Gewinnerbranchen der Jahre 2006 bis 2040 (Seite 915)
eröffnet am 10.12.06 16:57:17 von
neuester Beitrag 16.02.24 09:33:08 von
neuester Beitrag 16.02.24 09:33:08 von
Beiträge: 94.068
ID: 1.099.361
ID: 1.099.361
Aufrufe heute: 10
Gesamt: 3.535.966
Gesamt: 3.535.966
Aktive User: 0
Top-Diskussionen
| Titel | letzter Beitrag | Aufrufe |
|---|---|---|
| heute 18:36 | 8374 | |
| vor 1 Stunde | 6211 | |
| vor 52 Minuten | 5520 | |
| vor 1 Stunde | 4182 | |
| vor 1 Stunde | 3556 | |
| vor 39 Minuten | 3551 | |
| heute 20:58 | 3225 | |
| vor 1 Stunde | 3122 |
Meistdiskutierte Wertpapiere
| Platz | vorher | Wertpapier | Kurs | Perf. % | Anzahl | ||
|---|---|---|---|---|---|---|---|
| 1. | 3. | 18.159,50 | -0,16 | 205 | |||
| 2. | 5. | 193,94 | +15,24 | 117 | |||
| 3. | 4. | 2.334,81 | -0,14 | 58 | |||
| 4. | 19. | 65,95 | -2,66 | 50 | |||
| 5. | 6. | 7,9000 | +7,48 | 46 | |||
| 6. | 2. | 0,8300 | -29,66 | 39 | |||
| 7. | 39. | 15,116 | -5,73 | 35 | |||
| 8. | 33. | 0,1785 | -7,03 | 30 |
Beitrag zu dieser Diskussion schreiben
Antwort auf Beitrag Nr.: 59.587.031 von flying.kangaroo am 08.01.19 19:50:18zu VSTM:
What were the results presented at the 2018 ASH Annual Meeting?
The design of the DUO trial included a crossover portion, so patients treated with ofatumumab who experienced disease progression could then move on to duvelisib. At this meeting, I presented the updated data of the 90 patients who essentially crossed over to duvelisib monotherapy. The patients who received duvelisib tended to do well in general. We saw responses in about three-quarters of the patients. The PFS rates were comparable to what we saw in the earlier parts of the study. There did not appear to be any detriment to being treated with ofatumumab first in terms of duvelisib response. The drug was generally well tolerated for most patients, although there are some AEs that need to be monitored closely, such as diarrhea and colitis.
One of the biggest questions we had was whether patients with different molecular markers would spend differently. We were particularly curious about the patients with the high-risk del(17p) abnormalities. Fortunately, both PFS and response rates were very similar compared to the larger group.
What are the key takeaways from the trial?
Duvelisib appears to be an effective treatment option for relapsed CLL after 2 or more lines of therapy. Patients may receive other therapies and still do very well on duvelisib. Although we have many different options in CLL, there can never be too many new treatments.
What are some unanswered questions with duvelisib?
Going forward, we know the activity of this drug as a single agent. One of the things I would be excited to see is what this drug can do in combination with other agents. We have been running a frontline setting of duvelisib with FCR, and we are testing it with venetoclax in relapsed/refractory patients.
https://www.onclive.com/web-exclusives/crossover-to-duvelisi…
das sieht heute nach einem ziemlichen Hüpfer aus - sehr hohes ask, Donnerstag JPM-Konferenz, Ende der lock-out-period, sehr hohe short-quote!
greets cleara
What were the results presented at the 2018 ASH Annual Meeting?
The design of the DUO trial included a crossover portion, so patients treated with ofatumumab who experienced disease progression could then move on to duvelisib. At this meeting, I presented the updated data of the 90 patients who essentially crossed over to duvelisib monotherapy. The patients who received duvelisib tended to do well in general. We saw responses in about three-quarters of the patients. The PFS rates were comparable to what we saw in the earlier parts of the study. There did not appear to be any detriment to being treated with ofatumumab first in terms of duvelisib response. The drug was generally well tolerated for most patients, although there are some AEs that need to be monitored closely, such as diarrhea and colitis.
One of the biggest questions we had was whether patients with different molecular markers would spend differently. We were particularly curious about the patients with the high-risk del(17p) abnormalities. Fortunately, both PFS and response rates were very similar compared to the larger group.
What are the key takeaways from the trial?
Duvelisib appears to be an effective treatment option for relapsed CLL after 2 or more lines of therapy. Patients may receive other therapies and still do very well on duvelisib. Although we have many different options in CLL, there can never be too many new treatments.
What are some unanswered questions with duvelisib?
Going forward, we know the activity of this drug as a single agent. One of the things I would be excited to see is what this drug can do in combination with other agents. We have been running a frontline setting of duvelisib with FCR, and we are testing it with venetoclax in relapsed/refractory patients.
https://www.onclive.com/web-exclusives/crossover-to-duvelisi…
das sieht heute nach einem ziemlichen Hüpfer aus - sehr hohes ask, Donnerstag JPM-Konferenz, Ende der lock-out-period, sehr hohe short-quote!
greets cleara
Antwort auf Beitrag Nr.: 59.587.865 von clearasil am 08.01.19 21:32:48noch ein sehr guter zusammenfassender Artikel zu BVXP.
https://www.growthcompany.co.uk/bioventix-2593513/" target="_blank" rel="nofollow ugc noopener">
https://www.growthcompany.co.uk/bioventix-2593513/
Berichtigung: sie haben doch 16 Mitarbeiter.
https://www.growthcompany.co.uk/bioventix-2593513/" target="_blank" rel="nofollow ugc noopener">
https://www.growthcompany.co.uk/bioventix-2593513/
Berichtigung: sie haben doch 16 Mitarbeiter.

zu BVXP: mal mehr zu einer meiner stabilsten Aktien in 2018 mit in der Spitze über 40% Kursgewinnen plus einer stattlichen Dividende und Extradividende.
BVXP produziert mit 12 Mitarbeitern in einem kleinen Firmengebäude monoklonale Antikörper aus Schafen, normalerweise werden diese Antikörper aus Mäusen gewonnen (Morphosys). CEO Peter Harrison sagt, dass vielleicht wg. der Grösse und Länge der Schafe diese Antikörper qualitativ besser sind wie die von Mäusen.

das Video: https://www.youtube.com/watch?v=UheCnw9k78o
CEO Peter Harrison erklärt in diesem Video perfekt, was Bioventix macht, wie es funktioniert, wie damit nachhaltig sehr hohe Renditen erzielt werden, wo die Chancen für die Zukunft liegen (verbesserter Troponintest für Siemens Healthineers, der in den kommenden Jahren den Umsatz treiben wird, alte Maschinen werden nach und nach durch neue ersetzt). Obwohl die Umsätze langsamer kommen, wie erwartet - die Aktie blieb stabil.
Bioventix bekommt für jeden Blut-Test, der mit ihren Antikörpern durchgeführt wird eine royalty, z.B. 2 cents von einem Pfund Umsatz.
Bioventix beliefert alle großen Diagnosefirmen!: Roche, Siemens, Beckman-Coulter, Abott.
In der sehr langfristigen Entwicklung auch ein Antikörper für die Alzheimer-Forschung.
hier Infos und gutes Forum, in dem alle firmen-infos normalerweise früh auftauchen:https://uk.advfn.com/stock-market/london/bioventix-BVXP/shar…" target="_blank" rel="nofollow ugc noopener">
https://uk.advfn.com/stock-market/london/bioventix-BVXP/shar…
wie bereits gesagt: Aktie in 2018 weitgehend losgelöst von normalen Börsen-Schwankungen.
fact-sheet:
https://www.bioventix.com/wp-content/uploads/bvxp-presentati…" target="_blank" rel="nofollow ugc noopener">
https://www.bioventix.com/wp-content/uploads/bvxp-presentati…
hier noch ein guter blog, der ebenfalls bvxp hält:
https://maynardpaton.com/2019/01/01/year-in-review-2018/#mor…
Fazit: winzige Firma, sehr profitabel, genialer Gründer, nachhaltiges Geschäftsmodell in einer schwer zu kopierenden Nische. Ähnlich großartig wie Abcam.
greets cleara
BVXP produziert mit 12 Mitarbeitern in einem kleinen Firmengebäude monoklonale Antikörper aus Schafen, normalerweise werden diese Antikörper aus Mäusen gewonnen (Morphosys). CEO Peter Harrison sagt, dass vielleicht wg. der Grösse und Länge der Schafe diese Antikörper qualitativ besser sind wie die von Mäusen.


das Video: https://www.youtube.com/watch?v=UheCnw9k78o
CEO Peter Harrison erklärt in diesem Video perfekt, was Bioventix macht, wie es funktioniert, wie damit nachhaltig sehr hohe Renditen erzielt werden, wo die Chancen für die Zukunft liegen (verbesserter Troponintest für Siemens Healthineers, der in den kommenden Jahren den Umsatz treiben wird, alte Maschinen werden nach und nach durch neue ersetzt). Obwohl die Umsätze langsamer kommen, wie erwartet - die Aktie blieb stabil.
Bioventix bekommt für jeden Blut-Test, der mit ihren Antikörpern durchgeführt wird eine royalty, z.B. 2 cents von einem Pfund Umsatz.
Bioventix beliefert alle großen Diagnosefirmen!: Roche, Siemens, Beckman-Coulter, Abott.
In der sehr langfristigen Entwicklung auch ein Antikörper für die Alzheimer-Forschung.
hier Infos und gutes Forum, in dem alle firmen-infos normalerweise früh auftauchen:https://uk.advfn.com/stock-market/london/bioventix-BVXP/shar…" target="_blank" rel="nofollow ugc noopener">
https://uk.advfn.com/stock-market/london/bioventix-BVXP/shar…
wie bereits gesagt: Aktie in 2018 weitgehend losgelöst von normalen Börsen-Schwankungen.
fact-sheet:
https://www.bioventix.com/wp-content/uploads/bvxp-presentati…" target="_blank" rel="nofollow ugc noopener">
https://www.bioventix.com/wp-content/uploads/bvxp-presentati…
hier noch ein guter blog, der ebenfalls bvxp hält:
https://maynardpaton.com/2019/01/01/year-in-review-2018/#mor…
Fazit: winzige Firma, sehr profitabel, genialer Gründer, nachhaltiges Geschäftsmodell in einer schwer zu kopierenden Nische. Ähnlich großartig wie Abcam.
greets cleara
Antwort auf Beitrag Nr.: 59.586.950 von flying.kangaroo am 08.01.19 19:43:25Der beste Comment vom Autor selber zur gestrigen Options-NEWS (thx cbär):
SA surprisingly published the article without asking for any corrections. I would have added something about the hundreds of thousands of options the company gave to the top 5 directors at $3.51 yesterday. They had a very curious clause:
"2. The option vests as to: (a) 40% of the shares upon the date on which the closing price per share of the common stock is at least $10.00 on at least 20 (whether or not consecutive) of the prior 30 trading days, (b) 40% of the shares shall vest upon the date on which the closing price per share of the common stock is at least $15.00 on at least 20 (whether or not consecutive) of the prior 30 trading days, and (c) 20% of the shares shall vest upon the date on which the closing price per share of the common stock is at least $20.00 on at least 20 (whether or not consecutive) of the prior 30 trading days, provided that the Reporting Person continues to serve as an employee of or other service provider to the Issuer on each such vesting date."
investor.verastem.com/...
The directors cannot vest any of these new options below the stock price of $10. I see this as a brilliant incentive given by the board to directors and also gives us a glimpse at what they think the stock price should be at the moment.
SA surprisingly published the article without asking for any corrections. I would have added something about the hundreds of thousands of options the company gave to the top 5 directors at $3.51 yesterday. They had a very curious clause:
"2. The option vests as to: (a) 40% of the shares upon the date on which the closing price per share of the common stock is at least $10.00 on at least 20 (whether or not consecutive) of the prior 30 trading days, (b) 40% of the shares shall vest upon the date on which the closing price per share of the common stock is at least $15.00 on at least 20 (whether or not consecutive) of the prior 30 trading days, and (c) 20% of the shares shall vest upon the date on which the closing price per share of the common stock is at least $20.00 on at least 20 (whether or not consecutive) of the prior 30 trading days, provided that the Reporting Person continues to serve as an employee of or other service provider to the Issuer on each such vesting date."
investor.verastem.com/...
The directors cannot vest any of these new options below the stock price of $10. I see this as a brilliant incentive given by the board to directors and also gives us a glimpse at what they think the stock price should be at the moment.
https://seekingalpha.com/article/4232072-rout-happened-now
A Rout Happened, What Now?
Jan. 8, 2019 12:00 PM ET|
About: Verastem, Inc. (VSTM)
At the lowest point of this recent decline, Verastem had lost almost 70% of it's 2018 high valuation.
The company trades at the moment at a valuation of $280M with $279M cash end of Q3 and also eligible for $250M Japan and China milestone payments.
Speculative Bloomberg Symphony Copiktra script numbers show steady growth, which will become accumulative in time.
The company will now have revenues flowing in - something a lot of small cap/speculative bios hit hard by the market cannot say.
I continue now my coverage of the Needham, MA oncology singlet Verastem (VSTM) with my third article (first here, second here) about it since August 2018. A ride that has had days of glory and of utter despair - reaching a stock price of $10.3, the FDA approval of it's Leukemia drug Copiktra (Duvelisib) and partnership news in China and institutional partners in the US, only to be followed by a destruction of 70% of the company's stock valuation to €3.14 with seemingly no bad news. What were the causes and what can the future hold for brave longs still holding and new ones buying the seriously cheaper shares?
The Apocalypse and the Index Connection
"“Sure, everything is ending," Jules said, "but not yet.”" - Jennifer Egan
The bio industry and it's indexes were hit hard, very hard, when the stock market fell apart during this fall and early winter of 2018 amidst the Fed rising rates (money flees into safer bonds with less risk) and the Trump vs China trade war mayhem, causing the media hysteria and headlines to frighten people even more even with basically good news from the US economy. Could there be opportunities found in all this chaos?
For people not understanding the relation of indexes with single companies, I try to use the following chart examples. I do not claim to know the whole mechanism how indexes or especially Electronically Traded Funds (ETF) affect the companies that are in them but there certainly is some relation. In Verastem's case, one can day by day observe the ETF's that own the company's shares for example here:
Source: Fidelity
We can see from the situation now (1/6/2019), that the SPDR Biotech ETF XBI (XBI), which tracks small cap bios, owns at the moment around 7.27M shares (exposure divided by current price) of VSTM. On Dec 20th, the company also issued a press release of Verastem to be included into NBI, the Nasdaq's biggest and boldest bio index tracking mostly larger cap bios. The ETF tracking NBI is iShares Nasdaq Biotechnology ETF IBB (IBB), which now owns just under a million VSTM shares.
Roughly estimated, ETF's now own around 10M of Verastem shares, that is 13.6% of all outstanding shares. So in theory, if the ETF's trade the companies (you can trade ETF's like normal shares, which confirms some of this theory) in them and they start to go down, it can be a death sentence for a stock that does not have significant buying power towards it. Of course when the indexes go up, with this theory, it can be a positive for a stock. I also did some some very unscientific analysis of hours of tick by tick comparison and thought of seeing some pattern of ETF movement affecting a specific company a bit later. Of course it could also be that the movement of the companies in an ETF move the ETF value. Likely it's a bit of both. However, one thing is for certain, VSTM daily trades do not affect XBI daily values in any meaningful way - XBI exposure to VSTM is only 0.74%.
So how does the XBI chart look compared to the VSTM chart?
Source: Yahoo
XBI started its downtrend on August 31st. What is most curious, VSTM started its downtrend on the very same day:
Source: Yahoo
How did IBB fare?
Source: Yahoo
IBB managed to shortly bounce back but also continued its decline afterwards.
It would be very hard for one to spot the VSTM chart after Aug 31st from these three in a blind test and I'm sure that there are plenty of bios out there affected the same way. Pretty much all have, sooner or later, been impacted by this rout. The Dow Jones industrial index managed to survive a bit longer but you can also see the similarities with the larger cap bio IBB index:
Source: Yahoo
Another possibility is of course, that there are some algorithms that trade all, in this case bio stocks, which in turn affects all the indexes. I also read somewhere, that these algos are programmed to read headlines, which would explain the recent moves with mass panic abound..
But one thing is for sure, much of the recent small cap bio destruction is not attributable to company fundamentals.
Future Outlook
On Jan 3rd management issued a PR called "Verastem Oncology Outlines Strategic Priorities for 2019 and Highlights Recent Progress":
"“Since the launch of COPIKTRA, we’ve been encouraged by the positive feedback we are hearing from physicians and other healthcare providers about this important new oral monotherapy within the treatment landscape,” said Joseph Lobacki, Executive Vice President and Chief Commercial Officer of Verastem Oncology. “Following the approval, COPIKTRA was quickly added to the National Comprehensive Cancer Network® (NCCN) guidelines, which has led to its inclusion on formularies and extensive reimbursement coverage, including on the top national health plans, reaching approximately 75% of U.S. Pharmacy lives and providing critical access to treatment for appropriate patients. In 2019, the commercial team will be diligently working to engage with physicians and other health care professionals to focus on ensuring COPIKTRA reaches the patients who need it.”"
Source
The NCCN is the authority that medical doctors and insurance companies follow when deciding on treatments, making this great news. Also the reimbursement coverage and inclusion in the top national health plans is an important piece of news, something bears were skeptical of a few months ago. Sick people can now get this treatment with a smaller amount of their own personal money.
The company also got a Therapy Acceleration Program funding deal for PTCL from the The Leukemia & Lymphoma Society TAP program, making it the LLS's last collaboration of 2018 in very big company:
"Collaboration with The Leukemia & Lymphoma Society for Development of Duvelisib in Peripheral T-Cell Lymphoma – Duvelisib was selected for The Leukemia & Lymphoma Society’s (LLS) Therapy Acceleration Program® (TAP) which provides additional resources to support the development of therapies for patients with blood cancers. The Company plans to use the TAP funds to conduct certain translational and clinical activities relating to the development of duvelisib for the treatment of Peripheral T-Cell Lymphoma (PTCL). LLS and Verastem Oncology will share the cost of the PTCL development program, portions of which will be conducted in collaboration with Memorial Sloan Kettering Cancer Center, The Dana-Farber Cancer Institute, The Washington University in St. Louis and Stanford University."
Source
This will decrease quarterly burn as outsider money will fund some of the pipeline research.
A possible PTCL FDA approval would be huge for the company, since few efficient treatments exist and most patients relapse (I recommend reading this source link if you are interested in the subject). PTCL is also more prevalent in Asia, where VSTM has already partnerships. On Dec 3rd, the company posted new phase 1 combo data on duvelisib and romidepsin for PTCL. The results showed an ORR (Overall Response Rate) of 55% which is extremely impressive with the best competition rate being around 20-30%.
Source: Verastem Investor Presentation
The same information on recent treatments can also be found in that source link I recommended earlier.
"About 74,680 people (41,730 males and 32,950 females) will be diagnosed with NHL" (source) and "Between 10 percent and 15 percent of all patients with NHL have a T-cell lymphoma subtype (source). So around 7400-11202 new annual cases emerge in the US, a viable market opportunity for Duvelisib mono and combo.
They also commented on the balance sheet:
"Entering 2019 with a Strong Balance Sheet– In May 2018, Verastem Oncology successfully completed multiple fundraising transactions, including an underwritten registered offering in May 2018, a registered offering in June 2018, and a registered direct offering of 5.00% convertible senior notes in October 2018. The Company also raised funds through the sale of shares of common stock under its at-the-market equity offering program. The Company has approximately $279 million in cash and cash equivalents pro-forma to the close of the third quarter of 2018.".
Many stockholders were extremely agitated by managements decision to use convertible notes in the offering, since it might attract shorting as a delta hedge, but I really do not see it as a bigger problem. Convertible notes are very, very widely used and it would be madness to assume that all CEO's who decide to use them are suicidal with the stock prices they are responsible to increase.
2019 priorities listed by the management can be seen from below:
Source
LinkedIn states that the company has 169 employees at the moment, showing a recent increase.
The current pipeline looks like this:
Source
Revenue?
In this recent stock market rout, VSTM was very lucky to have just turned into a company doing actual revenue. One bear thesis on the recent bio drop was (as was with the FDA drop of VSTM), that very few companies produce revenue and should not be valued so high, which is a somewhat fair opinion. Is there any possible data out there to tell anything about future revenue? Seeking Alpha is a community share data mining site after all. Management has kept a lid on this information so far and we won't know for sure until the next ER.
User handle FEDERICO has posted some prescription numbers on the Yahoo conversation board of VSTM. They are apparently from the pay-to-use Bloomberg Intelligence Symphony database. More info on the subject for example in another SA article here. I have confirmed these numbers are really from the Symphony database and proof of it (Symphony screenshots, which I can't/won't post here), so it's not just some random numbers from a random guy from the internet. Below you can see the RETAIL numbers by week for Q3:
Apparently there are also INSTITUTIONAL numbers to count in also (Symphony screenshots confirm this), but I do not know how this works and in any case there is no data available of them on Symphony. I also do not know which of these are refills, since naturally the treatment does not last one month.
The Phase 3 DUO study had treatments vary from 12 to 36 months: "~40% of patients treated with duvelisib remained on treatment for over 18 months, with a median total follow-up of nearly 2 years " (source).
Now, I have seen some discussion on the numbers and also seen some comparisons of other bio companies with some recent quarterly ER data to compare them with and Symphony data seems to be generally a bit on the low side of the actualized numbers. So if we count the retail number of 55 prescriptions, add another 55 from the institutional side, we get 110 prescriptions in total for Q4.
110 x $11 800 (monthly list price) = $1.298M revenues for Q4.
I'm not using a discounted price since I'm not that familiar with it and also remember, insurance has turned out to cover most of the treatment costs.
Now this may seem low but is actually what I and some analysts have been expecting for Q4. Remember, Q4 basically has sales only, because Duvelisib got the FDA priority review. If the company can double the sales on every quarter on 2019, which can happen since these scripts slowly start to accumulate when people staying in the treatment get monthly refills, we can see the following:
Q1: $2.6M
Q2: $5.2M
Q3: $10.4M
Q4: $20.8M
2019: $39M
I have stated before, that I'm satisfied with $50M 2019 revenues, with the peak sales of course being much higher. $40-50M is also what many analysts are expecting for 2019. Operating expenses for Q3 were $37M. We have to remember that we now can deduct Copiktra development costs from that but also need add some employee costs. With $279M cash, Verastem can run for another 7 quarters with no revenues/milestone payments whatsoever, which of course will not be the case. The now confirmed Japan and China partnerships still have $250M of open milestone payments due at certain milestones.
If we want to value a small cap bio with ONLY the revenues it can create in 2019 (of course at some point one needs to start thinking of 2020 revenues also), I can get a following valuation using the price/sales ratio of 12 used also before:
12 x $39M = $468M / 73M shares = $6.4
A 12 month stock price estimate showing an almost 70% possible upside but still only is a fraction of Verastem's oncology singlet peer valuations which I discussed about in my former article(s) about Verastem.
A very conservative ratio number of 8 (average large cap pharma ratio is 5-7 depending where you look) would yield the following valuation for 2019, with the speculative script numbers and revenues calculated from them:
8 x $39M = $468M / 73M shares = $4.27
Now I would like to emphasize, I do not know the legitimacy of the script numbers and also that this does not value anything else in the company, cash or other pipeline, BO likelihood etc. In a stock market where all information would be available for everyone to use, there most likely would be no money to be made for anyone since everything would be perfectly valued.
The 6 analysts price targets have not changed, still showing an average 12 month target price of $15.8, an upside of 318%.
Source: MarketScreener
The Chart
Actually my favorite part of the company at the moment. Following a slight bounce in the general market, VSTM also bounced from it's earlier years support of 3ish and the stock has actually gone above the recent downtrend line for the first time since Dec 4th with a solid short term uptrend. The Relative Strength Index RSI is looking for a higher high after a higher low (as it is also with the actual short term chart), same for the Moving Average Convergence Divergence or MACD. But if no substantial news are to hit the company, this bounce is likely in the mercy of the recently very moody market gods. So far so good!
Source: Yahoo
The weekly chart is also recovering, with the RSI, MACD and Stochastics hitting the lowest points since early 2016.
Source: Yahoo
Float
Institutional ownership at the moment is at 49.85% which equals 36.8M shares. This data is for Q3 and will likely change for Q4 (which already is over), so I won't go over it now. I've seen some charts which have compared the money flow of retail and institutions this fall and as retail sold, institutions added, so there might be some positives to expect.
Source: Nasdaq
Shares short from the latest mid Dec data are 17.4M. Number show a slight decrease and new data for the last two weeks of December should come in the following days. It is very apparent that many new shorts were opened during the declining trend. I recommend to keep an eye on the situation.
Source: Nasdaq
If you add the shares in the Q3 institutional holdings (ETF's should be included), insider holdings and shares short, you get roughly 55M shares, leaving 18.8M shares to be traded around and only 11M with the Yahoo reported float of 66M.
Now what makes this interesting (if I understand the mechanism correctly) is, that there are hardly enough shares for the 17.43M shorts shares to cover. Shorts need to buy shares to cover - shares short are shares on loan from other owners (so they can be considered to be "stuck" shares) that need to be bought back to "cover" and return them to the original owner, hopefully lower and thus with a profit. This will make the near future quite interesting with a likelihood of a "short squeeze", where a covering panic with an increasing stock price forces the shorts to send the price higher quickly because they essentially need to buy higher and higher, possibly all available shares. One can also think of it as stop losses/margin calls hitting longs in an inverted, "normal" situation.
Conclusion
There are many things going for VSTM even at this bleak time in the general market with some light in the end of tunnel. There are plenty of catalysts for 2019, with several pipeline data dates and the management is actively seeking ex-us partnerships and EU approval for Copiktra.
The company valuation is low especially considering the company's cash position, which pretty much equals the market cap at the moment - something I don't know how common it is generally. Yes, the recent convertible note offering $150M can be considered debt but it still pays the bills and VSTM has cash to go on for years with absolutely no revenue, which will now slowly start to come in.
Some very speculative script numbers point to sales being made and growing, helping the company to become profitable step by step. Even with very meager 2019 sales the company seems very, very undervalued at the moment.
There have been some significant buy outs in the oncology space recently. Verastem's oncology singlet peer Tesaro (TSRO) was sold to GlaxoSmithKline (GSK) for $5.1B Dec 3rd 2018 and Bristol-Myers Squibb (BMY) threw $74B in cash and stocks at Celgene (CELG) last week. The most recent news came just today Monday morning 1/7, as I was proofreading this article: "Eli Lilly and Co (LLY.N) said on Monday it would buy Loxo Oncology Inc (LOXO.O) for about $8 billion in cash, buying into a portfolio of targeted medicines to treat cancers caused by rare gene mutations.", representing a 68% premium. Many were expecting 2018 to be the year of the bio buy outs but it seems the game is just getting heated.
So there is of course that horse in the race also. And the Verastem horse is cheap, cheap cheap even with a 100-300% premium.
There is significant cause to believe, not only the lack of bad news, that this most recent drop was likely mostly caused by the general market drop and is in relation to Verastem's 2018 additions into the Nasdaq's two largest Electronically Traded (bio) Funds XBI for small caps and IBB for larger caps.
All in all VSTM could now be a very nice dip play with very little other than general market/business risk, which IMO should also start to correct slowly to the upside in the coming months. The largest risk for Verastem at the moment seems to be the Q4 sales numbers. New owners can get the stock, much to the annoyance of longer holders, pretty much at the same price as before the FDA run with a large part of dilution etc risk removed from it. Also, we have yet to see that one very bad piece of news that would actually justify the current valuation.
A Rout Happened, What Now?
Jan. 8, 2019 12:00 PM ET|
About: Verastem, Inc. (VSTM)
At the lowest point of this recent decline, Verastem had lost almost 70% of it's 2018 high valuation.
The company trades at the moment at a valuation of $280M with $279M cash end of Q3 and also eligible for $250M Japan and China milestone payments.
Speculative Bloomberg Symphony Copiktra script numbers show steady growth, which will become accumulative in time.
The company will now have revenues flowing in - something a lot of small cap/speculative bios hit hard by the market cannot say.
I continue now my coverage of the Needham, MA oncology singlet Verastem (VSTM) with my third article (first here, second here) about it since August 2018. A ride that has had days of glory and of utter despair - reaching a stock price of $10.3, the FDA approval of it's Leukemia drug Copiktra (Duvelisib) and partnership news in China and institutional partners in the US, only to be followed by a destruction of 70% of the company's stock valuation to €3.14 with seemingly no bad news. What were the causes and what can the future hold for brave longs still holding and new ones buying the seriously cheaper shares?
The Apocalypse and the Index Connection
"“Sure, everything is ending," Jules said, "but not yet.”" - Jennifer Egan
The bio industry and it's indexes were hit hard, very hard, when the stock market fell apart during this fall and early winter of 2018 amidst the Fed rising rates (money flees into safer bonds with less risk) and the Trump vs China trade war mayhem, causing the media hysteria and headlines to frighten people even more even with basically good news from the US economy. Could there be opportunities found in all this chaos?
For people not understanding the relation of indexes with single companies, I try to use the following chart examples. I do not claim to know the whole mechanism how indexes or especially Electronically Traded Funds (ETF) affect the companies that are in them but there certainly is some relation. In Verastem's case, one can day by day observe the ETF's that own the company's shares for example here:
Source: Fidelity
We can see from the situation now (1/6/2019), that the SPDR Biotech ETF XBI (XBI), which tracks small cap bios, owns at the moment around 7.27M shares (exposure divided by current price) of VSTM. On Dec 20th, the company also issued a press release of Verastem to be included into NBI, the Nasdaq's biggest and boldest bio index tracking mostly larger cap bios. The ETF tracking NBI is iShares Nasdaq Biotechnology ETF IBB (IBB), which now owns just under a million VSTM shares.
Roughly estimated, ETF's now own around 10M of Verastem shares, that is 13.6% of all outstanding shares. So in theory, if the ETF's trade the companies (you can trade ETF's like normal shares, which confirms some of this theory) in them and they start to go down, it can be a death sentence for a stock that does not have significant buying power towards it. Of course when the indexes go up, with this theory, it can be a positive for a stock. I also did some some very unscientific analysis of hours of tick by tick comparison and thought of seeing some pattern of ETF movement affecting a specific company a bit later. Of course it could also be that the movement of the companies in an ETF move the ETF value. Likely it's a bit of both. However, one thing is for certain, VSTM daily trades do not affect XBI daily values in any meaningful way - XBI exposure to VSTM is only 0.74%.
So how does the XBI chart look compared to the VSTM chart?
Source: Yahoo
XBI started its downtrend on August 31st. What is most curious, VSTM started its downtrend on the very same day:
Source: Yahoo
How did IBB fare?
Source: Yahoo
IBB managed to shortly bounce back but also continued its decline afterwards.
It would be very hard for one to spot the VSTM chart after Aug 31st from these three in a blind test and I'm sure that there are plenty of bios out there affected the same way. Pretty much all have, sooner or later, been impacted by this rout. The Dow Jones industrial index managed to survive a bit longer but you can also see the similarities with the larger cap bio IBB index:
Source: Yahoo
Another possibility is of course, that there are some algorithms that trade all, in this case bio stocks, which in turn affects all the indexes. I also read somewhere, that these algos are programmed to read headlines, which would explain the recent moves with mass panic abound..
But one thing is for sure, much of the recent small cap bio destruction is not attributable to company fundamentals.
Future Outlook
On Jan 3rd management issued a PR called "Verastem Oncology Outlines Strategic Priorities for 2019 and Highlights Recent Progress":
"“Since the launch of COPIKTRA, we’ve been encouraged by the positive feedback we are hearing from physicians and other healthcare providers about this important new oral monotherapy within the treatment landscape,” said Joseph Lobacki, Executive Vice President and Chief Commercial Officer of Verastem Oncology. “Following the approval, COPIKTRA was quickly added to the National Comprehensive Cancer Network® (NCCN) guidelines, which has led to its inclusion on formularies and extensive reimbursement coverage, including on the top national health plans, reaching approximately 75% of U.S. Pharmacy lives and providing critical access to treatment for appropriate patients. In 2019, the commercial team will be diligently working to engage with physicians and other health care professionals to focus on ensuring COPIKTRA reaches the patients who need it.”"
Source
The NCCN is the authority that medical doctors and insurance companies follow when deciding on treatments, making this great news. Also the reimbursement coverage and inclusion in the top national health plans is an important piece of news, something bears were skeptical of a few months ago. Sick people can now get this treatment with a smaller amount of their own personal money.
The company also got a Therapy Acceleration Program funding deal for PTCL from the The Leukemia & Lymphoma Society TAP program, making it the LLS's last collaboration of 2018 in very big company:
"Collaboration with The Leukemia & Lymphoma Society for Development of Duvelisib in Peripheral T-Cell Lymphoma – Duvelisib was selected for The Leukemia & Lymphoma Society’s (LLS) Therapy Acceleration Program® (TAP) which provides additional resources to support the development of therapies for patients with blood cancers. The Company plans to use the TAP funds to conduct certain translational and clinical activities relating to the development of duvelisib for the treatment of Peripheral T-Cell Lymphoma (PTCL). LLS and Verastem Oncology will share the cost of the PTCL development program, portions of which will be conducted in collaboration with Memorial Sloan Kettering Cancer Center, The Dana-Farber Cancer Institute, The Washington University in St. Louis and Stanford University."
Source
This will decrease quarterly burn as outsider money will fund some of the pipeline research.
A possible PTCL FDA approval would be huge for the company, since few efficient treatments exist and most patients relapse (I recommend reading this source link if you are interested in the subject). PTCL is also more prevalent in Asia, where VSTM has already partnerships. On Dec 3rd, the company posted new phase 1 combo data on duvelisib and romidepsin for PTCL. The results showed an ORR (Overall Response Rate) of 55% which is extremely impressive with the best competition rate being around 20-30%.
Source: Verastem Investor Presentation
The same information on recent treatments can also be found in that source link I recommended earlier.
"About 74,680 people (41,730 males and 32,950 females) will be diagnosed with NHL" (source) and "Between 10 percent and 15 percent of all patients with NHL have a T-cell lymphoma subtype (source). So around 7400-11202 new annual cases emerge in the US, a viable market opportunity for Duvelisib mono and combo.
They also commented on the balance sheet:
"Entering 2019 with a Strong Balance Sheet– In May 2018, Verastem Oncology successfully completed multiple fundraising transactions, including an underwritten registered offering in May 2018, a registered offering in June 2018, and a registered direct offering of 5.00% convertible senior notes in October 2018. The Company also raised funds through the sale of shares of common stock under its at-the-market equity offering program. The Company has approximately $279 million in cash and cash equivalents pro-forma to the close of the third quarter of 2018.".
Many stockholders were extremely agitated by managements decision to use convertible notes in the offering, since it might attract shorting as a delta hedge, but I really do not see it as a bigger problem. Convertible notes are very, very widely used and it would be madness to assume that all CEO's who decide to use them are suicidal with the stock prices they are responsible to increase.
2019 priorities listed by the management can be seen from below:
Source
LinkedIn states that the company has 169 employees at the moment, showing a recent increase.
The current pipeline looks like this:
Source
Revenue?
In this recent stock market rout, VSTM was very lucky to have just turned into a company doing actual revenue. One bear thesis on the recent bio drop was (as was with the FDA drop of VSTM), that very few companies produce revenue and should not be valued so high, which is a somewhat fair opinion. Is there any possible data out there to tell anything about future revenue? Seeking Alpha is a community share data mining site after all. Management has kept a lid on this information so far and we won't know for sure until the next ER.
User handle FEDERICO has posted some prescription numbers on the Yahoo conversation board of VSTM. They are apparently from the pay-to-use Bloomberg Intelligence Symphony database. More info on the subject for example in another SA article here. I have confirmed these numbers are really from the Symphony database and proof of it (Symphony screenshots, which I can't/won't post here), so it's not just some random numbers from a random guy from the internet. Below you can see the RETAIL numbers by week for Q3:
Apparently there are also INSTITUTIONAL numbers to count in also (Symphony screenshots confirm this), but I do not know how this works and in any case there is no data available of them on Symphony. I also do not know which of these are refills, since naturally the treatment does not last one month.
The Phase 3 DUO study had treatments vary from 12 to 36 months: "~40% of patients treated with duvelisib remained on treatment for over 18 months, with a median total follow-up of nearly 2 years " (source).
Now, I have seen some discussion on the numbers and also seen some comparisons of other bio companies with some recent quarterly ER data to compare them with and Symphony data seems to be generally a bit on the low side of the actualized numbers. So if we count the retail number of 55 prescriptions, add another 55 from the institutional side, we get 110 prescriptions in total for Q4.
110 x $11 800 (monthly list price) = $1.298M revenues for Q4.
I'm not using a discounted price since I'm not that familiar with it and also remember, insurance has turned out to cover most of the treatment costs.
Now this may seem low but is actually what I and some analysts have been expecting for Q4. Remember, Q4 basically has sales only, because Duvelisib got the FDA priority review. If the company can double the sales on every quarter on 2019, which can happen since these scripts slowly start to accumulate when people staying in the treatment get monthly refills, we can see the following:
Q1: $2.6M
Q2: $5.2M
Q3: $10.4M
Q4: $20.8M
2019: $39M
I have stated before, that I'm satisfied with $50M 2019 revenues, with the peak sales of course being much higher. $40-50M is also what many analysts are expecting for 2019. Operating expenses for Q3 were $37M. We have to remember that we now can deduct Copiktra development costs from that but also need add some employee costs. With $279M cash, Verastem can run for another 7 quarters with no revenues/milestone payments whatsoever, which of course will not be the case. The now confirmed Japan and China partnerships still have $250M of open milestone payments due at certain milestones.
If we want to value a small cap bio with ONLY the revenues it can create in 2019 (of course at some point one needs to start thinking of 2020 revenues also), I can get a following valuation using the price/sales ratio of 12 used also before:
12 x $39M = $468M / 73M shares = $6.4
A 12 month stock price estimate showing an almost 70% possible upside but still only is a fraction of Verastem's oncology singlet peer valuations which I discussed about in my former article(s) about Verastem.
A very conservative ratio number of 8 (average large cap pharma ratio is 5-7 depending where you look) would yield the following valuation for 2019, with the speculative script numbers and revenues calculated from them:
8 x $39M = $468M / 73M shares = $4.27
Now I would like to emphasize, I do not know the legitimacy of the script numbers and also that this does not value anything else in the company, cash or other pipeline, BO likelihood etc. In a stock market where all information would be available for everyone to use, there most likely would be no money to be made for anyone since everything would be perfectly valued.
The 6 analysts price targets have not changed, still showing an average 12 month target price of $15.8, an upside of 318%.
Source: MarketScreener
The Chart
Actually my favorite part of the company at the moment. Following a slight bounce in the general market, VSTM also bounced from it's earlier years support of 3ish and the stock has actually gone above the recent downtrend line for the first time since Dec 4th with a solid short term uptrend. The Relative Strength Index RSI is looking for a higher high after a higher low (as it is also with the actual short term chart), same for the Moving Average Convergence Divergence or MACD. But if no substantial news are to hit the company, this bounce is likely in the mercy of the recently very moody market gods. So far so good!
Source: Yahoo
The weekly chart is also recovering, with the RSI, MACD and Stochastics hitting the lowest points since early 2016.
Source: Yahoo
Float
Institutional ownership at the moment is at 49.85% which equals 36.8M shares. This data is for Q3 and will likely change for Q4 (which already is over), so I won't go over it now. I've seen some charts which have compared the money flow of retail and institutions this fall and as retail sold, institutions added, so there might be some positives to expect.
Source: Nasdaq
Shares short from the latest mid Dec data are 17.4M. Number show a slight decrease and new data for the last two weeks of December should come in the following days. It is very apparent that many new shorts were opened during the declining trend. I recommend to keep an eye on the situation.
Source: Nasdaq
If you add the shares in the Q3 institutional holdings (ETF's should be included), insider holdings and shares short, you get roughly 55M shares, leaving 18.8M shares to be traded around and only 11M with the Yahoo reported float of 66M.
Now what makes this interesting (if I understand the mechanism correctly) is, that there are hardly enough shares for the 17.43M shorts shares to cover. Shorts need to buy shares to cover - shares short are shares on loan from other owners (so they can be considered to be "stuck" shares) that need to be bought back to "cover" and return them to the original owner, hopefully lower and thus with a profit. This will make the near future quite interesting with a likelihood of a "short squeeze", where a covering panic with an increasing stock price forces the shorts to send the price higher quickly because they essentially need to buy higher and higher, possibly all available shares. One can also think of it as stop losses/margin calls hitting longs in an inverted, "normal" situation.
Conclusion
There are many things going for VSTM even at this bleak time in the general market with some light in the end of tunnel. There are plenty of catalysts for 2019, with several pipeline data dates and the management is actively seeking ex-us partnerships and EU approval for Copiktra.
The company valuation is low especially considering the company's cash position, which pretty much equals the market cap at the moment - something I don't know how common it is generally. Yes, the recent convertible note offering $150M can be considered debt but it still pays the bills and VSTM has cash to go on for years with absolutely no revenue, which will now slowly start to come in.
Some very speculative script numbers point to sales being made and growing, helping the company to become profitable step by step. Even with very meager 2019 sales the company seems very, very undervalued at the moment.
There have been some significant buy outs in the oncology space recently. Verastem's oncology singlet peer Tesaro (TSRO) was sold to GlaxoSmithKline (GSK) for $5.1B Dec 3rd 2018 and Bristol-Myers Squibb (BMY) threw $74B in cash and stocks at Celgene (CELG) last week. The most recent news came just today Monday morning 1/7, as I was proofreading this article: "Eli Lilly and Co (LLY.N) said on Monday it would buy Loxo Oncology Inc (LOXO.O) for about $8 billion in cash, buying into a portfolio of targeted medicines to treat cancers caused by rare gene mutations.", representing a 68% premium. Many were expecting 2018 to be the year of the bio buy outs but it seems the game is just getting heated.
So there is of course that horse in the race also. And the Verastem horse is cheap, cheap cheap even with a 100-300% premium.
There is significant cause to believe, not only the lack of bad news, that this most recent drop was likely mostly caused by the general market drop and is in relation to Verastem's 2018 additions into the Nasdaq's two largest Electronically Traded (bio) Funds XBI for small caps and IBB for larger caps.
All in all VSTM could now be a very nice dip play with very little other than general market/business risk, which IMO should also start to correct slowly to the upside in the coming months. The largest risk for Verastem at the moment seems to be the Q4 sales numbers. New owners can get the stock, much to the annoyance of longer holders, pretty much at the same price as before the FDA run with a large part of dilution etc risk removed from it. Also, we have yet to see that one very bad piece of news that would actually justify the current valuation.
Listenfutter
100 fastest growing companies.
http://fortune.com/100-fastest-growing-companies/" target="_blank" rel="nofollow ugc noopener">
http://fortune.com/100-fastest-growing-companies/
https://cts.businesswire.com/ct/CT?id=smartlink&url=https%3A…" target="_blank" rel="nofollow ugc noopener">
https://cts.businesswire.com/ct/CT?id=smartlink&url=https%3A…
100 fastest growing companies.
http://fortune.com/100-fastest-growing-companies/" target="_blank" rel="nofollow ugc noopener">
http://fortune.com/100-fastest-growing-companies/
https://cts.businesswire.com/ct/CT?id=smartlink&url=https%3A…" target="_blank" rel="nofollow ugc noopener">
https://cts.businesswire.com/ct/CT?id=smartlink&url=https%3A…
immer diese überteuerten glamouraktien. 
Der Online-Händler Amazon ist erstmals zum wertvollsten börsennotierten Unternehmen der Welt aufgestiegen. Die Aktie des amerikanischen Konzerns legte am Montag um 3,44 Prozent zu, Amazons Börsenwert erhöhte sich auf 797 Milliarden Dollar. Das Unternehmen überflügelte damit den bisherigen Spitzenreiter Microsoft.
Der amerikanische Softwaregigant hatte den Titel als wertvollstes Unternehmen erst Ende November vom kalifornischen Technologiekonzern Apple übernommen, der in der aktuellen Rangliste nur noch auf dem vierten Platz liegt.
https://www.faz.net/aktuell/wirtschaft/diginomics/amazon-ist…" target="_blank" rel="nofollow ugc noopener">
https://www.faz.net/aktuell/wirtschaft/diginomics/amazon-ist…

Der Online-Händler Amazon ist erstmals zum wertvollsten börsennotierten Unternehmen der Welt aufgestiegen. Die Aktie des amerikanischen Konzerns legte am Montag um 3,44 Prozent zu, Amazons Börsenwert erhöhte sich auf 797 Milliarden Dollar. Das Unternehmen überflügelte damit den bisherigen Spitzenreiter Microsoft.
Der amerikanische Softwaregigant hatte den Titel als wertvollstes Unternehmen erst Ende November vom kalifornischen Technologiekonzern Apple übernommen, der in der aktuellen Rangliste nur noch auf dem vierten Platz liegt.
https://www.faz.net/aktuell/wirtschaft/diginomics/amazon-ist…" target="_blank" rel="nofollow ugc noopener">
https://www.faz.net/aktuell/wirtschaft/diginomics/amazon-ist…
zu WYNN - sieht langsam besser aus. Wenn es zu einem Ende des Handelsstreits kommt, wird WYNN profitieren. Macau-Zahlen ohnehin gut.
https://www.ariva.de/chart/images/chart.png?z=a45411~A1~B38:…
https://www.ariva.de/chart/images/chart.png?z=a45411~A1~B38:…
zu INVA
+++
Strong Q3 Results
Innoviva reported earnings of $0.43 a share as revenue soared 26.8% to $61.68 million. The company’s balance sheet improved nicely after a $110 million Term B loan repayment. Royalty revenue from Glaxo Group (GSK) rose to $65.1 million, with $51.7 million coming from global sales of REVLAR/BREO ELLIPTA. Expenses fell by around half to $4.0 million, compared to $8.6 million last year. Even though last year’s costs included a $2.5 million proxy contest related litigation cost, management is finally driving sales higher.
+++
INVA shares trade at an annualized P/E of 10.3 times and a forward P/E of 8.8 times. In light of the strong quarterly report, the PEG of just 0.56 discounts INVA stock excessively.
Improving Balance Sheet
Innoviva ended the third quarter (as of Sep. 30, 2018) with $48.6 million cash on hand. But liabilities fell sharply: the company no longer has $25 million in its current portion of its long-term debt. Accrued interest payable fell sharply, from $5.92 million down to $1.775 million.
Considering expenses fell more than markets expected and interest expenses halved, chances are good that management may reinstate its dividend or initiate a share buyback. That would attract income investors back and would drive Innoviva’s share price back to $20 or above.
+++
https://seekingalpha.com/article/4231937-innoviva-50-percent…" target="_blank" rel="nofollow ugc noopener">
https://seekingalpha.com/article/4231937-innoviva-50-percent…
eine der wenigeren Aktien mit einem bullishen setting:

+++
Strong Q3 Results
Innoviva reported earnings of $0.43 a share as revenue soared 26.8% to $61.68 million. The company’s balance sheet improved nicely after a $110 million Term B loan repayment. Royalty revenue from Glaxo Group (GSK) rose to $65.1 million, with $51.7 million coming from global sales of REVLAR/BREO ELLIPTA. Expenses fell by around half to $4.0 million, compared to $8.6 million last year. Even though last year’s costs included a $2.5 million proxy contest related litigation cost, management is finally driving sales higher.
+++
INVA shares trade at an annualized P/E of 10.3 times and a forward P/E of 8.8 times. In light of the strong quarterly report, the PEG of just 0.56 discounts INVA stock excessively.
Improving Balance Sheet
Innoviva ended the third quarter (as of Sep. 30, 2018) with $48.6 million cash on hand. But liabilities fell sharply: the company no longer has $25 million in its current portion of its long-term debt. Accrued interest payable fell sharply, from $5.92 million down to $1.775 million.
Considering expenses fell more than markets expected and interest expenses halved, chances are good that management may reinstate its dividend or initiate a share buyback. That would attract income investors back and would drive Innoviva’s share price back to $20 or above.
+++
https://seekingalpha.com/article/4231937-innoviva-50-percent…" target="_blank" rel="nofollow ugc noopener">
https://seekingalpha.com/article/4231937-innoviva-50-percent…
eine der wenigeren Aktien mit einem bullishen setting:

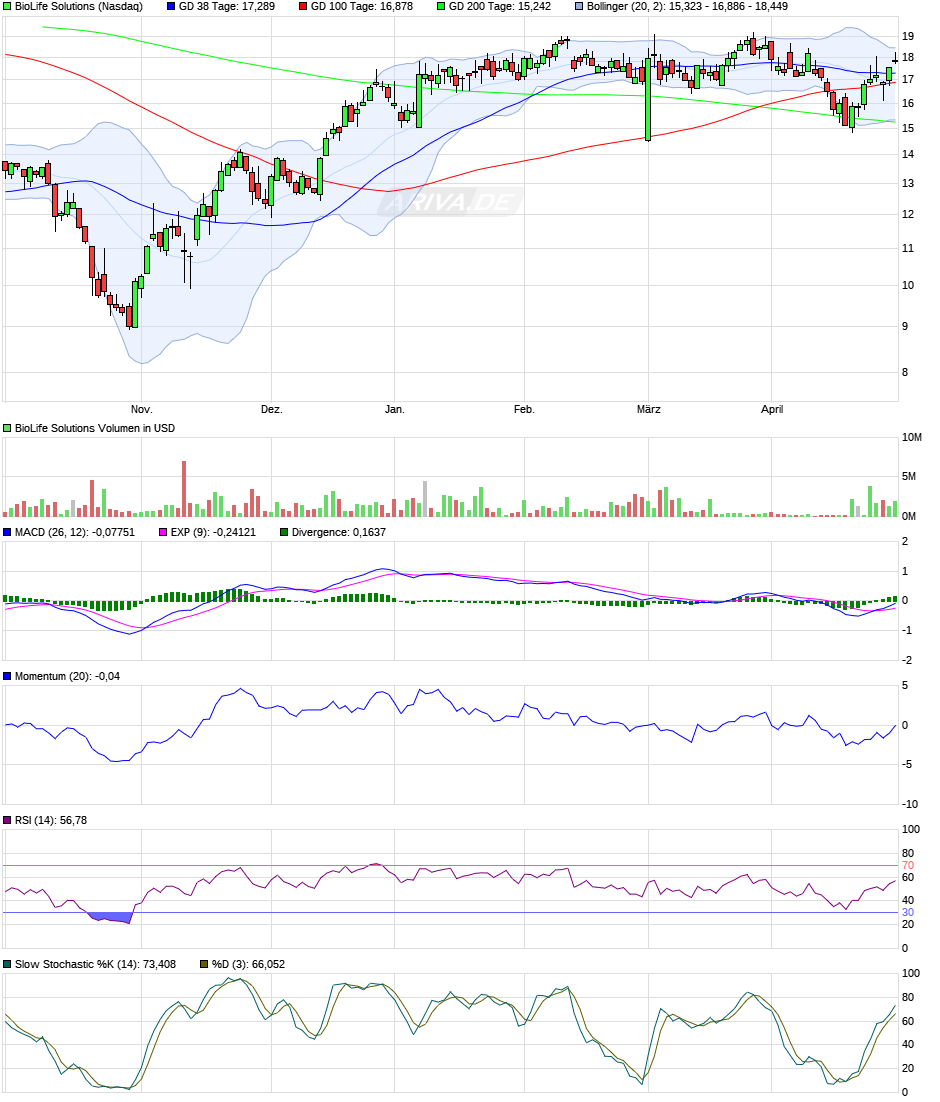
zu BLFS - comeback läuft.