Profiteur.In den kommenden Monaten dürfte sich die Aktie zum Highflyer entwickeln.
Medx's Molemate System wird weltweit zur Anwendung kommen zur Hautkrebs Früherkennung die Aktie sollte kräftig steigen denn das Medx Produkt rettet Leben.
Your dermatologist will examine your moles using new technologies like MoleMate, an FDA-approved non-invasive, painless melanoma-screening device. This method eliminates the need for a biopsy, making the check-up process far less intimidating. The hand-held scanner uses light and a skin-imaging technology to view up to a depth of 2mm below the surface of suspicious moles and lesions. It measures the concentration and distribution of the skin’s melanin, collagen and haemoglobin to detect cancerous melanoma, and the images it generates are used by doctors to ascertain if a mole is benign or malignant. The technology is available across the UAE, including Aesthetics International, and is worth doing to give you peace of mind – plus, it could save your life. “If detected in its pre-cancerous stage, a mole can be treated before it develops into skin cancer,” says Dr Khan.
NEWS
10. Juli 2013
http://m.gulfnews.com/life/health/know-your-moles-1.120736
Your dermatologist will examine your moles using new technologies like MoleMate, an FDA-approved non-invasive, painless melanoma-screening device. This method eliminates the need for a biopsy, making the check-up process far less intimidating. The hand-held scanner uses light and a skin-imaging technology to view up to a depth of 2mm below the surface of suspicious moles and lesions. It measures the concentration and distribution of the skin’s melanin, collagen and haemoglobin to detect cancerous melanoma, and the images it generates are used by doctors to ascertain if a mole is benign or malignant. The technology is available across the UAE, including Aesthetics International, and is worth doing to give you peace of mind – plus, it could save your life. “If detected in its pre-cancerous stage, a mole can be treated before it develops into skin cancer,” says Dr Khan.
NEWS
10. Juli 2013
http://m.gulfnews.com/life/health/know-your-moles-1.120736
News lt. IR erwartet. Einige Produkt Zulassungen auf "Pending"
Eine News zur Marktreife bzw. FDA Zulassung wird erwartet. Meff ist in einem sehr spannenden Quartal. Krebsfrüherkennung mit MedX MoleMate die einzigartigen Phototherapie Produkte zahlreiche Produktentwicklungen kurz vor Zulassung oder MarktEinführung etc.
Medx steht vor interessantem Newsflow.
Die Frage ist wo eröffnet die Aktie bei Bekanntgabe. Die letzte Produktnews sorgte für über 350 % Kursgewinn an einem einzigen Tag.
Diesmal sind die Umstände aufgrund der stark steigenden Krebs Produkte deutlich besser. Medx bald 1 $ / Aktie

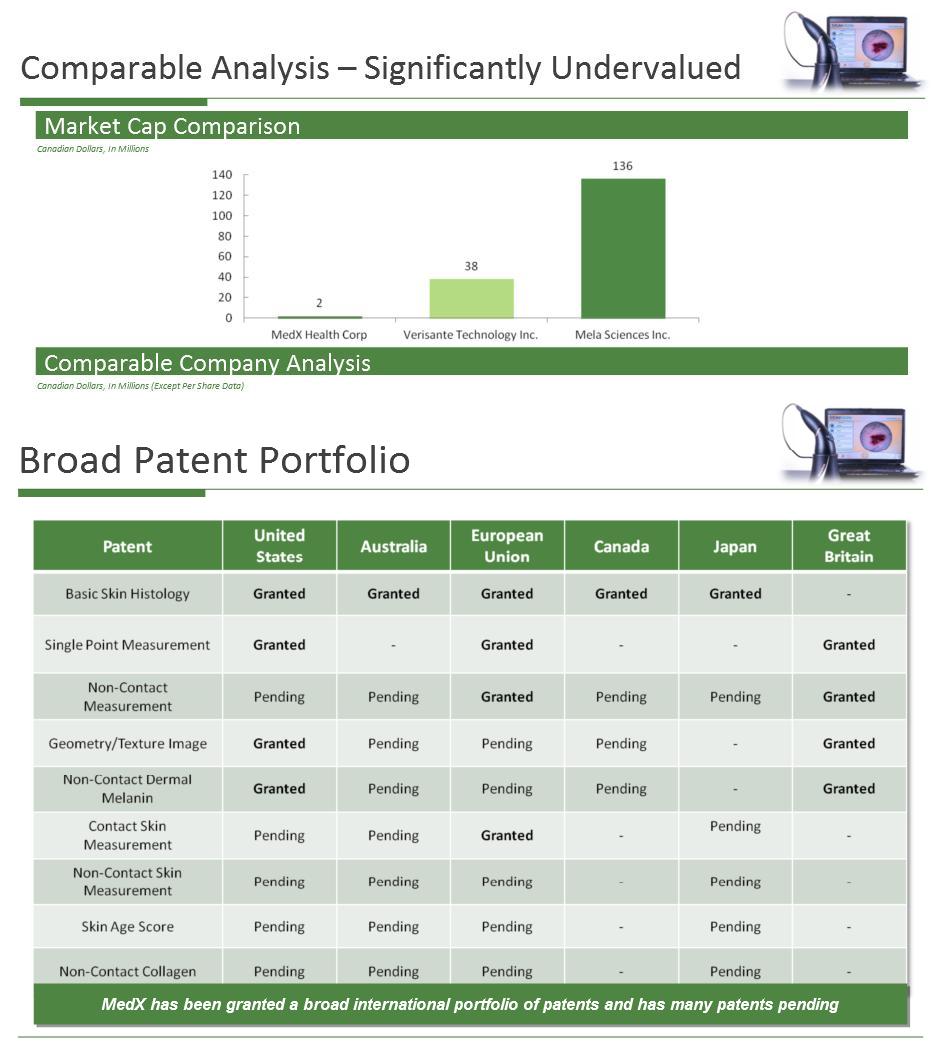
Eine News zur Marktreife bzw. FDA Zulassung wird erwartet. Meff ist in einem sehr spannenden Quartal. Krebsfrüherkennung mit MedX MoleMate die einzigartigen Phototherapie Produkte zahlreiche Produktentwicklungen kurz vor Zulassung oder MarktEinführung etc.
Medx steht vor interessantem Newsflow.
Die Frage ist wo eröffnet die Aktie bei Bekanntgabe. Die letzte Produktnews sorgte für über 350 % Kursgewinn an einem einzigen Tag.
Diesmal sind die Umstände aufgrund der stark steigenden Krebs Produkte deutlich besser. Medx bald 1 $ / Aktie

Zitat von Gesprengt: Medx's Molemate System wird weltweit zur Anwendung kommen zur Hautkrebs Früherkennung die Aktie sollte kräftig steigen denn das Medx Produkt rettet Leben.
Your dermatologist will examine your moles using new technologies like MoleMate, an FDA-approved non-invasive, painless melanoma-screening device. This method eliminates the need for a biopsy, making the check-up process far less intimidating. The hand-held scanner uses light and a skin-imaging technology to view up to a depth of 2mm below the surface of suspicious moles and lesions. It measures the concentration and distribution of the skin’s melanin, collagen and haemoglobin to detect cancerous melanoma, and the images it generates are used by doctors to ascertain if a mole is benign or malignant. The technology is available across the UAE, including Aesthetics International, and is worth doing to give you peace of mind – plus, it could save your life. “If detected in its pre-cancerous stage, a mole can be treated before it develops into skin cancer,” says Dr Khan.
NEWS
10. Juli 2013
http://m.gulfnews.com/life/health/know-your-moles-1.120736
Beitrag zu dieser Diskussion schreiben
Zu dieser Diskussion können keine Beiträge mehr verfasst werden, da der letzte Beitrag vor mehr als zwei Jahren verfasst wurde und die Diskussion daraufhin archiviert wurde.
Bitte wenden Sie sich an feedback@wallstreet-online.de und erfragen Sie die Reaktivierung der Diskussion oder starten Sie eine neue Diskussion.
Meistdiskutiert
| Wertpapier | Beiträge | |
|---|---|---|
| 48 | ||
| 44 | ||
| 28 | ||
| 22 | ||
| 17 | ||
| 16 | ||
| 14 | ||
| 13 | ||
| 10 | ||
| 10 |
| Wertpapier | Beiträge | |
|---|---|---|
| 9 | ||
| 8 | ||
| 7 | ||
| 7 | ||
| 7 | ||
| 6 | ||
| 6 | ||
| 5 | ||
| 5 | ||
| 5 |



















